Abstract
G protein-coupled receptors (GPCRs) play pivotal roles in cell proliferation, differentiation, and survival. Although many studies indicate that the stimulation of GPCRs leads to NF-κB activation, the molecular mechanism by which GPCRs induced NF-κB activation remains largely unknown. Bcl10 is an essential adaptor molecule connecting antigen receptor signaling cascades to NF-κB activation in lymphocytes. However, the function of Bcl10 in nonlymphoid cells remains to be determined. In this study, we demonstrated that the deficiency of Bcl10 resulted in the defect in NF-κB activation induced by either expressing the constitutively active mutant of G protein or stimulation of cells with lysophosphatidic acid or endothelin-1, which activate their GPCR. In contrast, TNF-α-, LPS-, and integrin-induced NF-κB activation was not affected in Bcl10-deficient cells. Together, our results provide genetic evidence showing that Bcl10 is a key signaling component mediating NF-κB activation induced by GPCRs in nonlymphoid cells.
Keywords: lysophosphatidic acid, signal transduction, endothelin-1
G protein-coupled receptors (GPCRs) constitute a large family of cell surface receptors and play important roles in regulating cell migration, differentiation, proliferation, and survival (1). GPCRs transduce environmental signals across the plasma membrane through their ability to stimulate guanine nucleotide exchange by heterotrimeric G proteins (2). Exchange of GDP for GTP results in activation of the Gα subunits and dissociation of the Gβγ subunits. The Gα subunits contain several subgroups, including Gi, Gs, Gq, G16, and G12/13. These G proteins can independently activate their downstream signaling cascades composed of scaffold/adaptor molecules and effector enzymes, leading to activation of various transcription factors, including NF-κB (3).
The NF-κB family of transcription factors plays critical roles in controlling expression of survival factors, cytokines, and proinflammatory molecules (4). Stimulation of various cell surface receptors, including receptors for proinflammatory cytokines, Toll-like receptors, antigen receptors, and GPCRs, induces different signaling pathways, leading to activation of the IκB kinase (IKK) complex. The activated IKK phosphorylates the inhibitory molecules, IκBs, triggering the rapid ubiquitination and proteolysis of IκBs, which unmasks the nuclear localization sequence of NF-κB. NF-κB is then rapidly translocated from the cytoplasm into the nucleus, where it regulates the transcription of its target genes (5). Although many GPCRs, such as the receptors for lysophosphatidic acid (LPA), endothelin-1 (ET-1), and angiotensin-II, are shown to effectively activate NF-κB, the molecular mechanism by which GPCRs activate NF-κB remains largely unknown.
LPA is a potent bioactive lipid that elicits a wide variety of biological actions, particularly as an inducer of cell proliferation, migration, and survival. It regulates tissue remodeling, neurogenesis, myelination, olfactions, immunity, and reproductive functions (6). In addition to its physiological functions, LPA is involved in pathophysiological processes such as would healing, cancer, and atherosclerosis (7). LPA binds to its cognate GPCRs, LPA1–4, on the cell surface. These GPCRs initiate several signaling pathways through Gq, Gi, and G12/13, leading to the activation of various transcription factors, including NF-κB (8). It has been shown that NF-κB is the key transcription factor mediating LPA-induced expression of cytokines and prosurvival factors (9). Although the molecular mechanism by which LPA induces NF-κB activation remains to be determined, earlier studies on signaling pathways induced by LPA receptors and other GPCRs suggest that PKC mediates GPCR-induced NF-κB activation (9, 10). However, how PKC activates the IKK complex after the stimulation of GPCRs has yet to be determined.
Recent studies on antigen receptor signaling pathways have demonstrated that a scaffold protein, CARMA1, is required for antigen receptor-induced NF-κB activation and functions downstream of PKC (11–13). After the stimulation of T cell receptor, it induces activation of PKCθ, a novel isoform of PKC family members. The activated PKCθ phosphorylates CARMA1 (14, 15), which, in turn, recruits Bcl10 and MALT1 to form a CARMA1–Bcl10–MALT1 (CBM) complex. The formation of the CBM complex has been shown to activate IKK through an ubiquitination-dependent pathway (16–19), leading to activation of NF-κB. Because the CBM complex functions downstream of PKCs in antigen receptor signaling pathways, we postulated that the CBM complex may also play an important role downstream of PKCs in GPCR-induced signaling pathways. In this study, we have tested this hypothesis and demonstrated that Bcl10 is required for GPCR-induced NF-κB activation after stimulation by LPA and ET-1.
