Abstract
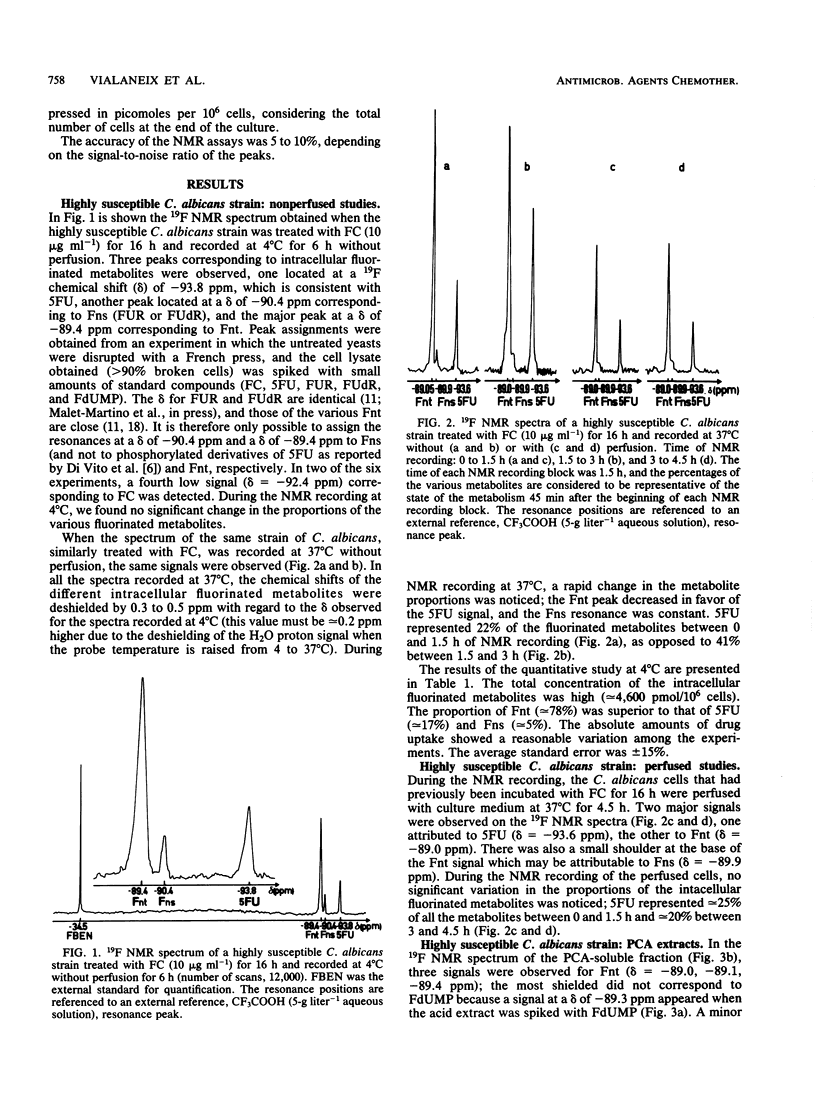
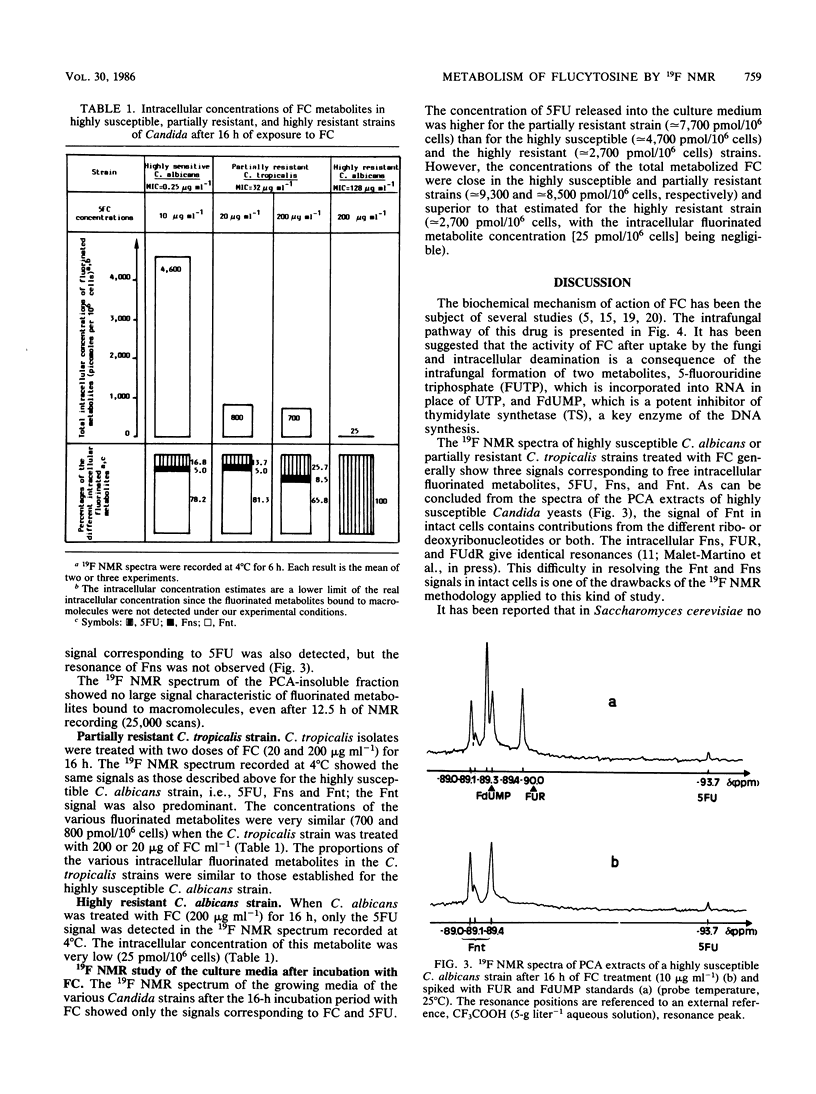
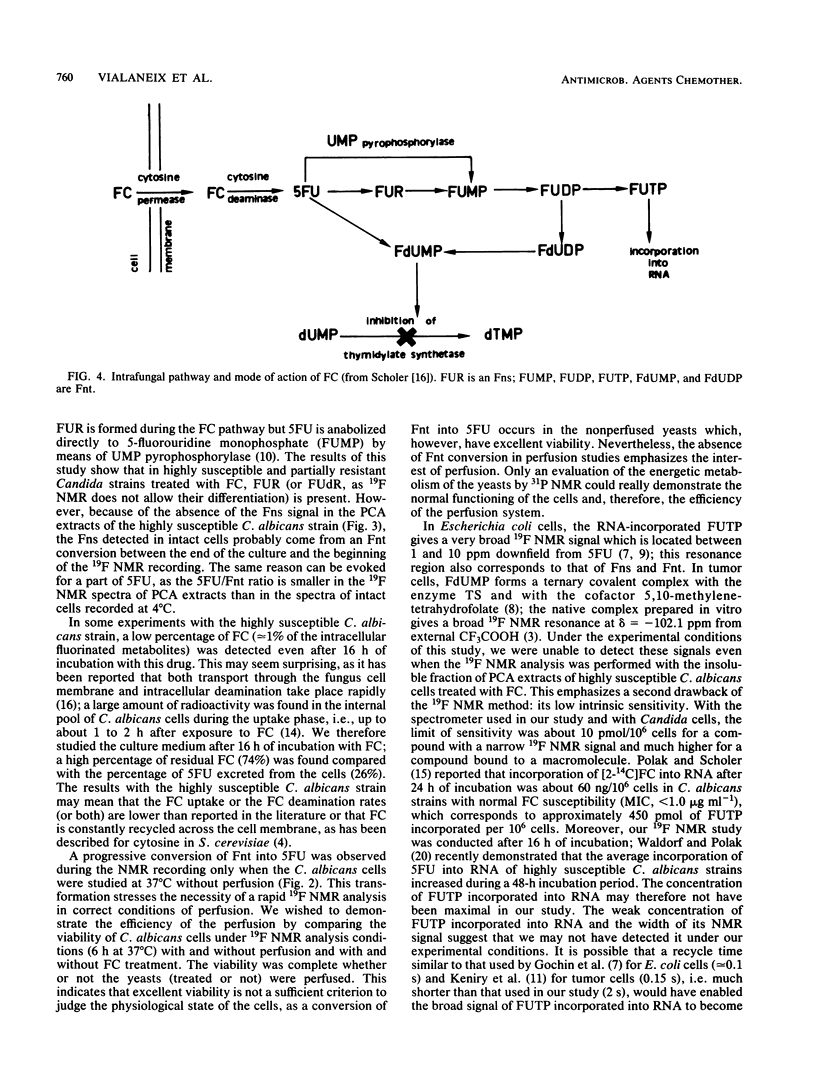
19F nuclear magnetic resonance was used for a noninvasive and quantitative study of flucytosine (FC; 5-fluorocytosine) metabolism in two strains of Candida albicans and one strain of Candida tropicalis with various susceptibilities to FC. Three intracellular fluorinated metabolites were detected in the highly susceptible strain, F-nucleotides (Fnt), F-nucleosides, and 5-fluorouracil (5FU). Fnt were partially converted into 5FU when the spectra of the yeasts were recorded at 37 degrees C without perfusion, but the intensities of the signals were not modified at 4 or 37 degrees C if the cells were perfused. In the acid extract, the Fnt signal was resolved into three distinct peaks; none of them was attributable to 5-fluoro-2'-deoxyuridine-5'-monophosphate. The same signals were detected in the partially resistant strain, but only 5FU was observed in the highly resistant strain; the resistance of the latter strain therefore was primarily due to a defect in UMP pyrophosphorylase. At the end of the incubation period, only FC and released 5FU were present in the culture media. The concentration of the intracellular fluorinated metabolites was increased if the strain was susceptible to FC. The total amount of metabolized FC was very similar for the highly susceptible and the partially resistant strains, but the percentage of Fnt was much higher in the former (38%) than in the latter (8%); the mild resistance of the partially resistant strain therefore was attributed to the decreased activity of UMP pyrophosphorylase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chevallier M. R., Jund R., Lacroute F. Characterization of cytosine