Abstract
Despite conceptual recognition that indirect effects initiated by large herbivores are likely to have profound impacts on ecological community structure and function, the existing literature on indirect effects focuses largely on the role of predators. As a result, we know neither the frequency and extent of herbivore-initiated indirect effects nor the mechanisms that regulate their strength. We examined the effects of ungulates on taxa (plants, arthropods, and an insectivorous lizard) representing several trophic levels, using a series of large, long-term, ungulate-exclusion plots that span a landscape-scale productivity gradient in an African savanna. At each of six sites, lizards, trees, and the numerically dominant order of arthropods (Coleoptera) were more abundant in the absence of ungulates. The effect of ungulates on arthropods was mediated by herbaceous vegetation cover. The effect on lizards was simultaneously mediated by both tree density (lizard microhabitat) and arthropod abundance (lizard food). The magnitudes of the experimental effects on all response variables (trees, arthropods, and lizards) were negatively correlated with two distinct measures of primary productivity. These results demonstrate strong cascading effects of ungulates, both trophic and nontrophic, and support the hypothesis that productivity regulates the strength of these effects. Hence, the strongest indirect effects (and thus, the greatest risks to ecosystem integrity after large mammals are extirpated) are likely to occur in low-productivity habitats.
Keywords: bottom-up, top-down, ecosystem engineers, food webs, trophic cascades
The publication more than 40 years ago of two seminal papers on the role of indirect interactions in food webs (1, 2) charted a course for community ecology and paved the way for an immense amount of research and debate. These early studies focused on the paramount role of predators, describing interactions that were later relabeled “trophic cascades” and “keystone predation” (3–5). Perhaps in part for these historical reasons, the discussion of indirect or “cascading” effects in ecological interaction webs has remained largely top-down and predator-centric (6), with much recent activity focusing on the mechanisms that determine the relative strength of trophic cascades across ecosystems (7, 8) and the importance of nonlethal, trait-mediated predator effects (9, 10).
Of course, this emphasis on the influence of high-level predators is not without justification: empirical studies have convincingly demonstrated “ecological meltdown” in the absence of top predators (11, 12). There are theoretical reasons to expect this, because top predators couple distinct energy channels in food webs (13). Nevertheless, the apparent neglect of herbivores as potential initiators (sensu ref. 5) of interaction chains is troubling. Ungulates are particularly likely to serve in this capacity because of their large body sizes and energy requirements; Paine, whose work was critical in establishing the concepts of keystone predation (2) and trophic cascades (4), recently predicted that mammalian herbivores would be found to exert “rampant indirect effects” and urged ecologists to test for them (14). Few authors have heeded this call, however, and experimental studies of the indirect effects of large herbivores on species of other trophic levels remain rare (ref. 15; but see also refs. 16–19).
If large herbivores do indeed exert rampant indirect effects, then it is important to understand the mechanisms regulating the strength of these effects. Primary productivity is one factor likely to influence food-web properties, including the strength of indirect effects (20). Although the role of productivity in strengthening or dampening predator-initiated trophic cascades is still unclear (21–23), such a role may be more pronounced when herbivores are the initiators. Because the direct effects of herbivory decrease with increasing productivity in grassland systems (24), we predicted that the strength of indirect effects would follow suit.
We excluded native and domestic ungulates (see Methods) from experimental plots in six blocks that were replicated across a landscape-scale gradient in primary productivity in an African savanna (Fig. 1), and we measured the responses of three taxa (plants, arthropods, and the arboreal, insectivorous, and numerically dominant lizard, Lygodactylus keniensis) relative to paired controls. Our results provide clear and compelling evidence of far-reaching indirect effects exerted by terrestrial large herbivores and further indicate that the strength of these effects is negatively correlated with productivity, with the strongest indirect effects at the lowest-productivity sites.
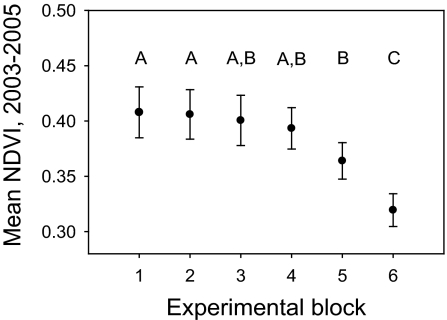
Fig. 1.
