Abstract
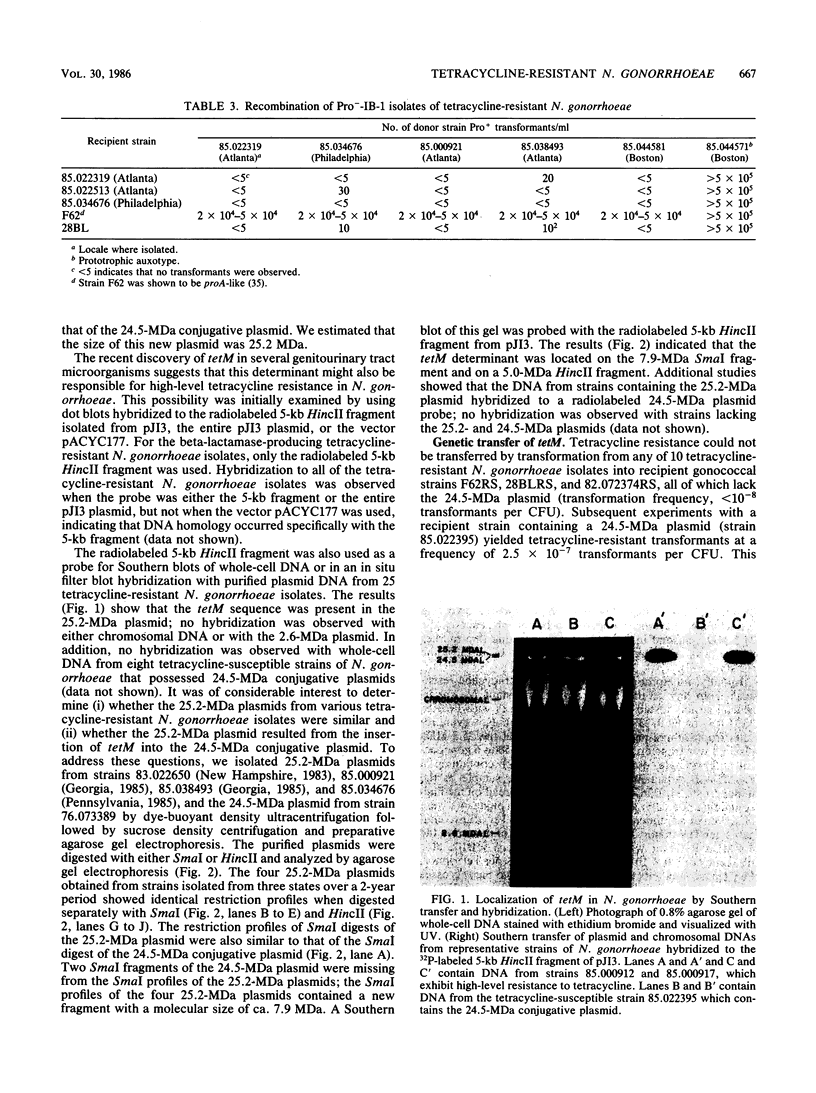
Recently, strains of Neisseria gonorrhoeae have been isolated which are highly resistant to tetracycline (MICs of 16 to 64 micrograms/ml). This resistance was due to the acquisition of the resistance determinant tetM, a transposon-borne determinant initially found in the genus Streptococcus and more recently in Mycoplasma hominis, Ureaplasma urealyticum, and Gardnerella vaginalis. In N. gonorrhoeae, the tetM determinant was located on a 25.2-megadalton plasmid. This plasmid arose from the insertion of tetM into the 24.5-megadalton gonococcal conjugative plasmid. The tetM determinant could be transferred to suitable recipient strains of N. gonorrhoeae by both genetic transformation and conjugation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddle J. W., Swenson J. M., Thornsberry C. Disc agar diffusion antimicrobial susceptibility tests with beta-lactamase producing Neisseria gonorrhoeae. J Antibiot (Tokyo) 1978 Apr;31(4):352–358. doi: 10.7164/antibiotics.31.352. [DOI] [PubMed] [Google Scholar]
- Biswas G. D., Graves J. F., Sox T. E., Tenover F. C., Sparling P. F. Marker rescue by a homologous recipient plasmid during transformation of gonococci by a hybrid Pcr plasmid. J Bacteriol. 1982 Jul;151(1):77–82. doi: 10.1128/jb.151.1.77-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J Bacteriol. 1986 Feb;165(2):564–569. doi: 10.1128/jb.165.2.564-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Reyn A. Neisseria gonorrhoeae isolated from disseminated and localised infections in pre-penicillin era. Auxotypes and antibacterial drug resistances. Br J Vener Dis. 1982 Jun;58(3):158–165. doi: 10.1136/sti.58.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Faur Y. C., Weisburd M. H., Wlson M. E., May P. S. NYC medium for simultaneous isolation of Neisseria gonorrhoeae, large-colony mycoplasmas, and T-mycoplasmas. Appl Microbiol. 1974 Jun;27(6):1041–1045. doi: 10.1128/am.27.6.1041-1045.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett F., Humphreys G. O., Saunders J. R. Intraspecific and intergeneric mobilization of non-conjugative resistance plasmids by a 24.5 megadalton conjugative plasmid of Neisseria gonorrhoeae. J Gen Microbiol. 1981 Jul;125(1):123–129. doi: 10.1099/00221287-125-1-123. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco C. A., Knapp J. S., Clark V. L. Conjugation of plasmids of Neisseria gonorrhoeae to other Neisseria species: potential reservoirs for the beta-lactamase plasmid. J Infect Dis. 1984 Sep;150(3):397–401. doi: 10.1093/infdis/150.3.397. [DOI] [PubMed] [Google Scholar]
