Abstract
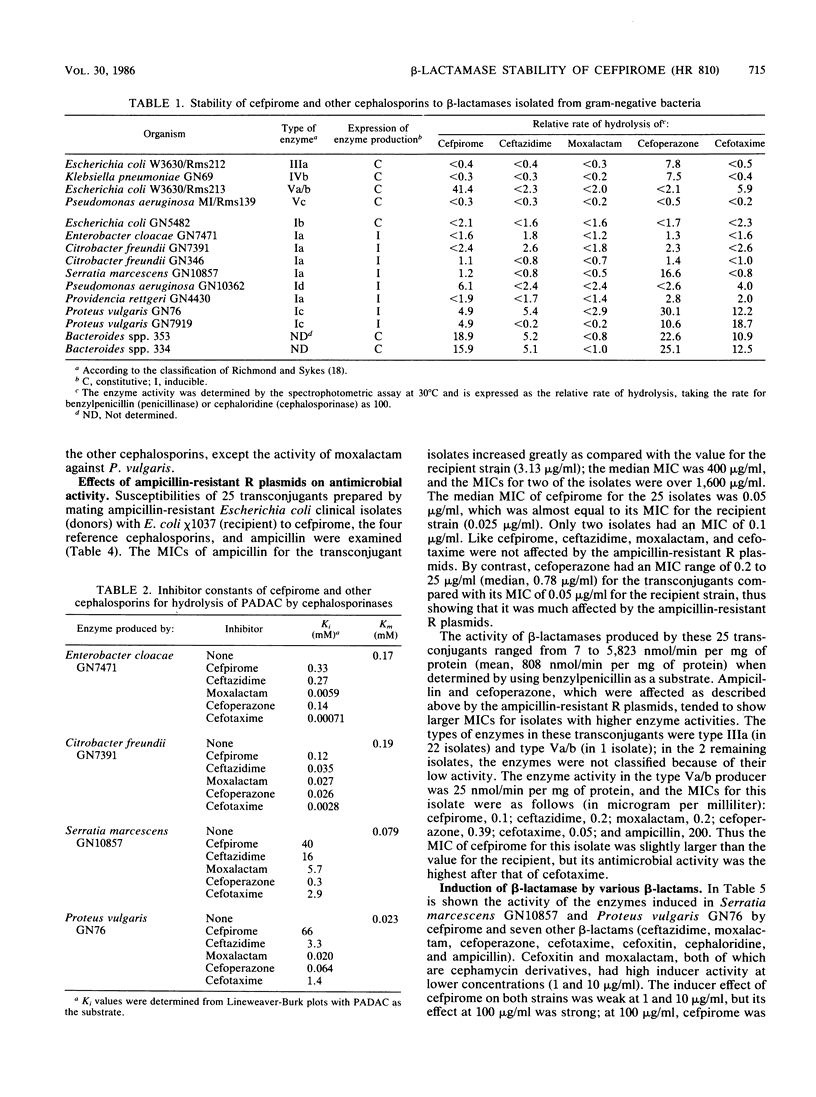
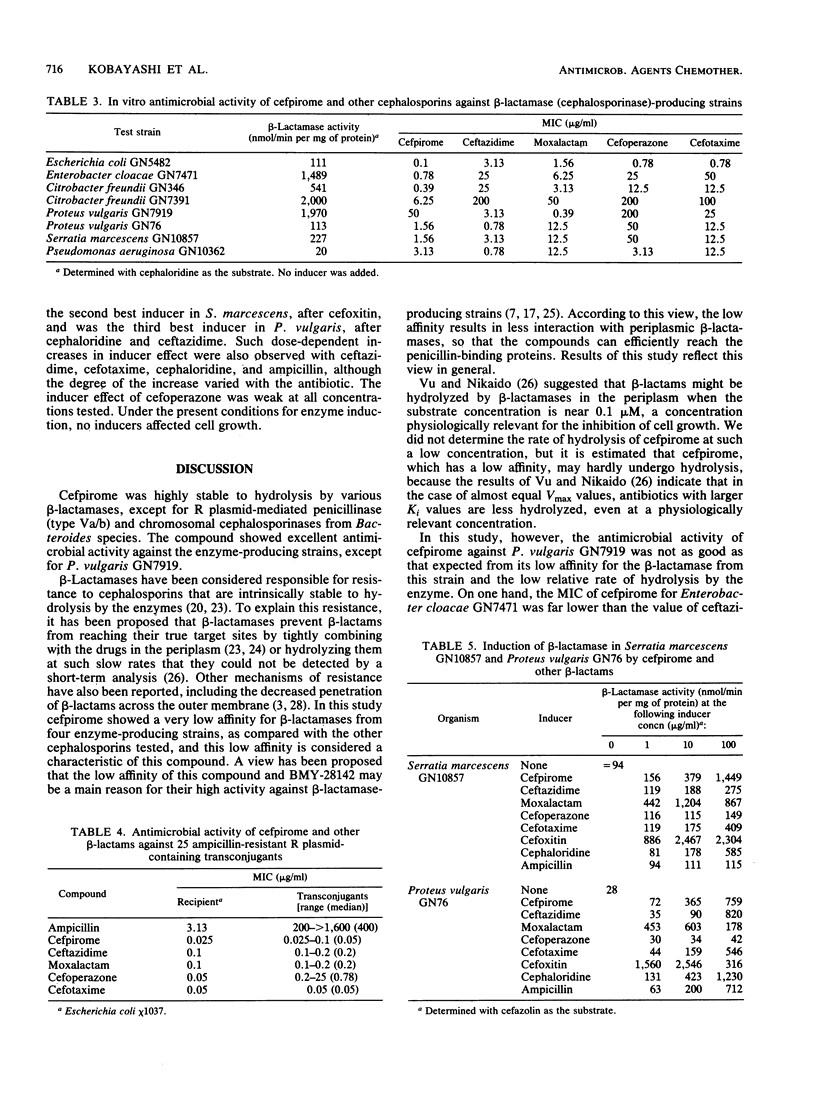
Cefpirome was highly stable to hydrolysis by various beta-lactamases, although it was hydrolyzed to some extent by R plasmid-mediated penicillinase of Richmond-Sykes type Va/b and by chromosomal cephalosporinases from Bacteroides species. The compound had a very low affinity for cephalosporinases from Enterobacter cloacae, Citrobacter freundii, Serratia marcescens, and Proteus vulgaris. Cefpirome showed strong antimicrobial activity against eight beta-lactamase (cephalosporinase)-producing strains which have become resistant to broad-spectrum cephalosporins; especially against E. cloacae and C. freundii, it had the highest activity among the cephalosporins used. Its activity against ampicillin-resistant R plasmid-containing transconjugant isolates of Escherichia coli was as high as that against the recipient strain E. coli chi 1037. The inducer activity of cefpirome in S. marcescens and P. vulgaris increased dose dependently, whereas cephamycin derivatives showed high inducer activity at low concentrations. A relatively low affinity of cefpirome for beta-lactamases is considered to be one of the reasons for its high antimicrobial activity against such enzyme-producing strains. In addition, other factors such as good penetration through the outer membrane and affinity for the target sites may also be involved in the high activity of cefpirome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauernfeind A. Susceptibility of gram-positive aerobic cocci to the new cephalosporin HR 810. Eur J Clin Microbiol. 1983 Aug;2(4):354–355. doi: 10.1007/BF02019468. [DOI] [PubMed] [Google Scholar]
- Bertram M. A., Bruckner D. A., Young L. S. In vitro activity of HR 810, a new cephalosporin. Antimicrob Agents Chemother. 1984 Aug;26(2):277–279. doi: 10.1128/aac.26.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Opferkuch W., Stieglitz M. Relation between beta-lactamase production and antimicrobial activity: comparison of the new compound HR 810 with cefotaxime. Eur J Clin Microbiol. 