Abstract
Environmental factors are thought to play a major role in the development of rheumatoid arthritis. Because the use of ethanol is widespread, we assessed the role of ethanol intake on the propensity to develop chronic arthritis. Collagen type II-immunized mice were given water or water containing 10% (vol/vol) ethanol or its metabolite acetaldehyde. Their development of arthritis was assessed, as well as the impact of ethanol on leukocyte migration and activation of intracellular transcription factors. Mice exposed daily to this dose of ethanol did not display any liver toxicity, and the development of erosive arthritis was almost totally abrogated. In contrast, the antibody-mediated effector phase of collagen-induced arthritis was not influenced by ethanol exposure. Also, the major ethanol metabolite, acetaldehyde, prevented the development of arthritis. This antiinflammatory and antidestructive property of ethanol was mediated by (i) down-regulation of leukocyte migration and (ii) up-regulation of testosterone secretion, with the latter leading to decreased NF-κB activation. We conclude that low but persistent ethanol consumption delays the onset and halts the progression of collagen-induced arthritis by interaction with innate immune responsiveness.
Keywords: inflammation, cytokines, sex hormones, antibodies, immunity
Excessive alcohol consumption depresses the immune system and increases the propensity to severe bacterial infections, including pneumonia (1), tuberculosis (2), and bacterial peritonitis (3), and to viral infections (4–6). However, some epidemiological studies have suggested that light to moderate ethanol consumption has protective effects against several diseases including chronic heart diseases (7, 8) and ischemic stroke (9).
Ethanol consumption has also been implicated in the pathogenesis of systemic lupus erythematosus (SLE), a complex autoimmune disease in the development of which environmental and genetic factors interact. Indeed, some investigators have found that exposure to ethanol is associated with a lower risk for developing SLE (10, 11), whereas others did not observe any effect of alcohol consumption on the incidence of SLE (12, 13). The association between ethanol and rheumatoid arthritis (RA) has been discussed in some studies, but definite conclusions could not be drawn (14–16). Studies so far have been of an epidemiological nature and therefore have not provided any hints about the biological mechanisms of environmental stimuli in the development of autoimmune disease. Our aim here was to assess whether low but persistent consumption of ethanol in quantities nontoxic to liver might affect the incidence and disease manifestation of collagen type II (CII)-induced arthritis (CIA), an established model of human RA. Our results suggest that ethanol intake delays the onset and halts the progression of destructive arthritis.
Results
Effect of Ethanol Consumption on Development of CIA.
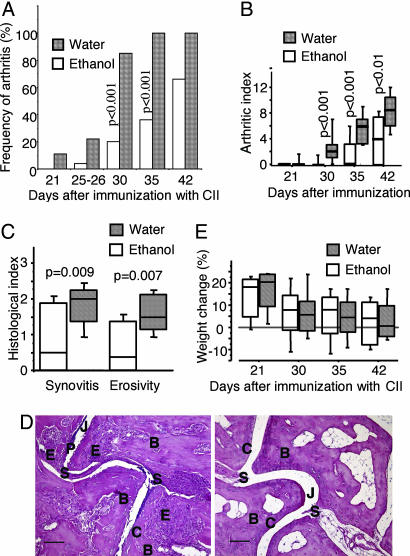
To assess whether ethanol drinking has any impact on the development of CIA, CII-immunized mice were provided with either 10% (vol/vol) ethanol in drinking water or water alone. Nine days after booster immunization with CII, only 5 of 25 mice (20%) that drank ethanol showed signs of arthritis, whereas almost all control mice (23 of 27 or 85%) had ongoing arthritis (P < 0.001) (Fig. 1A). As shown in Fig. 1B, mice that drank 10% ethanol displayed a significantly lower severity (P < 0.001) of arthritis than did the control mice. Histological sections from mice confirmed that ethanol in drinking water led to less severe arthritis. Importantly, the destruction of bone and cartilage was significantly decreased in the ethanol-drinking group compared with the control mice (Fig. 1 C and D). In contrast, no significant differences in weight were observed during the course of these experiments (Fig. 1E). Ethanol in drinking water did not affect liver function, as shown by analyses of circulating levels of serum alanine and aspartate aminotransferases (s-ALAT and s-ASAT, respectively) and γ-glutamyltransferase (γ-GT). Serum levels of ethanol were only moderately increased in mice exposed to ethanol compared with control mice exposed to water alone (data not shown). To assess the impact of the major ethanol metabolite acetaldehyde on the development of arthritis, we provided the mice with 1% acetaldehyde in drinking water. The acetaldehyde displayed an ameliorating effect on the development of arthritis, with a median arthritic index of 0 [interquartile range (IQR) of 0–0] for the acetaldehyde-drinking mice, compared with 3.5 (IQR of 3.0–6.0, P = 0.004) for the water-drinking control mice, on day 32 after CII immunization.