NDVI at our six experimental blocks, indicating a gradient of aboveground primary productivity. Points represent mean NDVI derived from MODIS (Moderate Resolution Imaging Spectroradiometer) images taken throughout 2003–2005. Error bars represent 95% confidence intervals. Blocks are arranged in order of decreasing productivity. Blocks 1–3 are underlain by vertisols with high clay content; blocks 4–6 are located on adjacent sandy loams. Points not connected by the same letter were significantly different (P < 0.05) in Tukey's honestly significant difference post hoc comparisons.
Results
Direct and Indirect Effects of Ungulate Exclusion.
Ungulates exerted a strong top-down effect on tree density and a weaker, marginally significant effect on herbaceous cover (P = 0.059; Table 1). There was no discernible effect of ungulate exclusion on arthropod abundance overall (Table 1); however, the abundance of at least some arthropod taxa did increase significantly in the absence of ungulates (multivariate ANOVA, F6,5 = 7,400, P < 0.0001). Univariate effect tests revealed that coleopterans (i.e., beetles, the numerically dominant arthropod in our samples, and the most common taxon found in lizard stomach contents) were nearly twice as abundant in exclosure plots as in paired control plots (Table 1). However, no other major order responded significantly to ungulate exclusion (all P > 0.1). Experimental block (i.e., location) was a significant term in all univariate models (Table 1), with abundances of all response variables increasing from less-productive to more-productive sites.
Table 1.
Means of response variables by treatment and results of ANOVA effect tests
| Response variable ANOVA model effect | Mean ± SE |
Effect tests |
||||
|---|---|---|---|---|---|---|
| Control | Exclosure | df | MS | F | P | |
| Lizard density, indiv per ha | 295 ± 99 | 475 ± 123 | ||||
| Herbivore treatment | 1 | 97,000 | 12.8 | 0.016 | ||
| Block location | 5 | 142,000 | 18.8 | 0.003 | ||
| Error | 5 | 7,570 | ||||
| Tree density, indiv per ha | 841 ± 126 | 1,150 ± 138 | ||||
| Herbivore treatment | 1 | 286,000 | 14.8 | 0.012 | ||
| Block location | 5 | 190,000 | 9.6 | 0.013 | ||
| Error | 5 | 19,300 | ||||
| Herbaceous cover, % | 66 ± 13 | 82 ± 8 | ||||
| Herbivore treatment | 1 | 0.0740 | 5.91 | 0.059 | ||
| Block location | 5 | 0.131 | 10.5 | 0.011 | ||
| Error | 5 | 0.0125 | ||||
| Total arthropods, indiv per effort | 23 ± 7 | 26 ± 5 | ||||
| Herbivore treatment | 1 | 26.8 | 1.10 | >0.3 | ||
| Block location | 5 | 406 | 17.3 | 0.004 | ||
| Error | 5 | 23.4 | ||||
| Coleopterans, indiv per effort | 3.7 ± 2.8 | 6.9 ± 1.5 | ||||
| Herbivore treatment | 1 | 31.0 | 26.0 | 0.004 | ||
| Block location | 5 | 27.2 | 22.8 | 0.002 | ||
| Error | 5 | 1.19 | ||||
MS, mean square; indiv, individuals.
On average, lizard density was 61% greater in the absence of ungulates than in paired control plots (Table 1). However, the response varied across individual blocks from 24% to 214%. The block term was again a significant main effect, with lizard density being nearly twice as great in the two most productive blocks as in the less productive blocks.
Drivers of Abundance.
Understanding the mechanisms underlying these experimental effects requires knowledge of the principal determinants of abundance for each of the response variables. Herbaceous cover was a strong predictor of arthropod abundance (F1,9 = 61.2, P < 0.0001), but tree density was not (F1,9 = 3.1, P = 0.1); a linear model of arthropod abundance including only herbaceous cover as a predictor explained 84% of the variation in arthropod abundance. Herbaceous cover also explained most of the variation in the abundance of coleopterans alone, which was the arthropod order that displayed a significant response to ungulate exclusion (F1,10 = 24.4, P < 0.001, R2 = 0.68).