Results
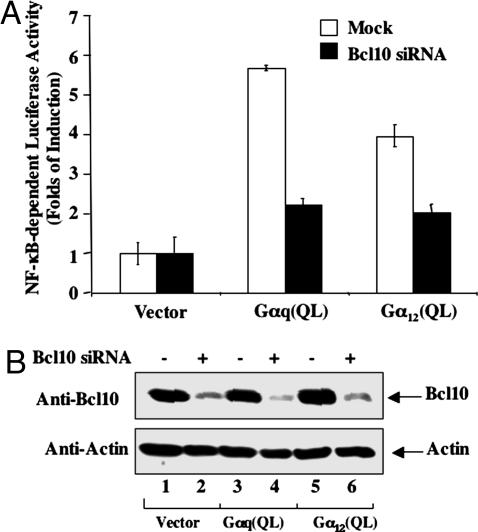
To examine whether Bcl10 is required for NF-κB activation induced by GPCRs, we first expressed the constitutively active mutants of Gαq and Gα12, which have been shown to mediate GPCR-induced NF-κB activation (20), in human embryo kidney 293T cells together with or without siRNA for Bcl10 (Fig. 1A). We found that expression of Gαq and Gα12 effectively activated NF-κB, whereas the transfection of Bcl10 siRNA reduced the expression of Bcl10 (Fig. 1B) and inhibited Gαq- and Gα12-induced NF-κB activation (Fig. 1A). Together, these results suggest that Bcl10 may be involved in G protein-mediated NF-κB activation.
Fig. 1.
Effect of Bcl10 siRNA on Gα-induced NF-κB activation. Human embryo kidney 293T cells were treated with transfection reagent alone or siRNA against Bcl10 (50 nM) for 48 h, then cells were transfected with NF-κB-dependent luciferase reporter together with Gαq(Q209L), Gα12(Q229), or pCMV5 vector DNA. At 6 h after transfection, the cells were starved for ≈16–18 h. Whole-cell lysates were prepared from these cells. (A) Luciferase activities were determined by dual-luciferase reporter assay. Data are the mean of triplicates from a representative experiment. (B) Cell lysates were subjected to 10% acrylamide SDS/PAGE followed by Western blot using the indicated antibodies.
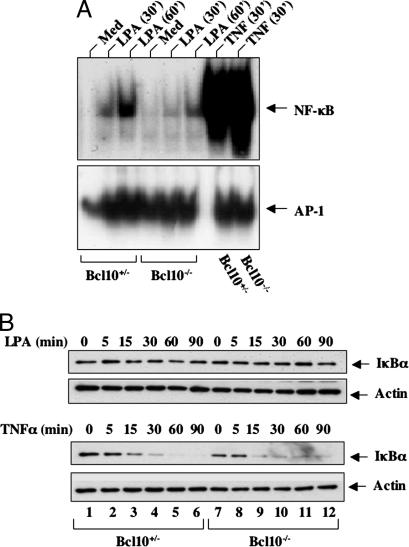
To further investigate whether Bcl10 is required for GPCR-induced NF-κB activation after a physiological stimulation, we made mouse embryonic fibroblasts (MEFs) from Bcl10+/− and Bcl10−/− mice. These cells were stimulated with or without LPA, and the nuclear extracts were prepared. LPA-induced NF-κB activation was examined by EMSA. Although LPA effectively induced NF-κB activation in Bcl10+/− MEF cells, this induction of NF-κB activation was significantly impaired in Bcl10−/− MEF cells (Fig. 2A). This defect was specific for LPA-induced NF-κB pathway, because TNFα-induced NF-κB activation was not affected in Bcl10−/− cells (Fig. 2A). In the same experiment, we observed that activator protein-1 (AP-1) activation was not defected in Bcl10−/− cells, although the constitutive level of AP-1 activity was much higher in Bcl10−/− cells than in Bcl10+/− cells (Fig. 2A), which may be because NF-κB can negatively regulate AP-1 activity (21, 22). We next examined the degradation of IκBα after LPA or TNF-α stimulation. As shown in Fig. 2B, LPA stimulation slightly induced some degradation of IκBα in Bcl10+/− MEF cells (Fig. 2B Upper). In contrast, the IκBα level remained almost unchanged after LPA stimulation in Bcl10−/− MEF cells (Fig. 2B Upper), whereas TNF-α effectively induced IκBα degradation in