Fig. 1.
Development of arthritis in DBA/1 mice immunized with CII and supplied with 10% ethanol or water. (A and B) Frequency (A) and severity (B) of arthritis in mice followed for 5–6 weeks after immunization. Values from two experiments were pooled. The ethanol-drinking group contained 26 mice, and the water-drinking group contained 27 mice except on day 42, when both groups contained 12 mice. Statistical evaluation was made by using the χ2 test or the Mann–Whitney U test. Bold lines indicate medians. (C) Histological signs of synovitis and erosivity in CII-immunized DBA/1 mice 6 weeks after the start of ethanol drinking. A histological scoring system was used to evaluate synovial hypertrophy and degradation of cartilage and bone. Scores were set as follows: 1, mild; 2, moderate; and 3, severe synovitis and joint damage. Each group contained 12 mice. (D Left) Micrograph of heavily inflamed tarsal joints from a CII-immunized DBA/1 mouse that drank water only for 6 weeks. Note frequent bone and cartilage erosions. (Right) Micrograph of histologically apparently intact tarsal joints from a CII-immunized DBA/1 mouse that was provided with 10% ethanol in drinking water. Hematoxylin/eosin staining was used. B, Bone; C, cartilage; E, erosion of bone and cartilage; J, joint cavity; P, pannus tissue formation; S, synovial tissue. (Scale bar: 100 μm.) (E) Weight development in DBA/1 mice after immunization with CII. The ethanol-drinking group contained 26 mice and the water-drinking control group contained 27 mice on days 21–35. Each group contained 12 mice on day 42. Statistical evaluation was made by using the Mann–Whitney U test.
Impact of Ethanol on Inflammatory Immune Responses.
To assess the mechanisms related to the ameliorative effects of ethanol on arthritis, we analyzed serum acute phase and antibody responses. Five weeks after CII immunization, circulating IL-6 levels were significantly decreased in ethanol-drinking mice as compared with water-drinking controls (30 ± 2 pg/ml versus 115 ± 26 pg/ml, P < 0.0002). One week later, these differences were almost gone because of the decrease of serum IL-6 levels in control animals (80 ± 23 pg/ml versus 74 ± 5 pg/ml). The antiinflammatory cytokine IL-10 was also measured in the sera 6 weeks after the start of the experiment. Levels of IL-10 were three times higher in ethanol-drinking animals than in control mice, but, because of uneven distribution, the data were not significant (N.S.) (9 ± 5 pg/ml in ethanol-drinking mice and 3 ± 0 pg/ml in control mice). Levels of circulating anti-CII antibodies were similar in both groups: 0.38 ± 0.05 mg/ml and 0.39 ± 0.03 mg/ml (N.S.) at week 5 and 0.67 ± 0.12 mg/ml and 1.14 ± 0.31 mg/ml (N.S.) at week 6 for ethanol- and water-drinking mice, respectively.
Effects of Ethanol Drinking on the Effector Phase of CIA.
To further investigate the role of ethanol in CII-specific immunity, naive DBA/1 mice were provided with 10% ethanol in drinking water or water alone. Four weeks after the start of the experiment, all of the mice were injected with a mixture of four monoclonal anti-CII antibodies to induce collagen antibody-induced arthritis (17). Two weeks after the injection of antibodies, 7 of 9 ethanol-drinking mice and 7 of 10 water-drinking mice developed arthritis of equal severity. These data suggest that ethanol affects the initiation, rather than the effector phase, of immune responsiveness during CIA.
Impact of Ethanol on in Vivo Cell-Mediated Inflammatory Reactions.
Does long-term intake of 10% ethanol affect the acute/subacute T cell, macrophage, or granulocyte capacity to cause inflammation? Ethanol-drinking mice had delayed-type hypersensitivity reactions similar to those of water-drinking mice [i.e., 0.275 ± 0.014 mm versus 0.259 ± 0.008 mm (N.S.)]. Olive oil-induced inflammation, which is granulocyte-mediated, was 0.676 ± 0.056 mm for ethanol-drinking mice compared with 0.804 ± 0.034 mm for water-drinking mice (N.S.). Thus, these acute/subacute inflammatory reactions were not influenced by long-term exposure to ethanol.
Impact of Ethanol on the Expression of Phenotype of Major Leukocyte Populations.
Cell counts of white blood cells and platelets in peripheral blood did not indicate any significant differences (data not shown). In addition, flow cytometry analyses of spleen cells and bone marrow cells did not show any differences with respect to frequencies of T and B cells in spleen or in bone marrow.
Impact of Ethanol on ex Vivo Cytokine Production by Spleen Cells.