permeation in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):629–641. doi: 10.1128/jb.122.2.629-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vito M., Podo F., Torosantucci A., Carpinelli G., Whelan W. L., Kerridge D., Cassone A. A 19F nuclear magnetic resonance study of uptake and metabolism of 5-fluorocytosine in susceptible and resistant strains of Candida albicans. Antimicrob Agents Chemother. 1986 Feb;29(2):303–308. doi: 10.1128/aac.29.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diasio R. B., Bennett J. E., Myers C. E. Mode of action of 5-fluorocytosine. Biochem Pharmacol. 1978 Mar 1;27(5):703–707. doi: 10.1016/0006-2952(78)90507-5. [DOI] [PubMed] [Google Scholar]
- Gochin M., James T. L., Shafer R. H. In vivo 19F-NMR of 5-fluorouracil incorporation into RNA and metabolites in Escherichia coli cells. Biochim Biophys Acta. 1984 May 22;804(1):118–124. doi: 10.1016/0167-4889(84)90105-8. [DOI] [PubMed] [Google Scholar]
- Hills D. C., Cotten M. L., Horowitz J. Isolation and characterization of two 5-fluorouracil-substituted Escherichia coli initiator methionine transfer ribonucleic acids. Biochemistry. 1983 Mar 1;22(5):1113–1122. doi: 10.1021/bi00274a019. [DOI] [PubMed] [Google Scholar]
- Jund R., Lacroute F. Genetic and physiological aspects of resistance to 5-fluoropyrimidines in Saccharomyces cerevisiae. J Bacteriol. 1970 Jun;102(3):607–615. doi: 10.1128/jb.102.3.607-615.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry M., Benz C., Shafer R. H., James T. L. Noninvasive spectroscopic analysis of fluoropyrimidine metabolism in cultured tumor cells. Cancer Res. 1986 Apr;46(4 Pt 1):1754–1758. [PubMed] [Google Scholar]