1983 Aug;2(4):350–352. doi: 10.1007/BF02019466. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Antibacterial activity of ceftizoxime, a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1980 Apr;17(4):583–590. doi: 10.1128/aac.17.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. The role of inducible beta-lactamases in the antagonism seen with certain cephalosporin combinations. J Antimicrob Chemother. 1981 Jan;7(1):104–107. doi: 10.1093/jac/7.1.104. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Barry A. L., Thornsberry C. Evaluation of the in vitro activity of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 May;27(5):679–682. doi: 10.1128/aac.27.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C., Barry A. L. In vitro evaluation of HR810, a new wide-spectrum aminothiazolyl alpha-methoxyimino cephalosporin. Antimicrob Agents Chemother. 1984 Jun;25(6):710–718. doi: 10.1128/aac.25.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machka K., Braveny I. In vitro activity of HR 810, a new broad-spectrum cephalosporin. Eur J Clin Microbiol. 1983 Aug;2(4):345–349. doi: 10.1007/BF02019465. [DOI] [PubMed] [Google Scholar]
- Masuyoshi S., Arai S., Miyamoto M., Mitsuhashi S. In vitro antimicrobial activity of cefotaxime, a new cephalosporin. Antimicrob Agents Chemother. 1980 Jul;18(1):1–8. doi: 10.1128/aac.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Inoue M., Mitsuhashi S. Purification and properties of a cephalosporinase from Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Dec;18(6):853–857. doi: 10.1128/aac.18.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S., Yotsuji A., Inoue M., Mitsuhashi S. Induction of beta-lactamase by various beta-lactam antibiotics in Enterobacter cloacae. Antimicrob Agents Chemother. 1980 Sep;18(3):382–385. doi: 10.1128/aac.18.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonogi K., Kuno M., Kida M., Mitsuhashi S. Beta-lactamase stability and antibacterial activity of cefmenoxime (SCE-1365), a novel cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):171–175. doi: 10.1128/aac.