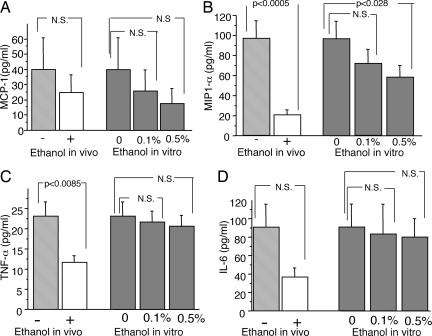
After 2 months of ethanol consumption, spleen cells from NMRI mice were assessed for their ability to produce proinflammatory cytokines. Spleen cells from ethanol-drinking mice produced significantly less macrophage inflammatory protein 1α (MIP-1α) and TNF-α than did cells from control mice. In contrast, no differences in the production of monocyte chemoattractant protein 1 (MCP-1) or IL-6 were found (Fig. 2). Upon addition of 0.5% ethanol to naive spleen cells from water-drinking mice, the chemokine MIP-1α was significantly reduced, whereas the production of all other cytokines analyzed was largely unaffected (Fig. 2).
Fig. 2.
Ex vivo production of MCP-1 (A), MIP-1α (B), TNF-α (C), and IL-6 (D) by spleen cells from NMRI mice drinking 10% ethanol (open bars) or water (light gray bars) for 2 months. In vitro production of MCP-1 (A), MIP-1α (B), TNF-α (C), and IL-6 (D) by spleen cells of naive NMRI mice after incubation with 0.1% and 0.5% ethanol. Statistical evaluation was made by using Student's t test. Values are presented as mean ± SEM. P value of ≤0.05 was considered N.S.
Ethanol Affects Leukocyte Migration.
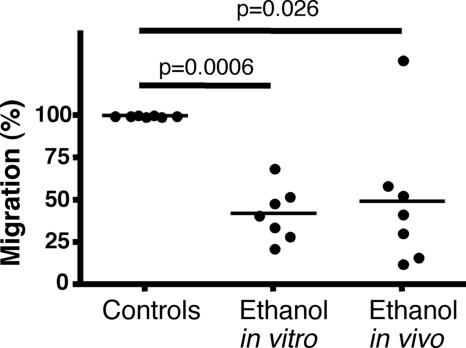
Cells collected from the peritoneal cavity of ethanol-drinking NMRI mice and of control mice were exposed to the hexapeptide WKYMVM at a concentration of 10−9 M (18) resuspended in Krebs–Ringer phosphate (KRG) buffer containing 0.1% BSA. We found that cells from ethanol-drinking mice migrated significantly less toward this chemotactic stimulus than did cells from the control mice (P = 0.0006) (Fig. 3). In addition, leukocytes exposed to ethanol in vitro showed a reduced migratory capacity (P = 0.05) compared with unexposed control cells (Fig. 3).
Fig. 3.
Migration of peritoneal leukocytes from NMRI mice, supplied with either 10% ethanol in drinking water or water alone for 8 weeks. Seven separate experiments were performed. Statistical evaluation was made by using the Mann–Whitney U test. Bars show the mean value.
Ethanol Down-Regulates the Nuclear Expression of Transcription Factors NF-κB and AP-1.
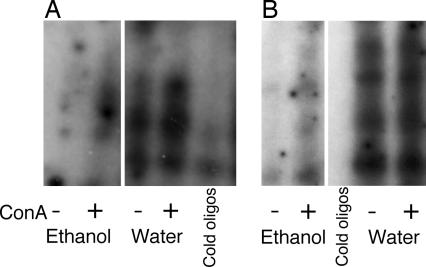
Binding of nuclear extracts to oligonucleotides containing DNA-binding sites specific for adaptor protein 1 (AP-1) or NF-κB was assessed by EMSA (Fig. 4). Spleen cells obtained from ethanol-drinking mice had significantly reduced levels of nuclear NF-κB and AP-1 transcription factors compared with those from control mice. Stimulation of leukocytes with Con A overcame the inhibitory effect of ethanol and increased the translocation of transcription factors in the control mice (Fig. 4). Interestingly, treatment of splenocytes from castrated mice with α-hydroxytestosterone down-regulated the spontaneous and Con A-induced activation of NF-κB and AP-1 (data not shown). These observations clearly demonstrate the in vivo inhibitory effect of long-time ethanol exposure on the activation of the two main inflammation-associated signaling pathways resulting in the reduction of NF-κB and AP-1 activity. Analogous inhibitory effects could be achieved by short-time treatment with α-hydroxytestosterone in vitro.
Fig. 4.
The reduction of NF-κB (A) and AP-1 (B) nuclear activity in the ethanol-drinking mice. NMRI mice were exposed in vivo to a continuous intake of 10% ethanol during a period of 8 weeks. Spleen cell cultures of ethanol-drinking and water-drinking control mice were stimulated with Con A (1.25 μg/ml). Nuclear extracts were prepared after 2 h of stimulation. EMSA was performed by using probes specific to the NF-κB and AP-1 binding sites, and after 20 min at room temperature the complexes were resolved by electrophoresis through a 2.5% polyacrylamide gel. In vivo exposure of mice to ethanol resulted in a significant reduction of NF-κB (A) and AP-1 (B) expression in the nuclei.