both Bcl10+/− and Bcl10−/− MEF cells (Fig. 2B). Of note, LPA induced only a very small amount of IκBα degradation in comparison to TNF-α even in Bcl10−/− MEF cells, which is consistent with the fact that NF-κB activation induced by LPA is much weaker than that induced by TNF-α (Fig. 2A). However, LPA-induced degradation of IκBα is necessary for NF-κB activation, because inhibition of LPA-induced and proteasome-mediated IκBα degradation by proteasome inhibitor (MG132) completely abrogated LPA-induced NF-κB activation (data not shown). Together, these results indicate that although LPA induces significantly weaker NF-κB activation than TNF-α, Bcl10 is required for LPA-induced NF-κB activation.
Fig. 2.
Bcl10 is required for LPA-induced NF-κB activation. (A) Bcl10+/− or Bcl10−/− MEF cells were stimulated with LPA or TNF-α for 30 or 60 min. Nuclear extracts were prepared from these cells and used for EMSA with 32P-labeled NF-κB or AP-1 probes. Med, Medium. (B) Bcl10+/− or Bcl10−/− MEF cells were pretreated with cycloheximide for 30 min and then stimulated with LPA or TNF-α for the indicated time points. Cell lysates were prepared from these cells and subjected to SDS/PAGE and Western blot analysis with the antibodies as indicated.
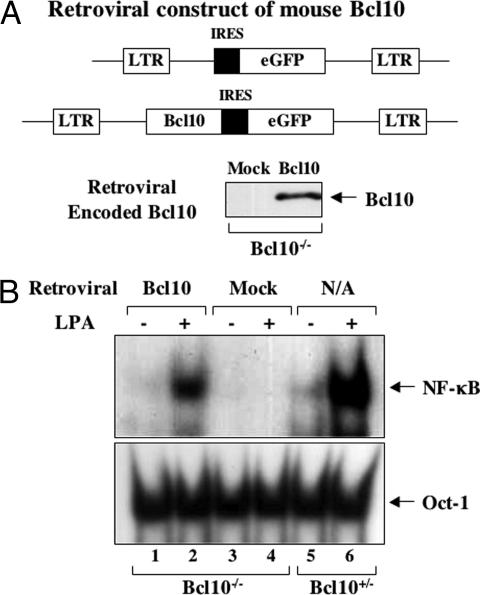
To further confirm that the defect of NF-κB activation induced by LPA in Bcl10−/− MEF cells is caused by the disruption of the Bcl10 gene, we constructed a retroviral vector encoding Bcl10 cDNA. In this construct, a GFP gene is under the control of an internal ribosome entry site (IRES) cassette downstream of Bcl10 gene (Fig. 3A) and is used to monitor the infection rate. These vectors were transfected into packaging cell lines to generate virus particles that were used to infect Bcl10−/− MEF cells. Although <30% of cells were infected (data not shown), Bcl10 expression was readily detected in the infected cells (Fig. 3B). Importantly, the expression of Bcl10 restored LPA-induced NF-κB activation in Bcl10−/− MEF cells (Fig. 3C). These results confirmed that Bcl10 is indeed a critical signaling molecule for LPA-induced NF-κB activation.
Fig. 3.
Retroviral-transduced Bcl10 rescues the defect in Bcl10−/− cells. (A) Schematic presentation of retroviral vectors encoding GFP or Bcl10–IRES–GFP. (B) Bcl10−/− MEF cells were infected with retrovirus encoding GFP (Mock) or Bcl10–IRES–GFP (Bcl10). Cell lysates were prepared and subjected to immunoblot with anti-Bcl10 antibodies. (C) Nuclear extracts were prepared from the retroviral-infected Bcl10−/−MEFs or Bcl10+/− MEF cells after LPA stimulation. NF-κB and Oct-1 activities were examined by EMSA with 32P-labeled NF-κB and AP-1 probes. N/A, noninfected.
