Abstract
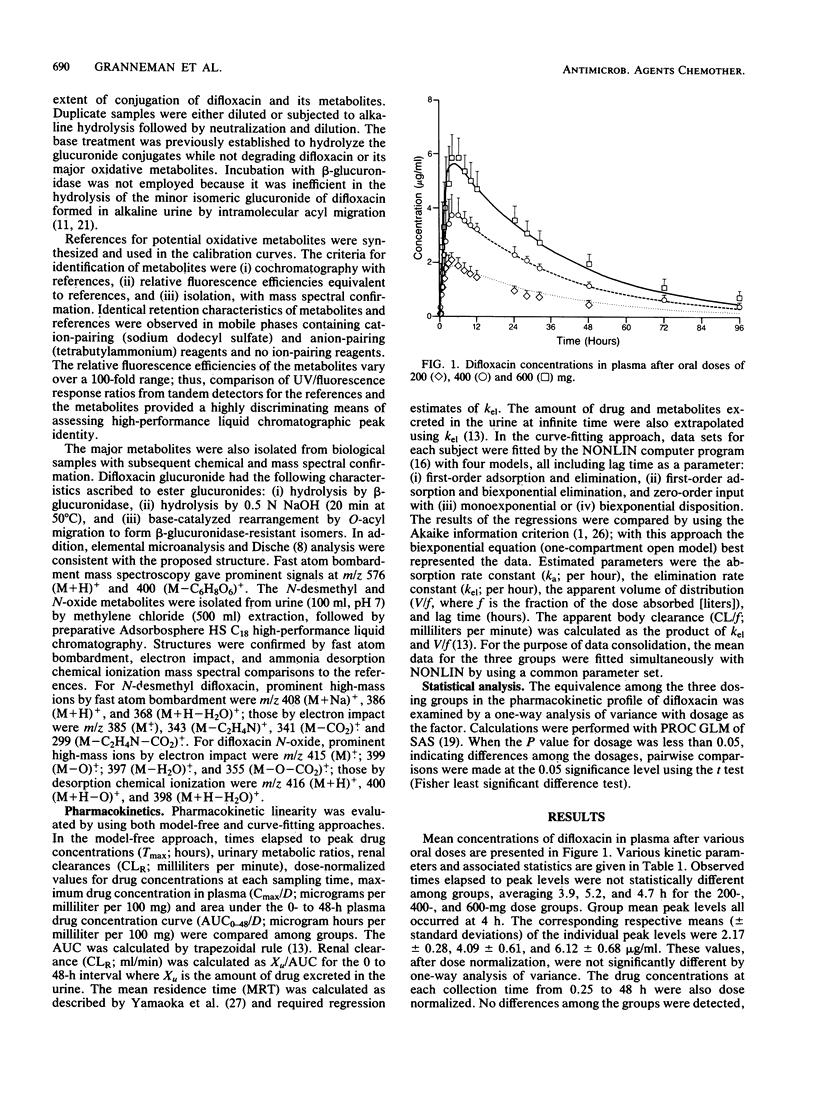
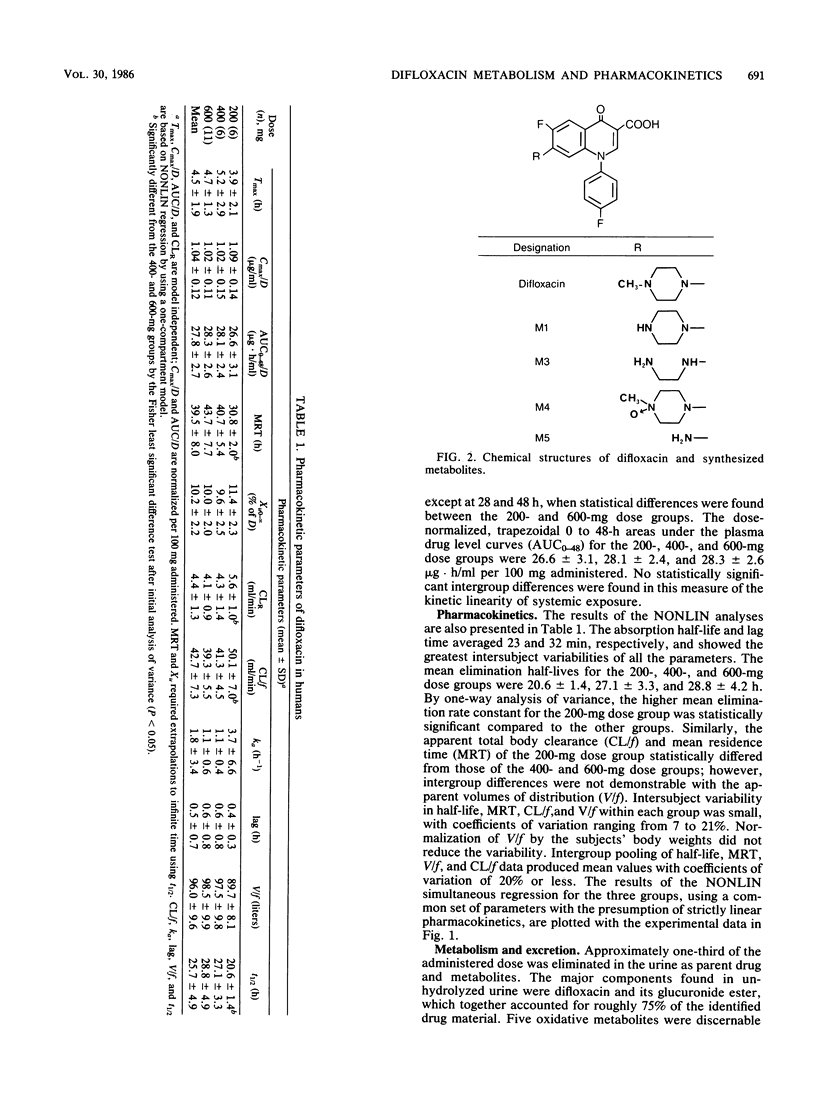
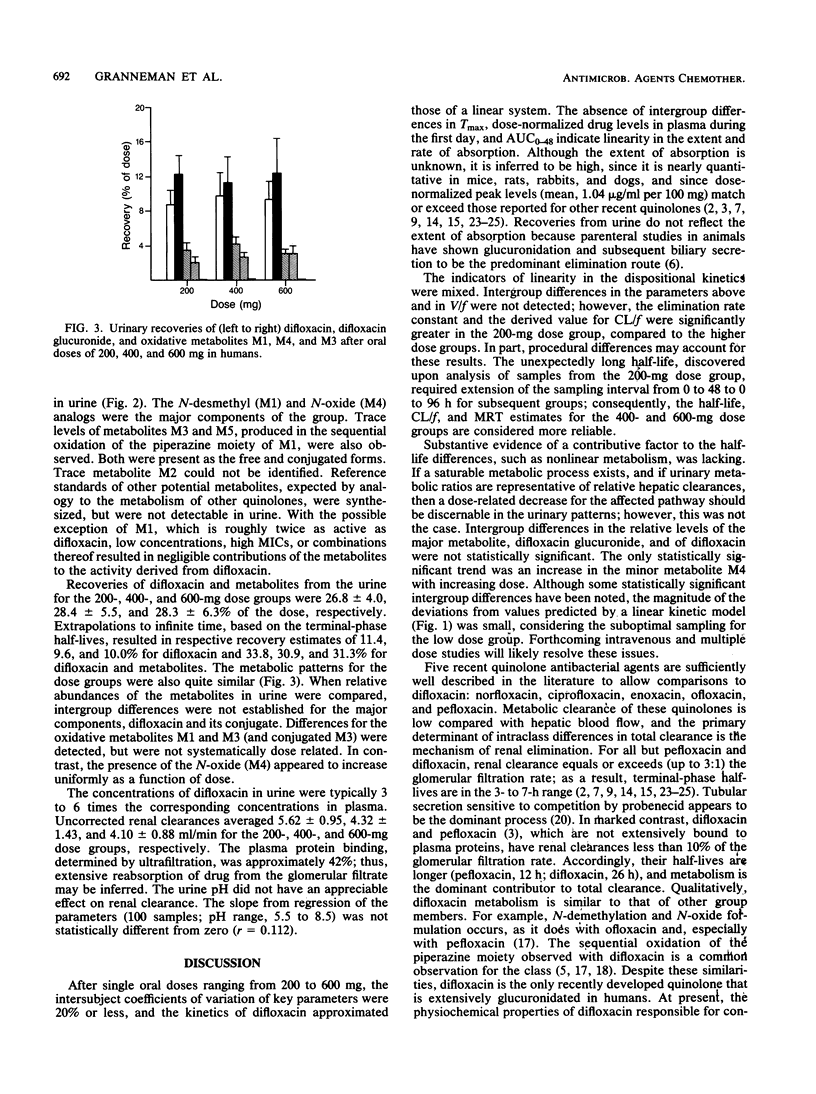
By using high-performance liquid chromatography, the metabolism and pharmacokinetics of difloxacin were characterized in humans after single oral doses of 200, 400, and 600 mg. Group mean peak levels in plasma were obtained 4 h after administration. The means of the individual peak levels for the 200-, 400-, and 600-mg groups were 2.17, 4.09, and 6.12 micrograms/ml, respectively. The mean respective terminal-phase half-lives were 20.6, 27.1, and 28.8 h; the mean half-life for all subjects was 25.7 h. Within the dose range studied, the behavior of difloxacin could be well described by a set of linear pharmacokinetic parameters with a one-compartment open model. Levels of unconjugated metabolites in plasma were negligible. The major urinary components were difloxacin and its glucuronide, each accounting for roughly 10% of the dose. Also present were the N-desmethyl and N-oxide metabolites, accounting for 2 to 4%. Trace levels of other metabolites were observed. Group mean renal clearances ranged from 4.1 to 5.6 ml/min, indicating extensive reabsorption from the glomerular filtrate. As a result, the terminal phase half-life and the dose-normalized area under the curve were substantially greater than those of other members of the class.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. E., Kenner C. H., Sloan R. S., Pottratz S. T. Multiple-dose ciprofloxacin kinetics in normal subjects. Clin Pharmacol Ther. 1984 Sep;36(3):384–388. doi: 10.1038/clpt.1984.192. [DOI] [PubMed] [Google Scholar]