Both tree density and arthropod abundance were strong predictors of lizard density (F1,9 = 14.7 and 11.0, respectively; P < 0.01 for both), so we retained both of these variables in our model of lizard density (adjusted R2 = 0.78). Further strengthening this inference, the log-response-ratio effect sizes (see Methods) of the experimental treatment on lizard and tree densities were positively correlated across blocks (n = 6, R2 = 0.64, F1,4 = 9.7, P = 0.04), as were the effect sizes of lizard density and arthropod abundance (n = 6, R2 = 0.85, F1,4 = 28.7, P = 0.006). In other words, the response of lizards to ungulate exclusion in a given block was proportional to the responses of both their arboreal microhabitat and their arthropod prey (independent of each other, as evidenced by the lack of correlation [R2 < 0.05] between tree density and arthropod abundance).
Primary Productivity as a Driver of Effect Size.
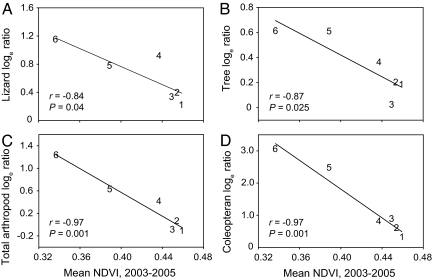
The effects of herbivore exclusion on lizards, trees, total arthropods, and coleopterans were all significantly negatively correlated with productivity, as measured by the Normalized Difference Vegetation Index (NDVI) (see Methods) (Fig. 2). Because the relationship between NDVI and productivity can sometimes be skewed by variation in soil color (25), we verified these relationships, using peak herbaceous cover as a second, independent, measure of productivity at each block (see Methods). When peak herbaceous cover was substituted for NDVI as the measure of productivity, we observed the same negative, statistically significant relationships with effect size for all response variables. (For all, n = 6. R2 = 0.63, 0.86, 0.72, and 0.80; and P = 0.04, 0.005, 0.02, and 0.01, for lizard density, tree density, total-arthropod abundance, and coleopteran abundance, respectively.)
Fig. 2.
Effect sizes (loge response ratios) of ungulates on four response variables regressed against aboveground primary productivity (as measured by NDVI): (A) lizard density, (B) tree density, (C) total-arthropod abundance, and (D) abundance of coleopterans only. Numbers represent individual blocks and correspond to those presented in Fig. 1. All relationships remain statistically significant after sequential Bonferroni corrections of α (0.05, 0.025, 0.017, and 0.013).
Discussion
Our results show that ungulate herbivores consistently depress the densities of trees, insectivorous lizards, and the dominant order of arthropods across a landscape-scale gradient in primary productivity. We infer that ungulate herbivory indirectly regulates lizard abundance by independently suppressing tree density (microhabitat availability) and beetle density (food availability). This chain of interactions involves both top-down effects (ungulate control of plant biomass) and bottom-up effects (resource control of arthropod and lizard densities); in this respect, our findings complement those of Croll et al. (26), who showed that foxes on islands exerted indirect effects via a pathway that comprised both top-down (fox predation on seabirds) and bottom-up (nutrient enrichment of island plants by seabird guano) processes. Furthermore, the strength of the direct and indirect effects documented here was greatest in low-productivity sites. Collectively, these results not only confirm the importance of large herbivores as “strong interactors” (14, 27) but also suggest that they will be stronger interactors where productivity is low.
Although there was no significant effect of ungulates on total arthropod abundance, beetles were nearly twice as abundant in exclosure plots as in control plots. Without greater taxonomic resolution, our data offer little insight as to why coleopterans were more sensitive to the presence of ungulates than other arthropod taxa (e.g., perhaps bruchid seed predators responded to increased seed production in exclosure plots). Nevertheless, coleopterans accounted for 22% of all arthropods in our samples and are the most abundant prey item in stomach contents of L. keniensis from this area (R.M.P., unpublished data). These data, and the strong positive correlation between the effect sizes of lizard density and arthropod abundance, strongly suggest that lizards' positive response to ungulate exclusion stems at least in part from increased prey availability.