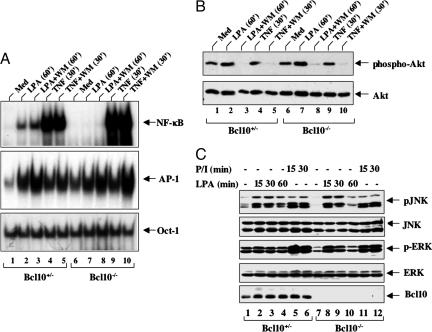
Previous studies also suggest that activation of phosphatidylinositol 3-kinase (PI3K) contributes to the activation of NF-κB induced by GPCRs (20). To investigate whether PI3K is involved in LPA-induced NF-κB activation, we pretreated MEF cells with or without a potent PI3K inhibitor, Wortmannin, which inhibits PI3K activation. LPA-induced activation of NF-κB and AP-1 was examined by EMSA. Interestingly, we found that NF-κB activation induced by both LPA and TNF-α was not inhibited with this inhibitor (Fig. 4A) in Bcl10+/− MEF cells, although this inhibitor significantly reduced the basal level of Akt phosphorylation (Fig. 4B). In contrast, LPA-induced NF-κB activation was significantly defected in Bcl10−/− MEF cells (Fig. 4A), Together these data indicate that the Bcl10-mediated, but not PI3K-Akt-mediated, pathway is required for LPA-induced NF-κB activation.
Fig. 4.
LPA-induced NF-κB activation depends on Bcl10, but not PI3K activation. (A) Bcl10+/− or Bcl10−/− MEF cells were stimulated with LPA and TNF-α in the absence or presence of Wortmannin (WM). Nuclear extracts and cell lysates were prepared for EMSA with 32P-labeled NF-κB, AP-1, or Oct-1 probes. Med, medium. (B) Lysates of these cells were subjected to Western blot analysis with the antibodies indicated. (C) Bcl10+/− or Bcl10−/− MEF cells were stimulated with LPA or PMA plus ionomycin (P/I) for various time points. Cell lysates were prepared and subjected to Western blot analysis with the antibodies indicated.
To investigate whether the requirement of Bcl10 in LPA-induced signaling pathways is specific for NF-κB activation, we determined MAP kinase activation after the stimulation of Bcl10+/− and Bcl10−/− MEF cells with LPA. The activation of JNK and ERK in these cells was examined by Western blotting analyses using antibodies against phospho-JNK or phospho-ERK. We found that LPA and phorbol-12-myristate-13-acetate (PMA)/ionomycin stimulation induced the phosphorylation of JNK and ERK in both Bcl10+/− and Bcl10−/− MEF cells (Fig. 4C). Therefore, these results indicate that the deficiency of Bcl10 does not affect the activation of MAP kinases induced by LPA.
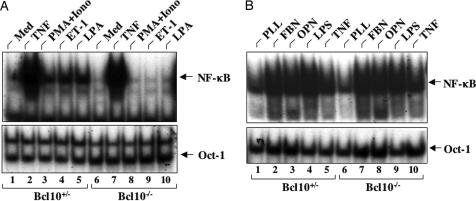
To examine whether Bcl10 is required for other GPCR-induced NF-κB activation, we stimulated Bcl10+/− and Bcl10−/− MEF cells with ET-1, another GPCR ligand, which also induces NF-κB activation. Similar to LPA, ET-1 effectively induced NF-κB activation in Bcl10+/− MEF cells, but it failed to do so in Bcl10−/− MEF cells (Fig. 5A). Consistent with the requirement of PKC isoforms in GPCR-induced NF-κB activation, although stimulation of Bcl10+/− MEF cells with PMA plus ionomycin, which activates PKCs, effectively activated NF-κB (Fig. 5A), this stimulation failed to induce NF-κB activation in Bcl10−/− MEF cells (Fig. 5A), suggesting that PKC-induced NF-κB activation depends on Bcl10. Because earlier studies suggest that PKC is also involved in integrin-induced NF-κB activation (23), we hypothesized that integrin-induced NF-κB also depends on Bcl10. Surprisingly, we found that osteopontin and fibronectin, which activate αvβ3 and αvβ1 integrin, respectively, effectively induced NF-κB activation in both Bcl10+/− and Bcl10−/− MEF cells (Fig. 5B). In addition, we examined whether Toll-like receptor-induced NF-κB depends on Bcl10 and found that LPS induced comparable levels of NF-κB activation in both Bcl10+/− and Bcl10−/− MEF cells (Fig. 2B). Together, these results indicate that Bcl10 is selectively involved in GPCR-induced NF-κB activation.
