Abstract
Lysophosphatidic acid (LPA) is a potent bioactive phospholipid that stimulates a variety of cellular responses by acting on cognate G protein-coupled receptors (GPCRs). There is increasing evidence that LPA signaling reprograms gene expression, but the GPCR-induced pathways connecting LPA receptor stimulation to downstream transcription factors are not well characterized. Here, we identify the adapter proteins Bcl10 and Malt1 as essential mediators of LPA-induced NF-κB activation. Both proteins were previously known to activate NF-κB in response to antigen receptor ligation on lymphocytes, but their functions in nonimmune cells are still largely undefined. By using murine embryonic fibroblasts from Bcl10- or Malt1-deficient mice as a genetic model, we report that Bcl10 and Malt1 are critically required for the degradation of IκB-α and the subsequent NF-κB induction in response to LPA stimulation. Bcl10 and Malt1 cooperate with PKCs selectively for LPA-induced NF-κB activation but are dispensable for the activation of the Jnk, p38, Erk MAP kinase, and Akt signaling pathways. In a biological readout, we demonstrate that LPA-induced IL-6 production is abolished in the absence of Bcl10. Thus, our results identify a NF-κB-inducing signaling pathway downstream of GPCRs and reveal previously unrecognized functions for Bcl10/Malt1 signaling in nonimmune cells.
Keywords: G protein-coupled receptor, signal transduction
NF-κB is a ubiquitously expressed dimeric transcription factor regulating inducible gene expression for cell proliferation, survival, differentiation and inflammation (1). It can be activated by a large variety of stimuli and plays critical roles in normal and disease physiology. Particularly, immune-cell-mediated pathologies and malignancies are causally connected to constitutive NF-κB activity (2–4). The mammalian NF-κB family contains five members: NF-κB1 (p105 and p50), NF-κB2 (p100 and p52), c-Rel, RelB, and RelA (p65). NF-κB dimers are retained in an inactive form in the cytoplasm by interactions with inhibitory IκB proteins. Most physiological and pathological signals for NF-κB activation depend on IκB kinase (IKK)-controlled events (1). Once activated, IKK phosphorylates IκB proteins on conserved serine residues, leading to ubiquitin-mediated IκB degradation and liberation of NF-κB, which then enters the nucleus to regulate the transcription of effector target genes.
A major challenge in the NF-κB field is to understand how distinct upstream stimuli activate IKK in a signal-specific manner (5). Recently, the adapter and scaffold proteins Bcl10 and Malt1 were identified as specific regulators of T cell receptor- and B cell receptor-mediated NF-κB activation (6). Originally, both molecules were isolated from chromosomal translocation breakpoints in mucosa-associated lymphoid tissue (MALT) lymphoma (7). Bcl10 is a caspase recruitment domain (CARD)-containing protein, and Malt1 is a molecule with structural relation to the caspase family of proteases. Bcl10 and Malt1 physically and functionally cooperate to couple antigen receptor-induced PKC signaling (PKC-θ in T cells or PKC-β in B cells) to IKK activation to mediate NF-κB induction (8–12). In addition, a recent report demonstrated essential functions for Bcl10 and Malt1 in FcεRI signaling (13). In mast cells, Bcl10 and Malt1 also operate downstream of PKC to selectively control FcεRI-induced NF-κB activation for proinflammatory cytokine production. The fact that Bcl10 and Malt1 also are expressed in nonimmune tissues suggests further still uncharacterized functions for Bcl10 and Malt1.
The largest family of cell surface receptors, which are expressed in virtually all mammalian tissues, are the G protein-coupled receptors (GPCRs) (14). In response to growth factors, hormones, neurotransmitters, or other stimuli, these receptors signal through a collection of heterotrimeric G proteins to activate downstream pathways for a broad spectrum of biological responses (15). There is increasing evidence that GPCRs are actively regulating gene transcription, and several GPCRs have been identified, which are able to activate NF-κB through unknown pathways (16). A prototypic GPCR agonist that can activate NF-κB is lysophosphatidic acid (LPA) (17, 18). LPA is a naturally occurring, water-soluble glycerophospholipid possessing growth factor-like activities. LPA can directly or indirectly (through the production of cytokines and chemokines) induce proliferation, migration, and survival of many cell types, normal and malignant (18). LPA activates distinct members of the endothelial differentiation gene (Edg) subfamily of GPCRs, which couple to at least three distinct G proteins (Gq, Gi, and G12/13) to feed into multiple effector systems, including NF-κB (18, 19).