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. In vitro antagonism of beta-lactam antibiotics by cefoxitin. Antimicrob Agents Chemother. 1982 Jun;21(6):968–975. doi: 10.1128/aac.21.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert G., Limbert M., Winkler I., Dick T. The Antibacterial activity in vitro and beta-lactamase stability of the new cephalosporin HR 810 in comparison with five other cephalosporins and two aminoglycosides. Infection. 1983 Sep-Oct;11(5):275–279. doi: 10.1007/BF01641262. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Sawai T., Ando T., Yamagishi S. Cefoxitin resistance by a chromosomal cephalosporinase in Escherichia coli. J Antibiot (Tokyo) 1980 Sep;33(9):1037–1042. doi: 10.7164/antibiotics.33.1037. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolxdorff-Neutzling R. M., Wiedemann B. HR 810, a cephalosporin with low affinity for Enterobacter cloacae beta-lactamase. Eur J Clin Microbiol. 1983 Aug;2(4):352–354. doi: 10.1007/BF02019467. [DOI] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Werner V., Sanders C. C., Sanders W. E., Jr, Goering R. V. Role of beta-lactamases and outer membrane proteins in multiple beta-lactam resistance of Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):455–459. doi: 10.1128/aac.27.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Matsuura S., Mayama M., Kameda Y., Kuwahara S. Moxalactam (6059-S), a novel 1-oxa-beta-lactam with an expanded antibacterial spectrum: laboratory evaluation. Antimicrob Agents Chemother. 1980 Mar;17(3):302–312. doi: 10.1128/aac.17.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuji A., Minami S., Araki Y., Inoue M., Mitsuhashi S. Inducer activity of beta-lactam antibiotics for the beta-lactamases of Proteus rettgeri and Proteus vulgaris. J Antibiot (Tokyo) 1982 Nov;35(11):1590–1593. doi: 10.7164/antibiotics.35.1590. [DOI] [PubMed] [Google Scholar]