The observed negative relationships between productivity and the effect sizes of ungulate removal would be expected in either of two nonexclusive scenarios. First, because compensatory regrowth of plants following herbivory is faster in high-productivity sites, herbivores have a relatively lower net impact on plant biomass in those areas (24). This process would be expected to dampen cascading effects as well, because more productive plant communities would absorb the impacts of herbivory and buffer the remainder of the community (21). However, a similar result might arise if plants differed systematically in edibility along the resource gradient [i.e., were better defended in higher productivity sites, where the effects of ungulate exclusion were weakest (21, 28)].
We consider the former scenario more likely in our system. Herbaceous species were clearly edible to arthropods in high-productivity blocks, as shown by the increase in arthropod abundance with increasing herbaceous productivity and percent cover. Moreover, there is a well established relationship between primary productivity and ungulate consumption rates in rangelands (29), which argues against the hypothesis that plants overall were less palatable in the high-productivity sites. However, we cannot conclusively rule out the latter scenario for the direct effect of ungulates on tree density (and hence for the tree-density-mediated component of the indirect effect of ungulates on lizards). Acacia drepanolobium, the dominant tree in the three highest productivity sites, is defended by symbiotic ants (30) and appears to suffer lower rates of elephant browsing than either Acacia brevispica or Acacia mellifera (R.M.P. and T.P.Y., unpublished observations). Because the three highest-productivity sites were also those where A. drepanolobium was dominant, it is possible that plant palatability contributed to the clumping of the high-productivity points in Fig. 2 A and B.
Differences in the intensity of predation on lizards are unlikely to have contributed strongly to the patterns we observed. Our experimental treatment did not exclude most potential predators of lizards. Indeed, snake densities increase approximately twofold when ungulates are absent (18), as does the presence and activity of the bird community, in which >70% of the species are insectivores or carnivores that are capable of preying on lizards (31). Moreover, although it is possible that the (marginally) greater grass cover in exclosure plots would protect lizards on the ground, movement between trees is rare in adult L. keniensis (32).
Complex interactions, such as those documented in this study, make it difficult to predict the community-wide ramifications of ecological perturbations, especially if the strength of indirect effects is highly sensitive to environmental variation. Our experimental blocks varied in resource availability (Fig. 1) and vegetation structure. The negative relationships between effect size and productivity across taxa, as well as the significant block terms in our models, suggest that indirect effects are highly sensitive to changes in these parameters. Thus, fully understanding the implications of major ecological perturbations, such as the extirpation of large mammals, may require examining whole communities at the landscape scale.
These conclusions are important in light of the progressive continent-wide declines of many African ungulate species (33, 34), and, indeed, of large herbivores worldwide (17). Our study indicates that such declines have cascading ramifications comparable with those observed in other systems after the loss of predators (35) and thus, that large-bodied herbivores, where they still exist, might be equally critical to ecosystem function. Moreover, we would expect these cascades to be most profound in areas of intrinsically low primary productivity, such as at the drier ends of rainfall gradients. Finally, we suggest that ungulate-initiated cascades were important in the history and evolution of ecosystems that today are bereft of large herbivores and that, although many of these cascades went extinct at the end of the Pleistocene along with the large herbivores that caused them, the legacies of the cascades may well remain (36, 37).
Methods
Study Sites and Experimental Design.
We conducted our research between May 2004 and December 2005 at the Mpala Research Centre (0°20′ N, 36°53′ E, 1,650–1,800 m above sea level) in the Laikipia District in central Kenya. Rainfall averages 450–550 mm/yr in a weakly trimodal annual pattern. The diverse ungulate fauna includes zebras (Equus burchelli and Equus grevyi), impalas (Aepyceros melampus), Grant's gazelles (Gazella granti), elands (Taurotragus oryx), elephants (Loxodonta africana), giraffes (Giraffa camelopardalis), hartebeests (Alcelaphus buselaphus), buffalos (Syncerus caffer), and cattle (Bos indicus). Native large preditors include lions (Panthera leo), leopards (Panthera pardus), cheetahs (Acinonyx jubatus), and hyenas (Crocuta crocuta and Hyaena hyaena).