Impact of Ethanol Consumption on Testosterone Production and Its Influence on Joint Inflammation During CIA.
Levels of testosterone and estrogen, as well as insulin-like growth factor (IGF1) and cortisol, were measured in plasma from DBA/1 mice 5 weeks after the start of the experiment. Levels of testosterone were significantly elevated in mice drinking 10% ethanol compared with control mice. In contrast, levels of IGF1 and cortisol were significantly decreased in ethanol-drinking mice. No significant differences in estrogen levels were detectable (Table 1).
Table 1.
Impact of 5 weeks of ethanol intake on circulating hormone levels in male DBA/1 mice
| Hormone | Drink provided |
P value | |
|---|---|---|---|
| 10% ethanol | Water | ||
| Testosterone, ng/ml | 2.05 ± 1.02 | 0.83 ± 0.31 | 0.045 |
| IGF1, ng/ml | 97.6 ± 3.6 | 121.6 ± 7.5 | 0.027 |
| Cortisol, nmol/liter | 4.14 ± 0.54 | 5.61 ± 0.34 | 0.036 |
| Estrogen, pg/ml | 16.13 ± 1.05 | 13.39 ± 1.66 | N.S. |
For each hormone shown, the number of mice observed was 28. Values shown are means ± SEM. P values of ≤0.05 were considered N.S.
These observations, considered together with the in vitro antiinflammatory properties of testosterone that lead to a decrease of NF-κB activation, point to testosterone as a potential link mediating the antiinflammatory effects of ethanol. To further analyze this link, DBA/1 mice were orchidectomized before induction of arthritis and treatment with ethanol. The absence of testosterone in orchidectomized mice totally abolished the antiinflammatory effect of ethanol: these mice showed similar development of arthritis, irrespective of treatment provided, throughout the whole experiment, with a median of 4.5 and an IQR of 2–6 for ethanol-drinking mice and a median of 2.0 and an IQR of 1–5 for water-drinking mice (N.S.) on day 32 after the immunization. Moreover, intact ethanol-drinking mice showed significantly less severe arthritis (median of 2.0 and IQR of 0–3) than did orchidectomized ethanol-drinking mice (P = 0.028).
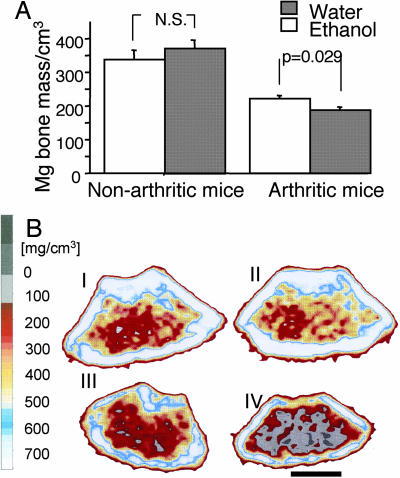
Ethanol Prevents the Arthritis-Induced Loss of Bone Mineral Density (BMD).
After the termination of the second experiment (i.e., 5 weeks after the start of ethanol exposure), the left femur of all mice was measured by peripheral quantitative computed tomography (pQCT) scan. Mice immunized with collagen and exposed to 10% ethanol in drinking water displayed significantly higher BMD than did the water-drinking control mice. In contrast, no differences between the groups were observed for cortical BMD. Femurs from healthy NMRI mice were also subjected to pQCT scan, and no significant differences were revealed between groups concerning the BMD of trabecular or cortical bone, irrespective of drink provided (Fig. 5).
Fig. 5.
Impact of ethanol drinking on bone mineral density. (A Right) Ethanol drinking decreases in vivo demineralization of trabecular bone by down-regulation of arthritis. (Left) Nonarthritic mice did not show any changes in bone mass as a function of ethanol consumption. Statistical evaluation was made by using the Mann–Whitney U test. (B) pQCT scans of one representative mouse from each group. Nonarthritic mice were represented by NMRI mice, and arthritic mice were represented by DBA/1 mice immunized with CII. Trabecular BMD was determined with a metaphyseal scan at a point 3% of the length of the femur from the growth plate, and the inner 45% of the area was defined as the trabecular bone compartment. (I) Nonarthritic mouse provided with 10% ethanol in drinking water (pQCT value of 367.7 mg/cm3). (II) Nonarthritic mouse drinking plain water (pQCT value of 398.8 mg/cm3). (III) Arthritic mouse provided with 10% ethanol in drinking water (pQCT value of 280.4 mg/cm3). (IV) Arthritic mouse drinking plain water (pQCT value of 119.1 mg/cm3). The gradient bar shows the density of the bone, from 0 (gray) to 750 (white) mg/cm3. (Scale bar: 1 mm.)