Because LPA-induced NF-κB activation involves PKCs (20), we tested the hypothesis that Bcl10 and Malt1 might be required for LPA signal transduction. By using murine embryonic fibroblasts (MEFs) as a model system, we show that Bcl10 and Malt1 are specifically required to mediate activation of NF-κB and subsequent cytokine production in response to LPA treatment. These results identify a novel NF-κB-inducing signaling pathway and indicate previously unrecognized functions for the Bcl10/Malt1 complex in nonimmune cells.
Results
Bcl10 Is Required for LPA-Induced NF-κB Signaling.
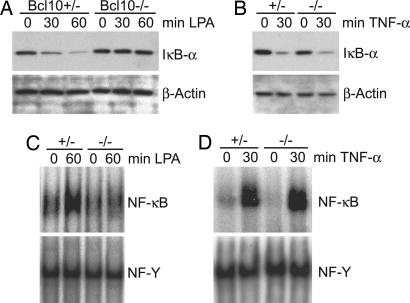
Because LPA can activate NF-κB in fibroblasts (17), we used MEFs as a model system to study a potential involvement of Bcl10 in LPA signaling (Fig. 1). We first stimulated MEFs from Bcl10-heterozygous (Bcl10+/−) or Bcl10-deficient (Bcl10−/−) mice with LPA and examined signal-induced degradation of IκB-α by Western blotting (Fig. 1A). Although we detected time-dependent degradation of IκB-α in Bcl10+/− MEFs, LPA stimulation did not induce IκB-α degradation in MEFs lacking Bcl10. As a control, we treated Bcl10+/− and Bcl10−/− cells with TNF-α, a strong NF-κB-inducing cytokine that signals independently of Bcl10 (9). In line with previous data, TNF-α treatment resulted in comparable IκB-α degradation in WT and in Bcl10+/− MEFs (Fig. 1B). Similar results were obtained with two independent Bcl10−/− cell lines (data not shown).
Fig. 1.
Bcl10 regulates LPA-induced NF-κB activation. (A and B) Bcl10+/− or Bcl10−/− MEFs were preincubated with cycloheximide and then stimulated with 10 μM LPA (A) or 10 ng/ml TNF-α (B) for the indicated times. Cell extracts were separated on 10% polyacrylamide gels, subjected to immunoblotting with anti-IκB-α antibody, and reprobed with anti-β-actin antibody to show equal loading. Experiments were repeated five times. (C and D) Bcl10+/− or Bcl10−/− MEFs were stimulated with LPA (C) or TNF-α (D) as described above; nuclear extracts were prepared and incubated with radiolabeled oligonucleotides specific for NF-κB or NF-Y as a loading control. Results are representative of three independent experiments.
The degradation of IκB-α frees NF-κB and allows its translocation into the nucleus and subsequent binding to DNA. To characterize further the role of Bcl10 in LPA-induced NF-κB activation, we performed gel retardation assays with nuclear extracts from stimulated Bcl10+/− and Bcl10−/− MEFs (Fig. 1C). Consistent with signal-induced IκB-α degradation, we observed an increase in NF-κB DNA-binding activity in Bcl10+/− MEFs after LPA stimulation. In contrast, LPA did not induce NF-κB activation in MEFs deficient for Bcl10 (Fig. 1C), whereas TNF-α was able to activate NF-κB regularly in these cells (Fig. 1D).
