Abstract
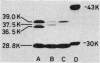
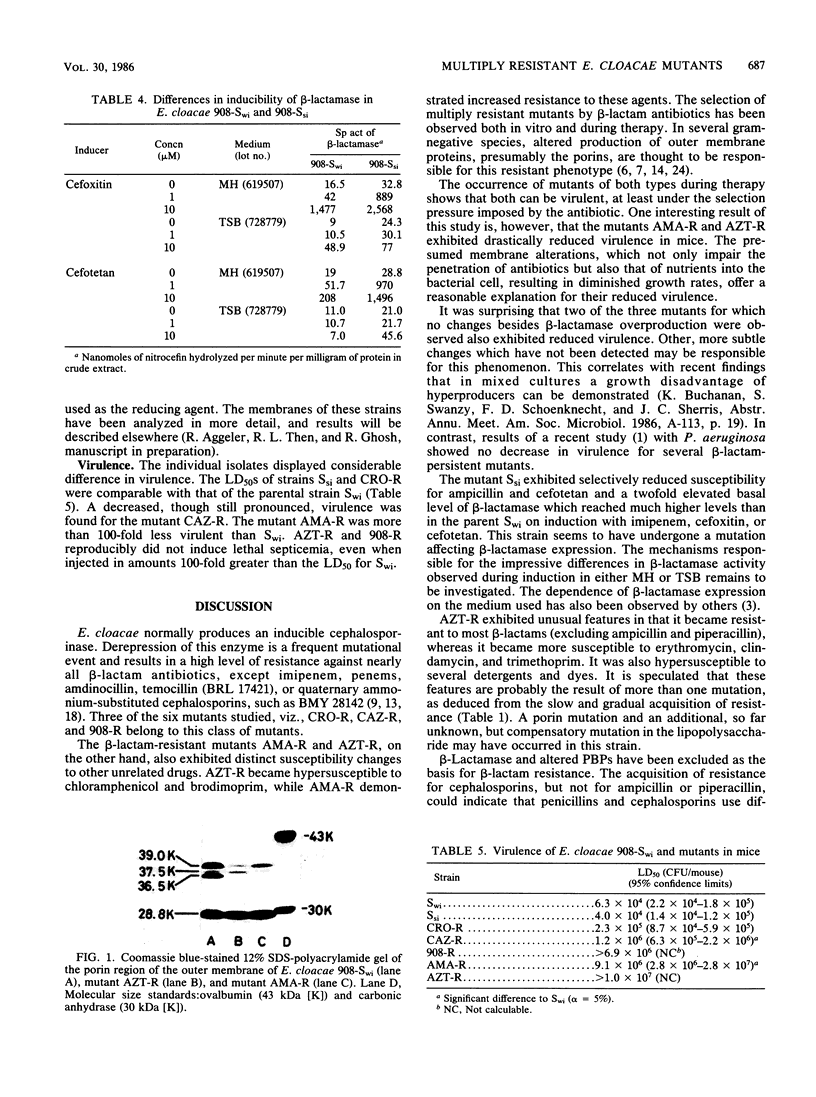
Mutants of Enterobacter cloacae, selected in vitro with ceftriaxone, ceftazidime, carumonam, or aztreonam, fell into several distinct classes. Three mutants highly resistant to nearly all beta-lactam antibiotics were stably derepressed for beta-lactamase production. Although no other changes could be detected, virulence in a mouse septicemia model was decreased in two of these mutants. One mutant, 908-Ssi, showed selectively decreased susceptibility to ampicillin and cefotetan. A change in beta-lactamase expression was thought to be responsible for this. Alterations in the production of two outer membrane proteins with molecular sizes of 36.5 and 39 kilodaltons were responsible for multiple antibiotic resistance in two mutants, both of which acquired a low level of resistance to beta-lactam antibiotics. Whereas one of the mutants, AMA-R, simultaneously acquired resistance to chloramphenicol and trimethoprim, the other, AZT-R, became hypersusceptible to these and other hydrophobic agents. Both strains had drastically reduced virulence in mice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E., Godfrey A. J., Schollardt T. Virulence of Pseudomonas aeruginosa strains with mechanisms of microbial persistence for beta-lactam and aminoglycoside antibiotics in a mouse infection model. Can J Microbiol. 1985 Apr;31(4):377–380. doi: 10.1139/m85-072. [DOI] [PubMed] [Google Scholar]
- Burnell E., van Alphen L., Verkleij A., de Kruijff B., Lugtenberg B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli. III. The outer membrane. Biochim Biophys Acta. 1980 Apr 24;597(3):518–532. doi: 10.1016/0005-2736(80)90224-2. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Dalhoff A., Dick W. Nonspecific induction of beta-lactamase in Enterobacter cloacae. J Gen Microbiol. 1984 Jul;130(7):1781–1786. doi: 10.1099/00221287-130-7-1781. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Govan J. R. Chromosomal loci associated with antibiotic hypersensitivity in pulmonary isolates of Pseudomonas aeruginosa. J Gen Microbiol. 1984 Apr;130(4):825–834. doi: 10.1099/00221287-130-4-825. [DOI] [PubMed] [Google Scholar]
- Giamarellou H., Koratzanis G., Kanellakopoulou K., Daikos G. K. Epidemiological study of Enterobacter cloacae resistant to 3rd generation cephalosporins: a preliminary report. Chemioterapia. 1985 Feb;4(1):43–46. [PubMed] [Google Scholar]