- Hartley D. L., Jones K. R., Tobian J. A., LeBlanc D. J., Macrina F. L. Disseminated tetracycline resistance in oral streptococci: implication of a conjugative transposon. Infect Immun. 1984 Jul;45(1):13–17. doi: 10.1128/iai.45.1.13-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison C., Glynn A. A., Bascomb S. Acquisition of new genes by oral Neisseria. J Clin Pathol. 1982 Oct;35(10):1153–1157. doi: 10.1136/jcp.35.10.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Bialasiewicz A. A., Blenk H. Evaluation of plasmids in tetracycline resistant strains of Neisseria gonorrhoeae and Ureaplasma urealyticum in a case of severe urethritis. Eur J Epidemiol. 1985 Dec;1(4):294–300. doi: 10.1007/BF00237105. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B., Roberts M., Smith A., Levy S. B. Homogeneity of transferable tetracycline-resistance determinants in Haemophilus species. J Infect Dis. 1984 Jun;149(6):1028–1029. doi: 10.1093/infdis/149.6.1028. [DOI] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Romig W. R. Self-transfer and genetic recombination mediated by P, the sex factor of Vibrio cholerae. J Bacteriol. 1972 Nov;112(2):707–714. doi: 10.1128/jb.112.2.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. J., Biddle J. W., JeanLouis Y. A., DeWitt W. E., Blount J. H., Morse S. A. Chromosomally mediated resistance in Neisseria gonorrhoeae in the United States: results of surveillance and reporting, 1983-1984. J Infect Dis. 1986 Feb;153(2):340–345. doi: 10.1093/infdis/153.2.340. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Dissemination of the tetM tetracycline resistance determinant to Ureaplasma urealyticum. Antimicrob Agents Chemother. 1986 Feb;29(2):350–352. doi: 10.1128/aac.29.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Koutsky L. A., Holmes K. K., LeBlanc D. J., Kenny G. E. Tetracycline-resistant Mycoplasma hominis strains contain streptococcal tetM sequences. Antimicrob Agents Chemother. 1985 Jul;28(1):141–143. doi: 10.1128/aac.28.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Piot P., Falkow S. The ecology of gonococcal plasmids. J Gen Microbiol. 1979 Oct;114(2):491–494. doi: 10.1099/00221287-114-2-491. [DOI] [PubMed] [Google Scholar]
- Short H. B., Ploscowe V. B., Weiss J. A., Young F. E. Rapid method for auxotyping multiple strains of Neisseria gonorrhoeae. J Clin Microbiol. 1977 Sep;6(3):244–248. doi: 10.1128/jcm.6.3.244-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Blackman E., Biswas G., Sparling P. F. Conjugative plasmids in Neisseria gonorrhoeae. J Bacteriol. 1978 Apr;134(1):278–286. doi: 10.1128/jb.134.1.278-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Cloning genes for proline biosynthesis from Neisseria gonorrhoeae: identification by interspecific complementation of Escherichia coli mutants. J Bacteriol. 1984 May;158(2):696–700. doi: 10.1128/jb.158.2.696-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Bonin P., Holmes K. K., Gutman L., Wiesner P., Alexander E. R. Isolation of viruses, bacteria and other organisms from venereal disease clinic patients: methodology and problems associated with multiple isolations. Health Lab Sci. 1973 Apr;10(2):75–81. [PubMed] [Google Scholar]
- Wilson C., Rose D. L., Tramont E. C. Increased antibiotic resistance of Neisseria gonorrhoeae in Korea. Antimicrob Agents Chemother. 1976 Apr;9(4):716–718. doi: 10.1128/aac.9.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]