Fig. 5.
Bcl10 is required for NF-κB activation induced by ET-1, LPA, and PMA/ionomycin, but not by LPS, TNF-α, and integrins. (A) Bcl10+/− or Bcl10−/− MEF cells were stimulated with or without ET-1, LPA, PMA (20 ng/ml) plus ionomycin (PMA+Iono; 200 ng/ml), or TNF-α (10 ng/ml). Nuclear extracts were prepared from these cells and subjected to EMSA with 32P-labeled NF-κB or Oct-1 probes. Med, medium. (B) Bcl10+/− or Bcl10−/− MEFs were plated onto dishes precoated with poly-l-Lys (PLL), osteopontin (OPN), or fibronectin (FBN) for 2 h. For TNF-α and LPS stimulation, cells were plated onto PLL-coated dishes for 1.5 h and then stimulated with TNF-α (10 ng/ml) or LPS (10 ng/ml) for 30 min. Nuclear extracts were prepared from these cells and subjected to EMSA with 32P-labeled NF-κB or Oct-1 probes.
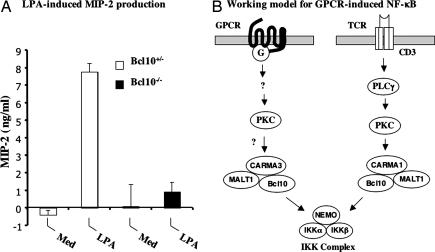
IL-8 is the major chemoattractant and activator for neutrophils and is a key component of innate immunity. Recently, it was reported that LPA-induced IL-8 production depends on NF-κB in human bronchial epithelial cells (10). Because Bcl10 mediates LPA-induced NF-κB activation, we examined whether LPA-induced IL-8 production would be defected in Bcl10−/− MEF cells. To test this hypothesis, supernatants from LPA-stimulated Bcl10+/− and Bcl10−/− MEF cells were analyzed for the production of macrophage inflammatory protein-2 (MIP-2), the murine homolog of human IL-8. Although LPA stimulation induced a significant increase of MIP-2 production in Bcl10+/− MEF cells, this induction was significantly reduced in Bcl10−/− MEF cells (Fig. 6A). Thus, the defect in LPA-induced NF-κB activation resulted in impaired production of MIP-2 in Bcl10−/− MEF cells after LPA stimulation.
Fig. 6.
LPA-induced MIP-2 production depends on Bcl10. (A) Bcl10+/− or Bcl10−/− MEF cells were subcultured into 10-cm dishes in DMEM supplemented with 10% FCS and antibiotics for 48 h. The cell cultures were stimulated with or without LPA for another 24 h. Cultured media were collected and cleared by centrifugation for ELISA analysis. MIP-2 level was determined by using the MIP-2 Quantikine kit from R&D Systems. Med, medium. (B) A schematic model of GPCR-induced and T cell receptor (TCR)-induced signaling cascades. In the GPCR pathway, proximal signaling events lead to activation of PKC, which, in turn, may regulate CARMA3 and Bcl10. The activated CARMA3 and Bcl10 may directly or indirectly regulate the IKK complex. In contrast, in the TCR pathway, PKC phosphorylates CARMA1, which induces the formation of the CARMA1–Bcl10–MALT1 complex, leading to activation of the IKK complex.