- Malet-Martino M. C., Armand J. P., Lopez A., Bernadou J., Béteille J. P., Bon M., Martino R. Evidence for the importance of 5'-deoxy-5-fluorouridine catabolism in humans from 19F nuclear magnetic resonance spectrometry. Cancer Res. 1986 Apr;46(4 Pt 2):2105–2112. [PubMed] [Google Scholar]
- Malet-Martino M. C., Martino R., Lopez A., Béteille J. P., Bon M., Bernadou J., Armand J. P. New approach to metabolism of 5'-deoxy-5-fluorouridine in humans with fluorine-19 NMR. Cancer Chemother Pharmacol. 1984;13(1):31–35. doi: 10.1007/BF00401443. [DOI] [PubMed] [Google Scholar]
- Polak A., Scholer H. J. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy. 1975;21(3-4):113–130. doi: 10.1159/000221854. [DOI] [PubMed] [Google Scholar]
- Spears C. P., Gustavsson B. G., Mitchell M. S., Spicer D., Berne M., Bernstein L., Danenberg P. V. Thymidylate synthetase inhibition in malignant tumors and normal liver of patients given intravenous 5-fluorouracil. Cancer Res. 1984 Sep;44(9):4144–4150. [PubMed] [Google Scholar]
- Stevens A. N., Morris P. G., Iles R. A., Sheldon P. W., Griffiths J. R. 5-fluorouracil metabolism monitored in vivo by 19F NMR. Br J Cancer. 1984 Jul;50(1):113–117. doi: 10.1038/bjc.1984.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. E., Shadomy S. Studies on the mode of action of 5-fluorocytosine in Aspergillus species. Chemotherapy. 1979;25(2):61–69. doi: 10.1159/000237824. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Polak A. Mechanisms of action of 5-fluorocytosine. Antimicrob Agents Chemother. 1983 Jan;23(1):79–85. doi: 10.1128/aac.23.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Kerridge D. Decreased activity of UMP pyrophosphorylase associated with resistance to 5-fluorocytosine in Candida albicans. Antimicrob Agents Chemother. 1984 Oct;26(4):570–574. doi: 10.1128/aac.26.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]