Discussion
Here we demonstrate that ethanol in quantities not toxic to liver delays the onset and alleviates the progression of CIA. Importantly, mice drinking 10% ethanol in water had a significantly slower onset of arthritis, but, even more importantly, the arthritis remained nondestructive even at late stages of the disease. Also, acetaldehyde, the main ethanol metabolite, displayed antiarthritic properties. Finally, we demonstrate that the beneficial effects of ethanol may be mediated by up-regulation of testosterone production, which in turn inhibits the NF-κB activation leading to decreased cytokine/chemokine production and decreased chemotactic activity of leukocytes.
RA is an autoimmune disease mediated by the concerted action of the innate and acquired immune systems that leads with time to a debilitating course of disease characterized by severe joint inflammation and both localized (cartilage and subchondral bone erosions) and systemic (osteoporosis) bone destruction (19). Chronic ethanol consumption is linked to a number of abnormalities of the immune system, including lymphopenia, and down-regulation of certain costimulatory molecules on the T cell surface (20), as well as to decreased interaction between leukocytes and endothelial cells (21). Chronic ethanol abuse will typically lead to severe medical consequences, but the potential of beneficial consequences of moderate alcohol intake have been discussed for decades, e.g., with respect to the epidemiology of cardiovascular diseases (22–25). Epidemiological studies have also analyzed the connection between alcohol consumption and RA (14–16). However, a direct beneficial effect of ethanol drinking on RA has not, to our knowledge, been reported. Interestingly, Mediterranean food has been shown to ameliorate the course of RA (26, 27). The mechanisms suggested for this amelioration include increased intake of unsaturated and single saturated fatty acids (28, 29), but the impact of daily exposure to low doses of ethanol, which is almost a standard of this diet, cannot be excluded.
The choice here of using doses of ethanol in quantities not toxic to liver was based on previous observations that mice exposed orally for a prolonged time to such a regimen did not suffer any visible damage to internal organs (30). This choice is indeed supported and extended by our study, which shows that at the dosage regimen used the mice did not suffer any metabolic consequences. Also, the numbers of leukocytes in peripheral and central lymphoid organs were not significantly affected by the ethanol intake. Finally, we were unable to detect any impact on acute and subacute T cell/macrophage- and neutrophil-dependent inflammatory responses in vivo, indicating that ethanol mediates its antiinflammatory properties in chronic inflammatory conditions.
How does prolonged ethanol drinking abolish the development of chronic, destructive inflammation? It is obvious that this effect was not mediated by the impact of ethanol on the expression of CII-specific antibodies. Indeed, during the course of the disease, the serum levels of CII antibodies were not affected by ethanol administration. Further support for this observation can be found in the fact that ethanol administration did not affect disease progression in a model of arthritis triggered by injection of preformed CII antibodies. These data collectively indicate that ethanol consumption does not affect the operation of this important effector mechanism in CIA. In contrast, it is clear that ethanol, both ex vivo as well as in vitro, profoundly affected NF-κB and AP-1 transcription factors. These systems are of major importance in mediating proinflammatory cytokine/chemokine production and destructive metalloproteinase transcription, respectively resulting in debilitating conditions. Indeed, data from our study indicate that the proinflammatory cytokine IL-6, but not the antiinflammatory cytokine IL-10, was significantly down-regulated in the circulation of ethanol-drinking mice. By the same token, in vitro data demonstrate that ethanol down-regulates production of the chemokine MIP-1α and the cytokine TNF-α. The latter data explain the potent ex vivo and in vitro antimigratory properties of ethanol we observed.
Are the effects of low doses of ethanol on the immune system mediated directly on immune cells or indirectly? The data presented suggest that indirect mediation is more probable. Indeed, our in vivo results strongly suggest that the increased production of endogenous testosterone may be of major importance to the beneficial effects of ethanol on the development of arthritis. Several lines of evidence support this hypothesis. First, ethanol-drinking mice had significantly higher levels (on average almost three times higher) of circulating testosterone compared with the controls. This difference was not due to testicular destruction because the weight of the seminal vesicles was not affected by ethanol consumption (data not shown). Second, treatment of severe arthritis with testosterone significantly decreases its severity (31). Finally, our data demonstrate that orchidectomized mice did not respond beneficially to ethanol exposure upon induction of CIA. The obvious question that needs to be addressed is to what extent the in vitro inhibitory impact of ethanol on the inflammatory cascade (e.g., NF-κB translocation) in the leukocyte environment, and in the absence of testosterone-producing Leydig cells, might be mediated. Schmidt et al. (32) clearly demonstrated that macrophages are able to produce nanomole quantities of testosterone and thereby act as a mobile source of this hormone. Another question that needs attention is the regulation of testosterone release by ethanol. Our data show that alcohol consumption leads concomitantly to down-regulation of IGF1 release and up-regulation of testosterone. It has been previously reported that suppression of IGF1 production leads to increased testosterone levels (33–35), suggesting that IGF1 might have an important role in the regulation of immune responses. Circulating cortisol levels were significantly decreased after the consumption of ethanol. This finding further underlines the importance of testosterone as an antiinflammatory molecule, even in a case of relative cortisol deficiency.