- Gutmann L., Chabbert Y. A. Different mechanisms of resistance to latamoxef (moxalactam) in Serratia marcescens. J Antimicrob Chemother. 1984 Jan;13(1):15–22. doi: 10.1093/jac/13.1.15. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Purification and characterization of two kinds of porins from the Enterobacter cloacae outer membrane. J Bacteriol. 1984 Jun;158(3):1179–1181. doi: 10.1128/jb.158.3.1179-1181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Yotsuji A., Inoue M., Mitsuhashi S. Induction of beta-lactamase by various beta-lactam antibiotics in Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Sep;18(3):382–385. doi: 10.1128/aac.18.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Microbial resistance to newer generation beta-lactam antibiotics: clinical and laboratory implications. J Infect Dis. 1985 Mar;151(3):399–406. doi: 10.1093/infdis/151.3.399. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe B., Basker M. J., Bentley P. H., Clayton J. P., Cole M., Comber K. R., Dixon R. A., Edmondson R. A., Jackson D., Merrikin D. J. BRL 17421, a novel beta-lactam antibiotic, highly resistant to beta-lactamases, giving high and prolonged serum levels in humans. Antimicrob Agents Chemother. 1981 Jul;20(1):38–46. doi: 10.1128/aac.20.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., Vaara M., Helander I. M., Viljanen P., Mäkelä P. H. New Salmonella typhimurium mutants with altered outer membrane permeability. J Bacteriol. 1984 Aug;159(2):704–712. doi: 10.1128/jb.159.2.704-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Hermann F. Properties of brodimoprim as an inhibitor of dihydrofolate reductases. Chemotherapy. 1984;30(1):18–25. doi: 10.1159/000238239. [DOI] [PubMed] [Google Scholar]
- Then R. L. Interaction of Ro 17-2301 (AMA-1080) with beta-lactamases. Chemotherapy. 1984;30(6):398–407. doi: 10.1159/000238300. [DOI] [PubMed] [Google Scholar]
- Then R. L., Kohl I. Affinity of carumonam for penicillin-binding proteins. Chemotherapy. 1985;31(4):246–254. doi: 10.1159/000238343. [DOI] [PubMed] [Google Scholar]
- Werner V., Sanders C. C., Sanders W. E., Jr, Goering R. V. Role of beta-lactamases and outer membrane proteins in multiple beta-lactam resistance of Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):455–459. doi: 10.1128/aac.27.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Tomiyama N., Hiruma R., Sawai T. Difference in pathway of Escherichia coli outer membrane permeation between penicillins and cephalosporins. FEBS Lett. 1985 Feb 11;181(1):143–148. doi: 10.1016/0014-5793(85)81130-3. [DOI] [PubMed] [Google Scholar]