Abstract
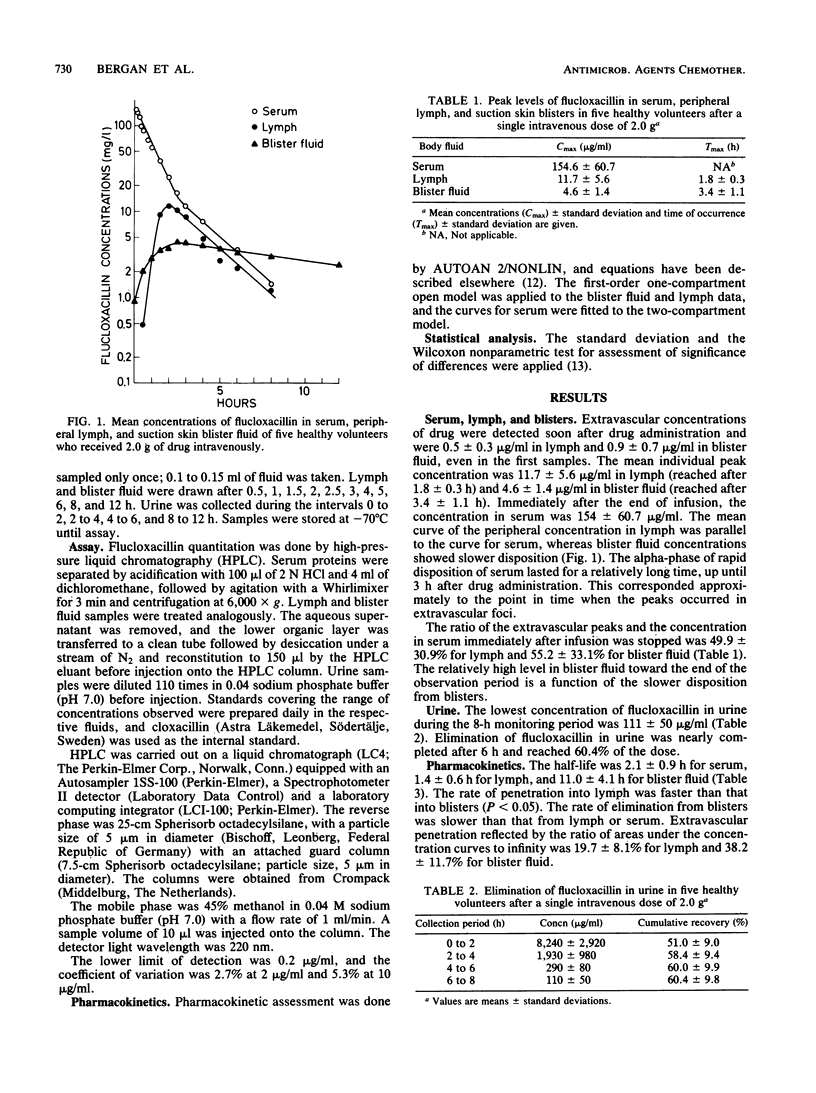
The pharmacokinetics of intravenously administered flucloxacillin (2.0 g to five volunteers) are described. The passage of flucloxacillin to peripheral lymph and suction skin blisters was monitored. This drug was selected because the high serum protein binding of 96% offered a particularly good opportunity for the study of the impact on tissue penetration. Flucloxacillin was assayed by high-pressure liquid chromatography, and pharmacokinetics were assayed by computerized curve fitting to accepted models. Penetration of flucloxacillin to extravascular foci was rapid. After 30 min the drug concentrations were 0.5 +/- 0.3 microgram/ml in lymph and 0.9 +/- 0.7 microgram/ml in blister fluid. The peak concentration was 11.7 +/- 5.6 micrograms/ml in lymph and 4.6 +/- 1.4 micrograms/ml in blister fluid. Concentrations in urine were above 111 +/- 50 micrograms/ml throughout the 8-h monitoring period, and urinary recovery was 60.4%. The half-life was 2.1 +/- 0.9 h in serum, 1.4 +/- 0.6 h in lymph, and 11.0 +/- 4.1 h in blister fluid. The differences in half-life were significant (P less than 0.05) between serum and blister fluid but not between lymph and serum. Penetration, as represented by the mean ratios of areas under the curve, was 19.7 +/- 8.1% to lymph and 38.2 +/- 11.7% to blister fluid. The flucloxacillin distribution volume during the phase of elimination was 36.4 +/- 16.0 liters and the total body clearance was 12.9 +/- 5.5 liters. Flucloxacillin showed good tissue penetration, considering its very high serum protein binding. High flucloxacillin levels in lymph and blister fluid were explained in part by drug affinity to extravascular albumin. The major impacts of high protein binding are (i) slightly slower passage into extravascular sites, (ii) slightly later peak concentration, and (iii) levels in extravascular fluid that are persistently below those in serum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T., Engeset A., Olszewski W., Josefsson K., Larsen N. Penetration of erythromycin into human peripheral lymph. J Antimicrob Chemother. 1982 Oct;10(4):319–324. doi: 10.1093/jac/10.4.319. [DOI] [PubMed] [Google Scholar]
- Bergan T., Engeset A., Olszewski W., Sjövall J. Distribution to human peripheral lymph of amoxycillin and of ampicillin from the oral prodrug bacampicillin. J Antimicrob Chemother. 1983 Nov;12(5):497–502. doi: 10.1093/jac/12.5.497. [DOI] [PubMed] [Google Scholar]
- Bergan T., Engeset A., Olszewski W., Solberg R. Pharmacokinetics of bacampicillin and bacmecillinam in plasma and peripheral lymph. Lymphology. 1979 Jun;12(2):85–94. [PubMed] [Google Scholar]
- Bergan T. Penicillins. Antibiot Chemother (1971) 1978;25:1–122. doi: 10.1159/000401058. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Suh B. Theory and practical impact of binding of antimicrobials to serum proteins and tissue. Scand J Infect Dis Suppl. 1978;(14):92–99. [PubMed] [Google Scholar]
- Craig W. A., Vogelman B. Changing patterns of hospital infections: implications for therapy. Changing concepts and new applications of antibiotic pharmacokinetics. Am J Med. 1984 Jul 31;77(1B):24–28. doi: 10.1016/s0002-9343(84)80092-3. [DOI] [PubMed] [Google Scholar]
- Engeset A., Hager B., Nesheim A., Kolbenstvedt A. Studies on human peripheral lymph. I. Sampling method. Lymphology. 1973 Mar;6(1):1–5. [PubMed] [Google Scholar]
- Hellum K. B., Solberg C. O. Human leucocyte migration: studies with an improved skin chamber technique. Acta Pathol Microbiol Scand C. 1977 Dec;85C(6):413–423. doi: 10.1111/j.1699-0463.1977.tb03663.x. [DOI] [PubMed] [Google Scholar]