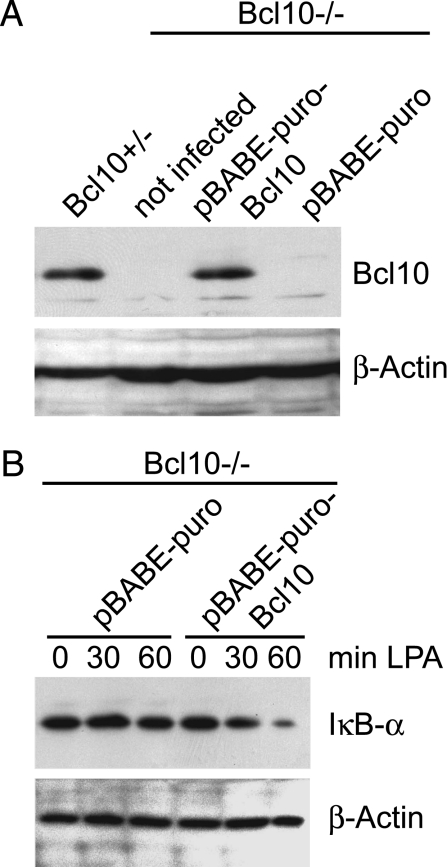
To study whether defective NF-κB signaling is a direct consequence of Bcl10 protein deficiency, we introduced the Bcl10 protein by retroviral expression into MEFs with a homozygous Bcl10 gene disruption. Control infections with a GFP-encoding retrovirus demonstrated a >80% efficiency of gene transfer into the cells (data not shown). Western blotting for Bcl10 expression revealed that retroviral gene transfer is able to induce Bcl10 expression to near normal levels (Fig. 2A). Both WT and Bcl10 reconstituted Bcl10−/− MEFs were then stimulated with LPA, and IκB-α protein degradation was monitored (Fig. 2B). Because reconstitution of Bcl10 in Bcl10−/− cells allowed IκB-α degradation (Fig. 2B), we conclude that Bcl10 directly controls NF-κB activation in response to LPA treatment via the classical pathway, depending on IκB-α degradation.
Fig. 2.
Retroviral reconstitution of Bcl10 gene-deficient MEFs rescues IκB-α degradation in response to LPA stimulation. (A) Bcl10-deficient MEFs were infected with the retroviral vector pBABE-puro encoding full-length Bcl10 (pBABE-puro-Bcl10) or an empty vector (pBABE-puro) as a negative control. Cell extracts were prepared and subjected to immunoblotting with anti-Bcl10-antibody and anti-β-actin antibody as a loading control. (B) Bcl10-deficient MEFs retrovirally infected with pBABE-puro or pBABE-puro-Bcl10 were stimulated with 10 μM LPA as described above, and cell extracts were subjected to immunoblotting by using antibodies against IκB-α and β-actin. Experiments were repeated three times.
Bcl10 Is Dispensable for LPA-Induced MAPK or Akt Activation.
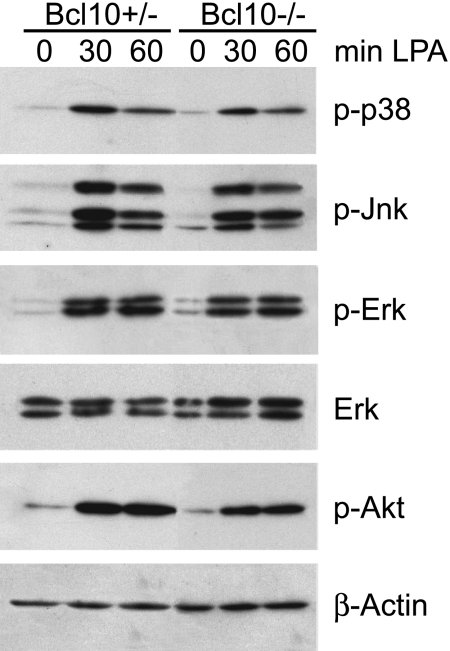
Previous work has indicated roles for Bcl10 in the activation of the Jnk and p38 MAPK kinase pathways in lymphocytes (6). To investigate whether Bcl10 regulates p38 or Jnk activation upon LPA stimulation, we treated WT and Bcl10−/− MEFs with LPA, and studied p38 and Jnk phosphorylation (Fig. 3). In parallel, we investigated the effects of the Bcl10 deletion on Akt and Erk signaling by performing Western blots with activation-specific phospho-antibodies against the respective kinases. We detected regular LPA-induced activation of the p38, Jnk, Erk, and Akt pathways in Bcl10−/− MEFs, demonstrating that Bcl10 is not involved in these signaling cascades.