Ethanol consumption has been shown to increase the risk for bone fractures (36). However, previous studies have failed to find a relationship between low-dose ethanol intake and loss of BMD. In fact, Nguyen et al. (37) claimed that moderate alcohol intake might be protective against osteoporosis. Our results clearly indicate that moderate doses of ethanol do not influence BMD in a healthy state. However, in the case of an arthritic background, ethanol will significantly prevent systemic trabecular bone loss by virtue of decreasing the inflammatory state. Indeed, we have recently demonstrated that down-regulation of arthritis severity will not only lead to local (i.e., cartilage and subchondral bone) but also systemic effects on BMD (38).
In conclusion, the consumption of ethanol prevents the development of the destructive form of arthritis by interfering with NF-κB-mediated mechanisms. This interference depends on up-regulation of testosterone production, which in turn down-regulates production of proinflammatory cytokines/chemokines and leukocyte migration.
Materials and Methods
Mice.
Male DBA/1 mice (Taconic Europe A/S, Ry, Denmark) were used in the CIA experiments. For in vivo cell-mediated inflammatory response and ex vivo studies of the effect of alcohol on the different organs, NMRI mice (B & K Universal, Sollentuna, Sweden) were used. Mice were provided with 10% ethanol in drinking water, except for the control mice, which drank water only. Mice were regularly weighed and checked for the development of arthritis. Ethical permission was obtained from the Animal Research Ethics Committee of Göteborg University.
Experimental Protocols of in Vivo Experiments.
Two separate in vivo experiments regarding the impact of ethanol on the expression of CIA were conducted. On the immunization day, mice were provided with 10% ethanol in their drinking water. Control mice received tap water alone. The experiments were terminated after 5–6 weeks. Blood was drawn for serological analyses of cytokines, anti-CII antibodies, and liver enzymes and for hormone analyses. Paws were processed for histological analyses. One femur was obtained for pQCT scan. To study the influence of ethanol on the effector phase of CIA, mice were exposed to ethanol and injected i.v. with a mixture of CII-specific antibodies as previously described (17). To study the interplay between ethanol and sex hormones on the development of CIA, 20 DBA/1 mice were orchidectomized, divided into two groups, and subjected to drinking 10% ethanol in water or water alone. In this experiment, another 20 intact mice were subjected to drinking either ethanol or water (10 mice per group). In an additional experiment, the DBA/1 mice (10 mice per group) were provided with 1% of the major ethanol metabolite, acetaldehyde (Sigma, St. Louis, MO), in the drinking water or with water alone as a control. After immunization with CII, an evaluation of arthritis was performed. NMRI mice were used for ex vivo analyses of the impact of exposure to ethanol for 6–8 weeks.
CIA.
Chicken CII (Sigma) was dissolved at a concentration of 2 mg/ml in 0.1 M acetic acid. Arthritis was induced by intradermal injection of DBA/1 mice at the base of the tail with 100 μg of chick CII emulsified in an equal volume of incomplete Freund's adjuvant (IFA) (Sigma), supplemented with 0.5 mg/ml Mycobacterium tuberculosis (Sigma). Booster immunization containing 100 μg of CII in IFA was administered 21 days after the priming.
Induction of Collagen Antibody-Induced Arthritis.
Six-week-old DBA/1 mice were given 10% ethanol in water or water alone. Four weeks later they were injected i.v. with four monoclonal collagen antibodies (1 mg each of M2139, CIIC1, CIIC2, and U1-1). Seven days later the mice were i.p. injected with 25 μg of LPS from Escherichia coli (Sigma) to enhance the incidence and severity of arthritis in accordance with a recent study (17).
Clinical Evaluation of Arthritis.
All DBA/1 mice were inspected at regular intervals to ascertain the presence of arthritis. To evaluate the intensity of arthritis, a clinical scoring system of 0–3 points for each paw was used: 0, no sign of inflammation; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema. The arthritic index was constructed by adding the scores from all four limbs for each animal.
Impact of Ethanol on in Vivo Cell-Mediated Inflammatory Responses.
After 6 weeks of ethanol drinking, cell-mediated inflammatory responses in vivo were assessed. Delayed-type hypersensitivity, a T cell- and macrophage-dependent reaction, was induced by epicutaneous application of 150 μl of a mixture of ethanol and acetone (3:1) containing 3% (vol/vol) 4-ethoxymethylene-2-phenyloxazolone (Sigma) on the abdomen skin as previously described (40).