With densities approaching 1,000 per ha in places, lizards are the most abundant group of vertebrates in this habitat. We focused on the small (3- to 4-cm snout–vent length, 1–2 g), arboreal, diurnal gecko L. keniensis Parker, which is by far the most abundant lizard in this community (≈94% of all individuals). This species forages for small arthropods both on its host trees and in brief forays to the ground. Coleopterans were the most abundant prey item in 14 gecko stomach contents examined as part of an ongoing study (R.M.P., unpublished data), whereas ants are actively avoided (32). Predators of L. keniensis include bushbabies (Galago senegalensis), snakes, and birds.
We quantified direct and indirect effects of large mammals, using six pairs of herbivore-exclusion and control plots (to which we refer throughout the paper as “exclosure” and “control”). All exclosure plots consisted of 2.4-m-high electric fences that exclude mammals >15 kg, but importantly, they do not exclude the saurophagous predators listed above. The locations of the six experimental blocks span ≈12 km. Three are located on volcanic clay vertisols, which are highly productive (200 × 200 m, established in 1995; see ref. 38). The other three are located on sandy loams derived from metamorphic basement rock, which are variable in productivity (70 × 70 m, established in 1999; see ref. 39) (Fig. 1). The vegetation communities in these two soil types share many of the same species, but at different relative abundances. Woody species common to both communities include A. brevispica, A. drepanolobium, A. mellifera, Balanites aegyptiaca, Boscia angustifolia, and Rhus natalensis. A. drepanolobium was dominant in the three blocks underlain by clay soils, whereas A. brevispica, A. mellifera, and Acacia etbaica were variously dominant in the three blocks underlain by sandy soils (see refs. 35 and 36 for full details of the exclosure plots and vegetation communities).
Intensive aerial wildlife censuses of Laikipia and repeated dung surveys in our study sites have indicated that the background densities of native ungulates are similar across the study communities (40, 41). Densities of cattle (the mammal with the greatest biomass density in our study area) were experimentally controlled at the three clay-soil blocks to match the stocking rates on the remainder of Mpala Ranch, where our other three blocks were located (41).
We used two indices of aboveground primary productivity at each of our blocks (i.e., exclosure–control pairs). Our preferred index was the NDVI, because it simultaneously reflects the production of both woody and herbaceous species, and because it has been shown to correlate closely with productivity per se at our study sites (N. Georgiadis, Mpala Research Centre, Kenya, personal communication; see also refs. 25 and 42). We calculated mean NDVI values from MODIS (Moderate Resolution Imaging Spectroradiometer) satellite images (250-m resolution) taken at 16-day intervals throughout the study period. However, because the relationship between NDVI and productivity can sometimes be skewed by soil color (25), we also used peak herbaceous cover (i.e., herbaceous cover measured in exclosure plots; see Vegetation and Arthropod Surveys) as a second measure of productivity at each block. NDVI and peak herbaceous cover were positively correlated across our sites (n = 6, R2 = 0.70). The NDVI data revealed a productivity gradient that spanned the six blocks, with greater values on average at the clay-soil blocks and broad variability across the sand-soil blocks (Fig. 1).
Lizard Censuses.
Within each exclosure and control plot, we randomly selected four 25 × 25 m study quadrats without replacement. We censused lizards within these quadrats (the first two quadrats per plot during June–September 2004 and the other two during June–September 2005), using the mark–resight procedure and analysis described by Heckel and Roughgarden (43), with the modifications incorporated by Schoener et al. (44). These censuses were randomly ordered within each field season to prevent any short-term temporal biases. Densities did not differ between years (F1,22 = 0.04, P > 0.8). We therefore took the mean of the density estimates from all four censuses in each plot to represent overall density for that plot (n = 12 plots). In all, we made >2,600 lizard observations.