Fig. 3.
Normal LPA-induced MAPK and Akt activation in Bcl10-deficient MEFs. Bcl10+/− or Bcl10−/− MEFs were stimulated with 10 μM LPA as indicated. Cell extracts were separated on polyacrylamide gels and immunoblotted with antibodies against phospho-p38, phospho-p44/p42 (phospho-Erk), phospho-Jnk, or phospho-Akt. After stripping, membranes were reprobed with antibodies against p44/p42 (Erk) or β-actin to control for equal loading. Results are representative of five independent experiments.
Pharmacological Blockage of PKC, but Not Akt, Inhibits LPA-Induced NF-κB Signaling.
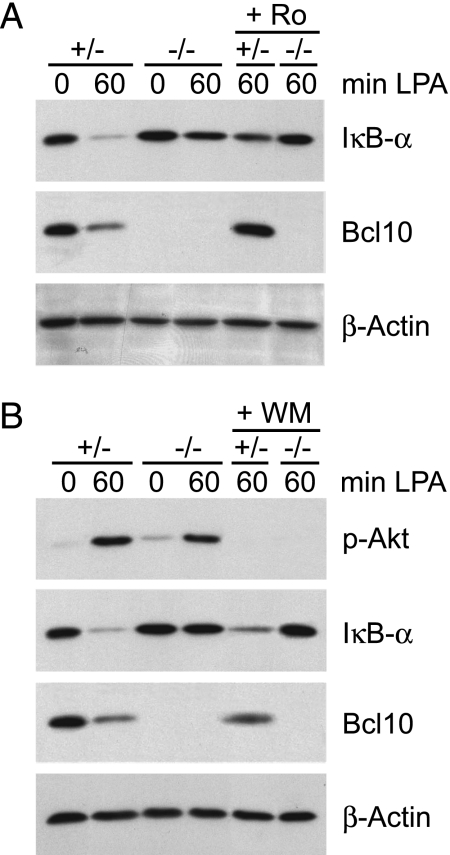
To study which upstream kinases might be involved in Bcl10-dependent LPA signaling to NF-κB, we preincubated MEFs with small-molecule PKC or phosphatidyl-inositol-3-kinase (PI3K) inhibitors (rottlerin for PKC and wortmannin for PI3K) 1 h before cell stimulation (Fig. 4). We chose to block these two enzymes because Bcl10 functions downstream of PKCs in immune cells and because PI3K-mediated Akt activation has recently been suggested to be involved in Bcl10-dependent activation of NF-κB in certain settings (21, 22). Consistent with previous data (17), PKC inhibition by rottlerin blocked LPA-induced degradation of IκB-α (Fig. 4A). However, blocking PI3K/Akt signaling did not inhibit LPA-induced activation of the NF-κB pathway, although PI3K-controlled Akt activation was impeded (Fig. 4B). Thus, the Bcl10-controlled NF-κB activation pathway engaged in response to LPA treatment is controlled by a PKC-dependent, Akt-independent mechanism.
Fig. 4.
PKC, not Akt, regulates LPA-induced NF-κB activation. Bcl10+/− or Bcl10−/− MEFs were treated for 1 h with the PKC inhibitor rottlerin (Ro, 3 μM) (A) or the PI3K inhibitor wortmannin (WM, 1 μM) (B) before stimulation with 10 μM LPA for the indicated times. Cell extracts were subjected to immunoblotting with antibodies against IκB-α, Bcl10, phospho-Akt, or β-actin as a loading control. Experiments were repeated independently three times.
LPA-Induced Activation of NF-κB Requires Malt1.