Discussion
GPCRs play pivotal roles in cell proliferation, differentiation, and survival. Although many studies indicate that stimulation of GPCRs leads to NF-κB activation, the molecular mechanism by which GPCRs induce NF-κB activation remains largely unknown. Bcl10 is an adaptor protein that mediates antigen receptor-induced NF-κB in lymphocytes. However, the physiological role of Bcl10 in nonlymphoid cells is completely unknown. In this study, we demonstrate that Bcl10 is required for GPCR-induced NF-κB activation. We have found that NF-κB activation induced by LPA or ET-1 is significantly defective in Bcl10−/− MEF cells (Figs. 2–5). This defect of NF-κB activation is very specific, because Bcl10 deficiency neither affects TNF-α-, LPS-, or integrin-induced NF-κB activation, nor GPCR-induced MAP kinase activation (Figs. 4 and 5). Although we only investigated NF-κB activation induced by two GPCRs, we were able to show that NF-κB activation induced by constitutively active mutants of Gα subunits was defective when endogenous Bcl10 expression was inhibited by Bcl10 siRNA (Fig. 1), suggesting that other GPCRs may also use the Bcl10-dependent pathway to activate NF-κB. Together, our results provide genetic evidence that GPCR-induced NF-κB activation depends on Bcl10.
Because Bcl10 forms a complex with MALT1 to activate the IKK complex through ubiquitination of the IKKγ/NEMO subunit of the IKK complex in antigen receptor signaling pathways, it is likely that Bcl10 also regulates IKK activation through forming a complex with MALT1 in GPCR-induced signaling pathways. Consistent with this hypothesis, Bcl10 has been shown to associate with MALT1 in nonlymphoid cells (24). When the expression level of MALT1 was partially inhibited by expressing MALT1 siRNA, we found that NF-κB activation induced by the constitutively active mutant of Gαq was also partially defective [supporting information (SI) Fig. 7], suggesting that MALT1 is also involved in GPCR-induced NF-κB activation. Nevertheless, additional studies, using cells deficient in MALT1, will be needed to definitely demonstrate whether MALT1 is required for GPCR-induced NF-κB activation.
Recent studies indicate that TAK1 is required for NF-κB activation induced by TNF receptor and Toll-like receptor family members (25, 26). Interestingly, it has been shown that TAK1 is inducibly associated with Bcl10 in B cells after the stimulation of BCR. Therefore, it is possible that TAK1 is also involved in GPCR-induced NF-κB activation through forming a complex with Bcl10. Consistent with this hypothesis, we found that suppressing the expression of TAK1 using TAK1 siRNA inhibited Gαq-induced NF-κB activation (data not shown), suggesting that TAK1 is required for GPCR-induced NF-κB activation. However, we will need to use TAK1−/− cells to confirm this finding.
Previous studies suggest that PI3K may play a role in GPCR-induced NF-κB activation. However, our data show that inhibition of the PI3K pathway has no effects on LPA-induced nuclear localization of NF-κB (Fig. 4), suggesting that the PI3K pathway is not involved in LPA-induced IKK activation. However, it remains to be determined whether PI3K signaling is involved in the regulation of NF-κB transcriptional activity inside nuclei after LPA stimulation. In addition, it will be important to investigate whether NF-κB activation induced by other GPCRs depends on the PI3K pathway.
Although it remains to be determined how GPCRs regulate Bcl10, previous studies in antigen receptor-induced signaling pathways have provided a potential mechanism by which Bcl10 is regulated by upstream signaling components. In lymphocytes, the stimulation of antigen receptors activates PKCs, which phosphorylate CARMA1. The phosphorylated CARMA1 further recruits Bcl10 into the antigen receptor complexes. The recruitment of Bcl10 activates MALT1 and TRAF6, leading to activation of IKK. Previous studies have shown that PKC is also involved in LPA-and other GPCR-induced NF-κB activation. Therefore, it is likely that Bcl10 may also be regulated in a similar manner in the GPCR signaling pathway. Because CARMA1 only expresses in lymphocytes, CARMA3, the CARMA1 homolog in nonlymphoid cells, may function downstream of PKC in GPCR signaling pathways. Therefore, the PKC–CARMA–Bcl10 module may constitute a common axis to transduce signals from PKC to IKK downstream of antigen receptors and GPCRs (Fig. 6B). Consistent with this model, we recently generated CARMA3-knockout mice and found that CARMA3 is required for GPCR-induced NF-κB activation (B. Grabiner and X.L., unpublished work). Together, these results provide genetic evidence that the PKC–CARMA–Bcl10–IKK signaling axis exists in multiple tissues and functions in a similar mechanism.