Vegetation and Arthropod Surveys.
In each study quadrat, we quantified two hypothesized determinants of lizard density: microhabitat availability (tree density) and prey availability (arthropod abundance). We also quantified herbaceous vegetation cover, which we used as a second measure of productivity (see Study Sites and Experimental Design), and which, along with tree density, was a hypothesized determinant of arthropod abundance.
We counted all trees ≥1-m tall in each quadrat (only 0.6% of the lizards sighted occupied trees <1-m tall). We estimated aerial arthropod abundance by walking two intersecting transects bisecting each quadrat on sunny days between 1000 and 1600 and making 30 sweeps per transect with a 39-cm-diameter sweep net at ≈0.5 m above ground level. We estimated terrestrial arthropod abundance, using pitfall traps (plastic cups of 9.5-cm diameter). Two traps per quadrat were deployed concurrently for three consecutive days. All arthropods were frozen, counted, and identified to order. The data from both sampling methods were added together for each quadrat and then averaged across the four quadrats in each plot to give an estimate of overall capture rate per unit effort for that plot. Ants, which are ubiquitous at our sites but are not eaten by L. keniensis (32), were excluded from analyses a priori. In all, we collected and identified to order >3,000 non-ant arthropods.
We used a 0.5-m frame with 10 pins to measure the percentage of herbaceous cover, counting presence vs. absence of vegetation for each pin. In the three clay-soil blocks, cover was measured four times (June and December 2004 and 2005) at 100 locations in the central ha of each 4-ha plot (1,000 pins per plot per survey) as part of ongoing monitoring (see ref. 41). In the three sand-soil blocks, we measured cover in June and September 2004 and 2005 at each of 36 locations in the central 40 × 40 m of each plot (360 pins per plot per survey).
Statistical Analyses.
Because our primary interest was at the landscape scale, and because we wanted to avoid potential spatial autocorrelation, we treated our data conservatively, averaging measurements from our four nested quadrats within each plot to obtain a single value for each response variable in that plot (n = 12 plots; one exclosure and one control in each of six blocks).
We tested for effects of the experimental treatment (herbivore exclusion), using ANOVA. Because our blocks were arrayed along a gradient in productivity (Fig. 1), we tested for treatment effects by using the model
in which μ…is the overall mean, Hi.. represents the ith experimental treatment (ungulate presence/absence), β.j. represents the jth experimental block, and εijk is the error term. Because there was no replication of treatments within each block, this model does not contain an interaction term (45). For arthropods, we first analyzed total abundance according to the model above. We then examined the five most abundant arthropod orders (Coleoptera > Orthoptera > Hemiptera > Araneae > Diptera, which collectively accounted for 78% of all non-ant arthropods), using multivariate ANOVA with treatment and block as factors. We report the Roy's Greatest Root test statistic because of its relatively high power and robustness when data satisfy the assumptions of univariate ANOVA (46). After a significant multivariate ANOVA, we analyzed the taxa individually according to the ANOVA model above (using sequential Bonferroni corrections; see ref. 47). We based inferences about the mechanisms underlying any treatment effects on arthropods and lizards on (i) the results from multiple regression models and (ii) correlations between the effect sizes of the response variables (see below).
We built two multiple-regression models to elucidate the drivers of arthropod abundance and lizard density, respectively. We hypothesized that arthropod abundance would be driven by tree density and herbaceous cover and that lizard density would be driven by tree density and arthropod abundance. If a predictor variable did not have a statistically significant effect, it was dropped from the model.
Finally, we used linear regression to examine the relationship between productivity and the strength of the treatment effects. Effect sizes were calculated as loGe ratios (48) of response variables in the absence and presence of herbivores:
Effect sizes of four response variables were regressed on NDVI, again by using sequential Bonferroni corrections to evaluate statistical significance. When these relationships were significant, we verified that the same was true and then used our alternative measure of productivity, peak herbaceous cover.