- Barre J., Houin G., Tillement J. P. Dose-dependent pharmacokinetic study of pefloxacin, a new antibacterial agent, in humans. J Pharm Sci. 1984 Oct;73(10):1379–1382. doi: 10.1002/jps.2600731014. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. In vitro evaluation of A-56619 and A-56620, two new quinolones. Antimicrob Agents Chemother. 1986 Jan;29(1):40–43. doi: 10.1128/aac.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boppana V. K., Swanson B. N. Determination of norfloxacin, a new nalidixic acid analog, in human serum and urine by high-performance liquid chromatography. Antimicrob Agents Chemother. 1982 May;21(5):808–810. doi: 10.1128/aac.21.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eandi M., Viano I., Di Nola F., Leone L., Genazzani E. Pharmacokinetics of norfloxacin in healthy volunteers and patients with renal and hepatic damage. Eur J Clin Microbiol. 1983 Jun;2(3):253–259. doi: 10.1007/BF02029528. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Moellering A. E., Reiszner E., Moellering R. C., Jr In vitro activities of the quinolone antimicrobial agents A-56619 and A-56620. Antimicrob Agents Chemother. 1985 Oct;28(4):514–520. doi: 10.1128/aac.28.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faed E. M. Properties of acyl glucuronides: implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab Rev. 1984;15(5-6):1213–1249. doi: 10.3109/03602538409033562. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Chu D. T., Bower R. R., Jarvis K. P., Ramer N. R., Shipkowitz N. In vivo evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):201–208. doi: 10.1128/aac.29.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffler D., Dalhoff A., Gau W., Beermann D., Michl A. Dose- and sex-independent disposition of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):363–366. doi: 10.1007/BF01977496. [DOI] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R., Yamaguchi T., Sekine Y., Hashimoto M. Determination of a new antibacterial agent (AT-2266) and its metabolites in plasma and urine by high-performance liquid chromatography. J Chromatogr. 