Materials and Methods
Reagents.
L-α-LPA (C18:1Cis-9) was from Sigma–Aldrich (St. Louis, MO). PMA was from Calbiochem (San Diego, CA). TNF-α was from Upstate Biotechnology (Lake Placid, NY). Antibodies against IκBα was a kind gift from Shao-Cong Sun (Pennsylvania State University, State College, PA). Antibodies against JNK, Erk, Akt, and their phosphorylated forms were from Cell Signaling (Beverly, MA). Antibodies specific for Bcl10, Gαq, and Actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bcl10 siRNA and relative transfection reagents were obtained from Dharmacon (Lafayette, CO). The target sequences for Bcl10 gene silencing are: GAAAUUUCUUGUCGAACAUUU (sense sequence) and 5′-PAUGUUCGACAAGAAAUUUCUU (antisense sequence). The plasmids encoding Gαq(Q209L) and Gα12(Q229L) were kindly provided by Richard D. Ye (University of Illinois, Chicago, IL).
Cell Culture and Transfection.
Mouse Bcl10+/− and Bcl10−/− MEF cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 units/ml). Cells were grown in 8% CO2 at 37°C. For all experiments, 1–2 × 106 cells were plated into 10-cm tissue culture plates. After 24–48 h, cells were starved in DMEM with 1% FBS and antibiotics for an additional 12–18 h. LPA (prepared as a stock of 1 mg/ml) in PBS containing 10 mg/ml essentially fatty acid-free BSA (Sigma) and other reagents or vehicles were added to achieve the concentrations specified. Wortmannin treatment was carried out by adding Wortmannin (Biosource, Inc., Camarillo, CA) to a final concentration of 8 μM for 1 h before LPA stimulation.
Human embryo kidney 293T cells were maintained in DMEM supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 units/ml). Cells were grown in 5% CO2 at 37°C and passed every 3 days. Cells (0.1 × 106 per well) were plated in 12-well plates the day before transfection. Bcl10 siRNA transfection was performed according to the manufacturer's instructions (Dharmacon, Inc.). Forty-eight hours after siRNA tranfection, cells were transfected with reporter plasmid encoding 5× NF-κB-luc (60 ng) and pEF-Renilla-luc (10 ng) together with active mutants of Gα, Gαq(Q209L), and Gα12(Q229L), or pCMV5 vector DNA (80 ng per sample) by using the calcium phosphate precipitation method. Six hours later, cells were serum-starved for 16–18 h. Cell lysates prepared for luciferase activities were measured with dual-luciferase assay kits (Promega, Madison, WI). NF-κB activities were determined by normalization of NF-κB-dependent Firefly luciferase to Renilla luciferase activity.
EMSA.
Nuclear extracts were prepared from stimulated MEF cells. Nuclear extracts (10 μg) were mixed with 32P-labeled NF-κB, AP-1, or Oct-1 probes (Promega) and incubated at room temperature for 15 min. The mixtures were then subjected to electrophoresis on a 6% polyacrylamide gel and autoradiography.
Integrin-Induced NF-κB Activation.
Six-well dishes were coated with recombinant mouse osteopontin (2 μg/ml), fibronectin (10 μg/ml), or poly-l-Lys (5 μg/ml) in PBS overnight at 4°C. The dishes were washed with PBS. A total of 2 × 106 serum-starved MEF cells were plated onto the dishes for 2 h. For TNF-α and LPS stimulation, 10 ng/ml TNF-α or 10 ng/ml LPS was added to poly-l-Lys-plated cells 30 min before harvest. After stimulation, the cells were collected and lysed, and nuclear extracts were prepared. Nuclear extracts were then subjected to EMSA for the detection of NF-κB activation.
Western Blot.