The assumptions of ANOVA and regression were satisfied by the untransformed data in all cases. The predictor variables in the two multiple regression models were not strongly collinear (variance inflation factor ≤1.35 for both pairs of predictors). All analyses were performed with JMP version 5.1 (SAS Institute, Cary, NC).
Acknowledgments
We thank M. Mohammed and J. Ekadeli for field assistance; D. J. Augustine, N. Georgiadis, N. Olwero, J. Roughgarden, G. C. Daily, F. Keesing, and the Office of the President of the Republic of Kenya for enabling various facets of the research; and P. R. Ehrlich, R. T. Paine, G. Ceballos, J. R. Goheen, T. M. Palmer, D. F. Doak, O. J. Schmitz, E. Pringle, L. Buckley, B. Brosi, A. Wolf, C. Lunch, C. Riginos, and K. Veblen for comments and discussion. This work was supported by the James Smithson Fund of the Smithsonian Institution (to A. Smith), National Geographic Society Grant 4691-91, National Science Foundation Grants BSR-97-07477 and BSR-03-16402, African Elephant Program of the U.S. Fish and Wildlife Service Grant 98210-0-G563 (all to T.P.Y), the William R. and Sara Hart Kimball graduate fellowships (to R.M.P.), and the Sherwood Family Foundation (to G. C. Daily).
Abbreviation
- NDVI
Normalized Difference Vegetation Index.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 5.
References
- 1.Hairston NG, Smith FE, Slobodkin LB. Am Nat. 1960;94:421–425. [Google Scholar]
- 2.Paine RT. Am Nat. 1966;100:65–75. [Google Scholar]
- 3.Paine RT. Am Nat. 1969;103:91–93. [Google Scholar]
- 4.Paine RT. J Anim Ecol. 1980;49:667–685. [Google Scholar]
- 5.Abrams PA, Menge BA, Mittelbach GG, Spiller DA, Yodzis P. In: Food Webs: Integration of Patterns and Dynamics. Polis GA, Winemiller KO, editors. New York: Chapman & Hall; 1996. pp. 371–395. [Google Scholar]
- 6.Kagata H, Ohgushi T. Ecol Res. 2006;21:26–34. [Google Scholar]
- 7.Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS. Ecol Lett. 2002;5:785–791. [Google Scholar]
- 8.Knight TM, McCoy MW, Chase JM, McCoy KA, Holt RD. Nature. 2005;437:880–883. doi: 10.1038/nature03962. [DOI] [PubMed] [Google Scholar]
- 9.Peacor SD, Werner EE. Proc Natl Acad Sci USA. 2001;98:3904–3908. doi: 10.1073/pnas.071061998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz OJ, Krivan V, Ovadia O. Ecol Lett. 2004;7:153–163. [Google Scholar]
- 11.Crooks KR, Soule ME. Nature. 1999;400:563–566. [Google Scholar]
- 12.Terborgh J, Lopez L, Nunez P, Rao M, Shahabuddin G, Orihuela G, Riveros M, Ascanio R, Adler GH, Lambert TD, et al. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 13.Rooney N, McCann K, Gellner G, Moore JC. Nature. 2006;442:265–269. doi: 10.1038/nature04887. [DOI] [PubMed] [Google Scholar]
- 14.Paine RT. J Mammal. 2000;81:637–648. [Google Scholar]
- 15.Cote SD, Rooney TP, Tremblay JP, Dussault C, Waller DM. Annu Rev Ecol Evol Syst. 2004;35:113–147. [Google Scholar]
- 16.Vazquez DP, Simberloff D. Ecol Monogr. 2004;74:281–308. [Google Scholar]
- 17.Feeley KJ, Terborgh JW. Ecology. 2006;87:144–150. doi: 10.1890/05-0652. [DOI] [PubMed] [Google Scholar]