Olive oil induces in vivo a strong granulocyte-mediated but T cell- and macrophage-independent inflammatory response (41). Inflammation was induced by injection of 30 μl of olive oil s.c. in the hind paw as previously described (41).
Analyses of Enzymes, Hormones, and Cytokines.
The liver enzymes serum alanine and aspartate aminotransferases (s-ALAT and s-ASAT, respectively) and γ-glutamyltransferase (γ-GT) were analyzed by using spectrophotometric methods (Roche, Stockholm, Sweden). Levels of serum ethanol were determined with a gas chromatograph (42, 43). For serum analyses of hormones, the following radioimmunoassays were used: IGF1 (Mediagnost, Reutlingen, Germany), testosterone (MP Biomedicals, Irvine, CA), cortisol (CIS Bio International, Marcoule, France), and estrogen (Diagnostic Systems Laboratories, Webster, TX).
Measurements of Antibody and Cytokine Levels.
Quantification of anti-CII antibodies in serum was performed as previously described (44). IL-6 levels in sera and supernatants were analyzed as described in detail elsewhere (45). By using ELISA kits, IL-10 (Biosite, San Diego, CA) was measured in sera and supernatants, and TNF-α (Biosite), MIP-1α, and MCP-1 (BioSource International, Camarillo, CA) were measured in supernatants.
Impact of Ethanol on in Vitro Cell Responses.
Leukocytes isolated from spleen and bone marrow were kept in complete medium [Iscove's modified Dulbecco's medium enriched with 50 μg/ml gentamicin (Sigma), 4 mM l-glutamine (Sigma), 50 mM mercaptoethanol (Sigma), and 10% FCS (Biological Industries, Beit Haemek, Israel)] until use.
Impact of Ethanol on Intracellular Signaling and Production of Proinflammatory Cytokines.
Nuclear extracts were prepared from unstimulated spleen cell cultures (107 cells per sample) and from those stimulated with Con A (1.25 μg/ml) for 2 h. To assess the ability of testosterone to suppress the translocation of transcription factors, spleen cells (107 cells per sample) of castrated male mice were treated in vitro with α-hydroxytestosterone (Sigma) (dose range of 10−6 to 10−10 M) for 2 h and then stimulated with Con A (1.25 μg/ml). For EMSA, the stimulation was stopped after 2 h by adding ice-cold PBS, and nuclear extracts were prepared and EMSA was performed as previously described (26). For competition studies, a 100 M excess of unlabeled double-stranded oligonucleotides was added to the reaction mixture and incubated for 20 min before the introduction of the 32P-labeled probe. For supershift assays, antiserum to the p65 subunit of NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA) was incubated with nuclear extracts for 15 min at room temperature. For the assessment of cytokines, supernatants were collected after 48 h of stimulation.
Flow Cytometry.
Isolated cells from spleen and bone marrow were stained with phycoerythrin (PE)-conjugated antibodies to CD19 (BD PharMingen, Franklin Lakes, NJ) and with allophycocyanin (APC)-conjugated antibodies to CD3 (BD PharMingen). The cells were analyzed in a FACSCalibur (BD Biosciences Europe, Erembodegem, Belgium) fluorescence-activated cell sorter. FlowJo version 6.2.1 (Tree Star, Ashland, OR) software was used for analyzing the data.
Histological Examination.
All four paws from DBA/1 mice in the first experiment were excised at the time of killing. Tissue sections were stained with hematoxylin/eosin. The sections were studied by a blinded examiner regarding synovitis and erosion of bone/cartilage. Synovial hypertrophy was defined as a membrane thickness of more than two cell layers (45). A histological scoring system was used as follows: 1, mild; 2, moderate; and 3, severe synovitis and joint damage (46). Knee joints, ankles, elbows, and wrists were inspected, and a mean score from all of the inspected paws per each animal was calculated.
Impact of Ethanol on BMD.
The left femurs from NMRI mice and from mice in one of the CIA experiments were stored for 3 days in 4% (vol/vol) buffered formaldehyde, which was then replaced by 95% (vol/vol) alcohol until analyses of BMD were performed. A pQCT scan with a Stratec pQCT XCT Research M (Norland, Fort Atkinson, WI) was performed as previously described (47). Trabecular BMD was determined with a metaphyseal scan at a point located 3% of the length of the femur from the growth plate. The inner 45% of the area was defined as the trabecular bone compartment. Cortical BMD was determined with a middiaphyseal scan, which contained only cortical bone.
In Vitro Migration Assay.