After different stimulations, MEF cells were rinsed with PBS and then lysed in ELB buffer (10 mM Hepes, pH 7.4/250 mM NaCl/1 mM EDTA/1% Nonidet P-40 and supplemented with the mixture of protease inhibitors (Roche, Indianapolis, IN). Cell lysates were then subjected to SDS/PAGE, transferred onto nitrocellulose, and probed with individual antibodies.
Retroviral Infection.
Cell lines, transfected with a retroviral vector that encodes Bcl10 or the empty retroviral vector, were provided by Demin Wang (The Blood Center of Southeastern Wisconsin, Milwaukee, WI). These virus-producing cells were kept in DMEM supplemented with 10% FCS and antibiotics. After 90% confluence, cultured media were collected every 24 h. For infections of MEF cells, 1 × 106 MEF cells were resuspended in freshly collected supernatant from retrovirus-generating cell lines and kept in culture, and culture medium was replaced with freshly collected retroviral supernatant every 24 h. At 48 h, MEF cells were starved in DMEM/1% FCS for 12–18 h, and LPA stimulation was carried out as described above.
ELISA.
A total of 1 × 106 MEF cells were plated into 10-cm dishes for 24 h in DMEM supplemented with 10% FCS and antibiotics. The medium was then replaced with DMEM/0.1% FCS and antibiotics for 18 h. LPA was then added to a final concentration of 10 μM. The cells were then cultured for another 24 h. Culture supernatants were collected and cleared by centrifugation and stored at −80°C for ELISA analysis. The MIP-2 level was determined by using the MIP-2 Quantikine kit from R&D Systems (Minneapolis, MN) according to the manufacturer's instruction manual.
Supplementary Material
Acknowledgments
We thank Dr. Richard Ye for expression vectors of Gα subunits. This work was partly supported by National Institutes of Health Grants AI05848 and GM065899 (to X.L.), AI052327 (to R.W.), CA87064 (to S.W.M.), and CA21765 (to L.X. and S.W.M.) and by the American Lebanese Syrian Associated Charities, St. Jude Children's Research Hospital. X.L. is a recipient of the Investigator Award of Cancer Research Institute, Inc. and the Scholar Award of the Leukemia and Lymphoma Society.
Abbreviations
- GPCR
G protein-coupled receptor
- IKK
IκB kinase
- LPA
lysophosphatidic acid
- ET-1
endothelin-1
- MEF
mouse embryonic fibroblast
- AP-1
activator protein-1
- IRES
internal ribosome entry site
- PI3K
phosphatidylinositol 3-kinase
- PMA
phorbol-12-myristate-13-acetate
- MIP-2
macrophage inflammatory protein-2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0601894104/DC1.
References
- 1.Marinissen MJ, Gutkind JS. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 2.Gilman AG. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 3.Ye RD. J Leukocyte Biol. 2001;70:839–848. [PubMed] [Google Scholar]
- 4.Ghosh S, May MJ, Kopp EB. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Karin M, Ben-Neriah Y. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 6.Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 7.Mills GB, Moolenaar WH. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 8.Moolenaar WH. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 9.Shahrestanifar M, Fan X, Manning DR. J Biol Chem. 1999;274:3828–3833. doi: 10.1074/jbc.274.6.3828. [DOI] [PubMed] [Google Scholar]
- 10.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J, Lin X. Nat Immunol. 2002;3:830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 12.Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M. Nat Immunol. 2002;3:836–843. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 13.Pomerantz JL, Denny EM, Baltimore D. EMBO J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Wang D, Lin X. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL, Rawlings DJ. Immunity. 2005;23:561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Ruland J, Duncan GS, Wakeham A, Mak TW. Immunity. 2003;19:749–758. doi: 10.1016/s1074-7613(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Wertz I, O'Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 19.Ruefli-Brasse AA, French DM, Dixit VM. Science. 2003;302:1581–1584. doi: 10.1126/science.1090769. [DOI] [PubMed] [Google Scholar]
- 20.Xie P, Browning DD, Hay N, Mackman N, Ye RD. J Biol Chem. 2000;275:24907–24914. doi: 10.1074/jbc.M001051200. [DOI] [PubMed] [Google Scholar]
- 21.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 22.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 23.Juliano RL. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 24.Uren AG, O'Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 26.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.