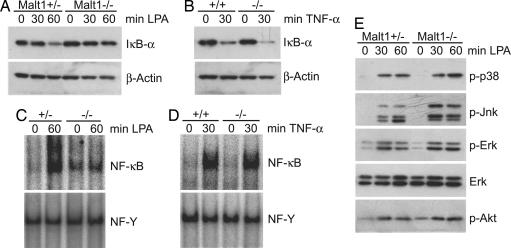
As cited in the introduction, Bcl10 can relay upstream signals to IKK via physical and functional interactions with Malt1, which play critical roles in immune-cell activation (6, 13). However, there are additional Bcl10 functions in nonimmune cells that do not require Malt1; for example, Bcl10-deficient embryos exhibit defects in neural tube closure, which are not observed in Malt1-deficient mice (8–10). To define whether Malt1 is involved in Bcl10-dependent LPA signaling, we stimulated MEFs from Malt1-deficient mice with LPA and studied signal-induced IκB-α degradation (Fig. 5A). LPA treatment induced degradation of IκB-α in Malt1+/− MEFs. However, IκB-α degradation was not observed in Malt1−/− MEFs, although IκB-α degradation in response to TNF-α stimulation was normal (Fig. 5B). Consistent with these results, we detected regular TNF-α-induced NF-κB DNA binding activity in Malt1−/− MEFs but defective LPA-induced NF-κB induction (Fig. 5 C and D). As shown before in Bcl10-deficient MEFs, activation of Akt and the MAP kinases p38, Erk1/2, and Jnk also was not affected by Malt1 deficiency, indicating that both Bcl10 and Malt1 are dispensable for MAPK and Akt activation (Fig. 5E).
Fig. 5.
Malt1 regulates LPA-induced NF-κB activation but not MAPK or Akt activation. (A and B) Malt1+/+, Malt1+/−, or Malt1−/− MEFs were preincubated with cycloheximide and then stimulated with 10 μM LPA (A) or 10 ng/ml TNF-α (B) for the indicated times. Total extracts were prepared and subjected to immunoblotting with an antibody against IκB-α and reprobed with anti-β-actin antibody for protein loading control. (C and D) Malt1+/+, Malt1+/−, or Malt1−/− MEFs were stimulated with LPA (C) or TNF-α (D) as described above; nuclear extracts were prepared and incubated with radiolabeled oligonucleotides against NF-κB or NF-Y as a loading control. (E) Malt1+/− or Malt1−/− MEFs were stimulated with 10 μM LPA as indicated, and cell extracts were immunoblotted with antibodies against phospho-p38, phospho-p44/p42 (phospho-Erk), phospho-Jnk, or phospho-Akt. After stripping, membranes were reprobed with antibodies against p44/p42 (Erk) as a loading control. Results are representative of four different experiments.
Bcl10 Signaling Controls LPA-Induced Production of the Cytokine IL-6.
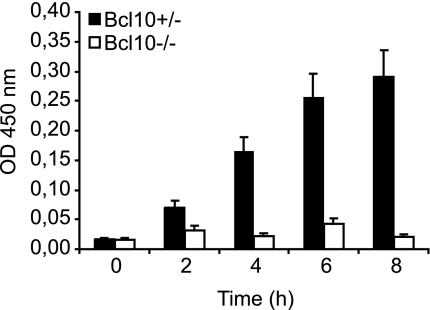
We next tested whether Bcl10-dependent NF-κB activation might control physiological outcomes of LPA stimulation. NF-κB is known to be required for LPA-induced expression of the proinflammatory cytokine IL-6 (19). Therefore, we analyzed the production of IL-6 in Bcl10+/− and Bcl10−/− MEFs in response to LPA treatment by ELISA (Fig. 6). Whereas WT cells produced IL-6 in a time-dependent fashion, LPA-induced IL-6 production was blocked in the absence of Bcl10. Thus, the Bcl10-controlled LPA signaling pathway is essential for physiological effects of LPA stimulation.
Fig. 6.
Impaired IL-6 production in Bc10-deficient MEFs. Bcl10+/− or Bcl10−/− MEFs were stimulated with 10 μM LPA for the indicated times. Supernatants were collected, and IL-6 concentrations were determined by ELISA. Data are given as means ± SEM and are representative of three independent experiments.