1983 Dec 9;278(2):321–328. doi: 10.1016/s0378-4347(00)84791-x. [DOI] [PubMed] [Google Scholar]
- Shimada J., Yamaji T., Ueda Y., Uchida H., Kusajima H., Irikura T. Mechanism of renal excretion of AM-715, a new quinolonecarboxylic acid derivative, in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1983 Jan;23(1):1–7. doi: 10.1128/aac.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. C., Hasegawa J., Langendijk P. N., Benet L. Z. Stability of acyl glucuronides in blood, plasma, and urine: studies with zomepirac. Drug Metab Dispos. 1985 Jan-Feb;13(1):110–112. [PubMed] [Google Scholar]
- Stamm J. M., Hanson C. W., Chu D. T., Bailer R., Vojtko C., Fernandes P. B. In vitro evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):193–200. doi: 10.1128/aac.29.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson B. N., Boppana V. K., Vlasses P. H., Rotmensch H. H., Ferguson R. K. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983 Feb;23(2):284–288. doi: 10.1128/aac.23.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender W., Graefe K. H., Gau W., Förster D., Beermann D., Schacht P. Pharmacokinetics of ciprofloxacin after oral and intravenous administration in healthy volunteers. Eur J Clin Microbiol. 1984 Aug;3(4):355–359. doi: 10.1007/BF01977494. [DOI] [PubMed] [Google Scholar]
- Wise R., Lockley R., Dent J., Webberly M. Pharmacokinetics and tissue penetration of enoxacin. Antimicrob Agents Chemother. 1984 Jul;26(1):17–19. doi: 10.1128/aac.26.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978 Dec;6(6):547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]