- 18.McCauley DJ, Keesing F, Young TP, Allan BF, Pringle RM. Ecology. 2006;87:2657–2663. doi: 10.1890/0012-9658(2006)87[2657:ieolho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Goheen JR, Keesing F, Allan BF, Ogada DL, Ostfeld RS. Ecology. 2004;85:1555–1561. [Google Scholar]
- 20.Persson L, Bengtsson J, Menge BA, Power ME. In: Food Webs: Integration of Patterns and Dynamics. Polis GA, Winemiller KO, editors. New York: Chapman & Hall; 1996. pp. 396–434. [Google Scholar]
- 21.Schmitz OJ. Proc Natl Acad Sci USA. 1994;91:5364–5367. doi: 10.1073/pnas.91.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chase JM. Oikos. 2003;101:187–195. [Google Scholar]
- 23.Shurin JB, Seabloom EW. J Anim Ecol. 2005;74:1029–1038. [Google Scholar]
- 24.Chase JM, Leibold MA, Downing AL, Shurin JB. Ecology. 2000;81:2485–2497. [Google Scholar]
- 25.Fensholt R, Sandholt I, Rasmussen MS. Remote Sens Environ. 2004;91:490–507. [Google Scholar]
- 26.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 27.Owen-Smith N. Paleobiology. 1987;13:351–362. [Google Scholar]
- 28.Leibold MA. Am Nat. 1989;134:922–949. [Google Scholar]
- 29.Augustine DJ, McNaughton SJ, Frank DA. Ecol Appl. 2003;13:1325–1337. [Google Scholar]
- 30.Palmer TM, Young TP, Stanton ML, Wenk E. Oecologia. 2000;123:425–435. doi: 10.1007/s004420051030. [DOI] [PubMed] [Google Scholar]
- 31.Misurelli D. Albany, NY: State University of New York; 2002. MS thesis. [Google Scholar]
- 32.Greer AE. Breviora. 1967;268:1–16. [Google Scholar]
- 33.Lamprey RH, Reid RS. J Biogeog. 2004;31:997–1032. [Google Scholar]
- 34.Owen-Smith N, Mills MGL. Ecol Monogr. 2006;76:73–92. [Google Scholar]
- 35.Terborgh J, Feeley K, Silman M, Nunez P, Balukjian B. J Ecol. 2006;94:253–263. [Google Scholar]
- 36.Owen-Smith N. Conserv Biol. 1989;3:405–412. doi: 10.1111/j.1523-1739.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 37.Donlan CJ, Berger J, Bock CE, Bock JH, Burney DA, Estes JA, Foreman D, Martin PS, Roemer GW, Smith FA, et al. Am Nat. 2006;168:660–681. doi: 10.1086/508027. [DOI] [PubMed] [Google Scholar]
- 38.Young TP, Okello B, Kinyua D, Palmer T. Afr J Range Forage Sci. 1998;14:92–104. [Google Scholar]
- 39.Augustine DJ, McNaughton SJ. J Appl Ecol. 2004;41:45–58. [Google Scholar]
- 40.Khaemba WM, Stein A, Rasch D, de Leeuw J, Georgiadis N. Afr J Ecol. 2001;39:374–382. [Google Scholar]
- 41.Young TP, Palmer TM, Gadd ME. Biol Conserv. 2005;122:351–359. [Google Scholar]
- 42.Oesterheld M, DiBella CM, Kerdiles H. Ecol Appl. 1998;8:207–212. [Google Scholar]
- 43.Heckel DG, Roughgarden J. Ecology. 1979;60:966–975. [Google Scholar]
- 44.Schoener TW, Spiller DA, Losos JB. Proc Natl Acad Sci USA. 2004;101:177–181. doi: 10.1073/pnas.0306887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potvin C. In: Design and Analysis of Ecological Experiments. Scheiner SM, Gurevitch J, editors. Oxford: Oxford Univ Press; 2001. pp. 63–76. [Google Scholar]
- 46.Scheiner SM. In: Design and Analysis of Ecological Experiments. Scheiner SM, Gurevitch J, editors. Oxford: Oxford Univ Press; 2001. pp. 99–115. [Google Scholar]
- 47.Rice WR. Evolution (Lawrence, Kans) 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 48.Hedges LV, Gurevitch J, Curtis PS. Ecology. 1999;80:1150–1156. [Google Scholar]