Discussion
Here, we identify a GPCR signal transduction pathway and discover functions for the Bcl10/Malt1 complex in nonimmune cells. By using MEFs as a model, we demonstrate on a genetic basis that Bcl10 and Malt1 are both essential to transduce signals upon LPA stimulation selectively to the canonical NF-κB pathway, which proceeds via degradation of IκB-α. In a biological readout for this cascade, we show that Bcl10-deficient MEFs have severe defects in the LPA-induced production of the NF-κB target cytokine IL-6.
Several previous reports have shown that cell stimulation with LPA can activate NF-κB (17, 19, 20, 23–26). LPA-induced NF-κB signaling controls the production of proinflammatory cytokines and chemokines in tumor cells, and can indirectly and directly contribute to the survival of malignant cells, indicating important pathophysiological functions for this pathway (18, 19, 23, 25, 27). We now provide mechanistic insights into this pathway. In line with recent work (20), we find that a pharmacological inhibition of PKCs with the pan-PKC inhibitor rottlerin, but not the inhibition of PI3K/Akt, blocked NF-κB induction by LPA. Several PKC isoforms can be activated by LPA stimulation in various cell types, including PKC-α, PKC-β, PKC-δ, PKC-ε, and PKC-ζ (20, 28). PKC-δ has been shown to control LPA-induced IL-8 production via NF-κB in bronchial epithelial cells (20). Yet the specific PKC isoform that regulates LPA signaling for NF-κB in fibroblast remains to be determined, for example, by using specific siRNAs. Because several PKCs are expressed in MEFs, it could be possible that there is redundancy at the PKC level. Nevertheless, the finding that a pan-PKC inhibition blocks NF-κB to a similar extent as the genetic deletion of Bcl10 or Malt1 indicates a nonredundant role for the Bcl10/Malt1 complex in the PKC-dependent LPA signaling. This model is consistent with the known essential roles of Bcl10 and Malt1 downstream of PKCs in immune cells.
Distinct PKC isoforms control NF-κB activation in response to T cell receptor, B cell receptor, or FcεRI signaling in a Bcl10-dependent manner (11–13, 29). Upon antigen receptor ligation, PKC-β (in B cells) or PKC-θ (in T cells) phosphorylate the Bcl10-binding molecule Carma1 (also called Card11/Bimp3). Carma1 is a member of a larger protein family additionally including Carma2 (Card14/Bimp2) and Carma3 (Card10/Bimp1). The Carma proteins possess a C-terminal CARD that can interact with the Bcl10 CARD, and they posses a coiled-coil domain and N-terminal MAGUK region (6). Carma1 is phosphorylated by PKCs in a region between the coiled coil and the MAGUK domain now referred to as the linker region (11, 12). The phosphorylation of Carma1 relieves inhibitory intramolecular interactions, allowing recruitment of Bcl10 and Malt1 to Carma1. These events initiate the assembly of higher-order signalosomes that contain additional proteins, including TRAF6, Tak1, Tak-associated proteins (Tab1, -2, or -3) and the ubiquitination enzymes Ubc13/Uev1A (30, 31). These proteins subsequently induce downstream IKK activation via processes that involve protein oligomerization and ubiquitinylation events (30, 31). We consider it likely that the LPA-induced NF-κB pathway also involves the assembly of Bcl10/Malt1 signalosomes, which, similar to the signalosomes in activated lymphocytes, could require a Carma molecule and include tumor necrosis factor receptor-associated factors, Tak1, and Tab proteins. Carma1 is specifically expressed in immune cells, and Carma2 is selectively expressed in the placenta (32, 33). Based on the broad expression of Carma3 in multiple tissues and the responsiveness of multiple cell lineages to LPA stimulation, we speculate that the Carma protein for PKC-dependent LPA signaling could be Carma3. The idea that Carma3 could physiologically regulate PKC-induced NF-κB signaling would be consistent with the findings that the Carma3 linker region can be phosphorylated by PKCs and that Carma3 can functionally replace Carma1 for PKC-dependent cell activation (11). However, Bcl10/Malt1 function for LPA signaling and Bcl10/Malt1 function for antigen receptor signaling differ in several aspects. Previous reports have indicated that p38 and Jnk are regulated by Bcl10 and Malt1 in response to antigen receptor signals (6). In contrast, we report here that LPA signaling to p38 and Jnk does not require Bcl10 or Malt1. Moreover, a recent study has indicated a function for PI3K/Akt signaling in Carma1-mediated NF-κB induction in T cells (21), whereas we do not detect effects of a PI3K/Akt inhibition on LPA-induced signaling to NF-κB. Together, these results indicate that the utilization of upstream and Bcl10/Malt1 effector pathways is cell-lineage and/or stimulus-specific and reinforces the necessity to investigate the function of this signaling complex in a context-dependent manner. Future experimental work is necessary to define the precise components and the regulation of Bcl10/Malt1-mediated NF-κB signaling in response to LPA stimulation.
LPA levels are significantly increased in various malignant effusions (18, 19), and LPA receptors are aberrantly expressed in several human cancers (18). Stimulation of tumor cells with LPA induces cell proliferation (34), cell survival, and drug resistance (35, 36) and promotes cell motility and invasion (37, 38) through still-undefined signaling pathways. Aberrant NF-κB signaling can mediate all these cellular outcomes (2). We show in conclusion that Bcl10/Malt1 signaling controls LPA-induced NF-κB activation. Based on these findings and the known roles of LPA in cancer, we suggest that the activity and function of Bcl10 and Malt1 signaling should be investigated in human malignancies beyond lymphomas.
Materials and Methods
Cell Culture and Cell Stimulation.
Murine embryonic fibroblasts (MEFs) from Bcl10+/−, Bcl10−/−, Malt1+/+, Malt1+/−, and Malt1−/− mice (9, 10) were cultured in DMEM supplemented with 10% (vol/vol) FCS/10 units/ml penicillin/10 μg/ml streptomycin/0.2 mM l-glutamine/50 μM 2-mercaptoethanol (all from GIBCO, Invitrogen, Carlsbad, CA). l-α-LPA (Sigma–Aldrich, St. Louis, MO), mouse tumor necrosis factor-α (TNF-α; Sigma-Aldrich), cycloheximide (Calbiochem, San Diego, CA), wortmannin (Calbiochem), or rottlerin (Calbiochem) were added as indicated.
Retroviral Infections.
Packaging cells (Phoenix E) were cultured in DMEM supplemented with 10% (vol/vol) FCS and transfected with a retroviral vector encoding full-length Bcl10 (pBABE-Puro-Bcl10) or an empty retroviral vector (pBABE-Puro). Virus-containing cell supernatant was collected 48 h later, and MEFs were spin-infected. Subsequently, puromycin was added to a final concentration of 1 μg/ml for cell selection.
Western Blot.
Cells were stimulated as indicated, rinsed with PBS, and lysed. Denatured proteins were separated on SDS/polyacrylamide gels, transferred to polyvinyldiflouride membranes, and subjected to immunoblotting with antibodies against Bcl10 (Santa Cruz Biotechnology, Santa Cruz, CA), IκB-α, phospho-p38, p38, phospho-p44/42, p44/42, phospho-JNK/SAPK, JNK/SAPK, phospho-Akt, Akt (all from Cell Signaling Technology, Danvers, MA), or β-actin (Sigma–Aldrich) and appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology).
Electromobility Shift Assays.
MEFs were subcultured on 10-cm, tissue-culture plates and stimulated with 10 μM LPA or 10 ng/ml TNF-α as indicated. Cells were washed with PBS, and nuclear extracts were prepared as previously described (9), incubated with 32P-end-labeled double-stranded oligonucleotide probes specific for NF-κB or NF-Y, separated on 5% polyacrylamide gels, and analyzed by phosphorimaging.
ELISA.
After stimulation of MEFs with LPA as indicated, cell supernatants were harvested and IL-6 concentration determined by an ELISA DuoSet (R & D Systems, Minneapolis, MN) as recommended by the manufacturer.
Acknowledgments
We thank Konstanze Pechloff for critically reading the manuscript. This work was supported by a Max-Eder-Program grant from Deutsche Krebshilfe and Sonderforschungsbereich grants from Deutsche Forschungsgemeinschaft (to J.R.).
Abbreviations
- CARD
caspase recruitment domain
- GPCR
G protein-coupled receptor
- IKK
IκB kinase
- LPA
lysophosphatidic acid
- MALT
mucosa-associated lymphoid tissue
- MEF
murine embryonic fibroblasts.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hayden MS, Ghosh S. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 2.Karin M. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Verma IM. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Yamamoto Y, Wang QM. Nat Rev Drug Discovery. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 5.Dixit V, Mak TW. Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- 6.Thome M. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson PG, Du MQ. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 8.Ruefli-Brasse AA, French DM, Dixit VM. Science. 2003;302:1581–1584. doi: 10.1126/science.1090769. [DOI] [PubMed] [Google Scholar]
- 9.Ruland J, Duncan GS, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, Millar DG, Bouchard D, Wakeham A, Ohashi PS, et al. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 10.Ruland J, Duncan GS, Wakeham A, Mak TW. Immunity. 2003;19:749–758. doi: 10.1016/s1074-7613(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 11.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, Rawlings DJ. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Wang D, Lin X. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Klemm S, Gutermuth J, Hultner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. J Exp Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 15.Marinissen MJ, Gutkind JS. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 16.Ye RD. J Leukocyte Biol. 2001;70:839–848. [PubMed] [Google Scholar]
- 17.Shahrestanifar M, Fan X, Manning DR. J Biol Chem. 1999;274:3828–33. doi: 10.1074/jbc.274.6.3828. [DOI] [PubMed] [Google Scholar]
- 18.Mills GB, Moolenaar WH. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 19.Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, et al. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 20.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 21.Narayan P, Holt B, Tosti R, Kane LP. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh PY, Kuo SH, Yeh KH, Chuang SE, Hsu CH, Chang WC, Lin HI, Gao M, Cheng AL. J Biol Chem. 2006;281:167–175. doi: 10.1074/jbc.M511014200. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Ye X, Mahanivong C, Bian D, Chun J, Huang S. J Biol Chem. 2005;280:10564–10571. doi: 10.1074/jbc.M412152200. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL. Am J Physiol. 2004;287:C1657–C1666. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 25.Raj GV, Sekula JA, Guo R, Madden JF, Daaka Y. Prostate. 2004;61:105–113. doi: 10.1002/pros.20083. [DOI] [PubMed] [Google Scholar]
- 26.Palmetshofer A, Robson SC, Nehls V. Thromb Haemostasis. 1999;82:1532–1537. [PubMed] [Google Scholar]
- 27.Zhao Y, Usatyuk PV, Cummings R, Saatian B, He D, Watkins T, Morris A, Spannhake EW, Brindley DN, Natarajan V. Biochem J. 2005;385:493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seewald S, Schmitz U, Seul C, Ko Y, Sachinidis A, Vetter H. Am J Hypertens. 1999;12:532–537. doi: 10.1016/s0895-7061(98)00269-6. [DOI] [PubMed] [Google Scholar]
- 29.Tan SL, Parker PJ. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, Merriam S, Glucksmann MA, DiStefano PS, Alnemri ES, et al. J Biol Chem. 2001;276:21405–21409. doi: 10.1074/jbc.M102488200. [DOI] [PubMed] [Google Scholar]
- 33.Bertin J, Wang L, Guo Y, Jacobson MD, Poyet JL, Srinivasula SM, Merriam S, DiStefano PS, Alnemri ES. J Biol Chem. 2001;276:11877–11882. doi: 10.1074/jbc.M010512200. [DOI] [PubMed] [Google Scholar]
- 34.Goetzl EJ, Dolezalova H, Kong Y, Hu YL, Jaffe RB, Kalli KR, Conover CA. Cancer Res. 1999;59:5370–5375. [PubMed] [Google Scholar]
- 35.Frankel A, Mills GB. Clin Cancer Res. 1996;2:1307–1313. [PubMed] [Google Scholar]
- 36.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, et al. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 37.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- 38.Sawada K, Morishige K, Tahara M, Kawagishi R, Ikebuchi Y, Tasaka K, Murata Y. Cancer Res. 2002;62:6015–6020. [PubMed] [Google Scholar]