Mice were injected i.p. with uric acid to promote in vivo influx of polymorphonuclear cells and monocytes in the peritoneal cavity as recently described (39). After 24 h, the peritoneal cavity was flushed with 2 ml of PBS, and the cellular content was aspirated. Collected cells were washed and resuspended in Krebs–Ringer phosphate (KRG) buffer (containing 10 nM glucose, 1 mM Ca2+, and 1.5 mM Mg2+ and supplemented with 0.1% BSA) to a final concentration of 106 cells per milliliter. Using the ChemoTx system (Neuro Probe, Gaithersburg, MD) with a pore size of 3 μm, we placed 3 × 105 cells in the upper chamber. As a positive chemotactic stimulus control, we used 10−9 M of hexapeptide WKYMVM (18), and as a negative control we used KRG buffer containing 0.1% BSA. Thirty microliters of each solution was placed in the lower chamber. After 90 min of incubation at 37°C and 5% (vol/vol) CO2, the remaining cell suspension was removed, and the plate was centrifuged for 10 min at 300 × g. Migrated cells were fixed by the addition of 4% (vol/vol) formaldehyde, stained with trypan blue, and enumerated by using an inverted microscope.
Statistical Analysis.
Statistical evaluation was made by using the Mann–Whitney U test, the χ2 test or Student's t test. Values are reported as medians and IQR or means ± SEM.
Acknowledgments
We thank Sylvie Amu, Berit Ericsson, Anette Hansevi, Caroline Jochems, Maud Petersson, and Fariba Zare for suggestions and assistance. This work was supported by the Göteborg Medical Society, Swedish Association Against Rheumatism, King Gustav V's Foundation, Swedish Medical Research Council, Nanna Svartz Foundation, University of Göteborg, A.-G. Crafoord Foundation, Börje Dahlin Foundation, European Union grants, Inflammation Network, A. M. E. Wolff Foundation, and Göteborg Association Against Rheumatism.
Abbreviations
- RA
rheumatoid arthritis
- CII
collagen type II
- CIA
CII-induced arthritis
- IQR
interquartile range
- N.S.
not significant
- IGF1
insulin-like growth factor
- pQCT
peripheral quantitative computed tomography
- BMD
bone mineral density.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Boe DM, Nelson S, Zhang P, Bagby GJ. J Infect Dis. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- 2.Bernardo J. Hosp Pract. 1991;26:195–208. doi: 10.1080/21548331.1991.11705315. [DOI] [PubMed] [Google Scholar]
- 3.Rosa H, Silverio AO, Perini RF, Arruda CB. Am J Gastroenterol. 2000;95:1290–1293. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson SJ, Bird SM, Goldberg DJ. Clin Gastroenterol Hepatol. 2005;3:1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 5.Cooper CL, Cameron DW. Clin Infect Dis. 2005;41(Suppl 1):S105–S109. doi: 10.1086/429506. [DOI] [PubMed] [Google Scholar]
- 6.Adams HG, Jordan C. Med Clin N Am. 1984;68:179–200. doi: 10.1016/s0025-7125(16)31249-4. [DOI] [PubMed] [Google Scholar]
- 7.Corrao G, Bagnardi V, Zambon A, La Vecchia C. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, De Gaetano G. Circulation. 2002;105:2836–2844. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 9.Djousse L, Ellison RC, Beiser A, Scaramucci A, D'Agostino RB, Wolf PA. Stroke. 2002;33:907–912. doi: 10.1161/hs0402.105245. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson AA, Rylander L, Hagmar L, Nived O, Sturfelt G. Rheumatology (Oxford) 2002;41:563–571. doi: 10.1093/rheumatology/41.5.563. [DOI] [PubMed] [Google Scholar]
- 11.Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Ann Rheum Dis. 1998;57:451–455. doi: 10.1136/ard.57.8.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Formica MK, Palmer JR, Rosenberg L, McAlindon TE. J Rheumatol. 2003;30:1222–1226. [PubMed] [Google Scholar]
- 13.Ghaussy NO, Sibbitt WL, Jr, Qualls CR. J Rheumatol. 2001;28:2449–2453. [PubMed] [Google Scholar]
- 14.Cerhan JR, Saag KG, Criswell LA, Merlino LA, Mikuls TR. J Rheumatol. 2002;29:246–254. [PubMed] [Google Scholar]
- 15.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Ann Rheum Dis. 1990;49:980–982. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Epidemiology. 1994;5:525–532. [PubMed] [Google Scholar]
- 17.Nandakumar KS, Backlund J, Vestberg M, Holmdahl R. Arthritis Res Ther. 2004;6:R544–R550. doi: 10.1186/ar1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bylund J, Samuelsson M, Collins LV, Karlsson A. Exp Cell Res. 2003;282:70–77. doi: 10.1016/s0014-4827(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 19.Walsh NC, Gravallese EM. Curr Opin Rheumatol. 2004;16:419–427. doi: 10.1097/01.bor.0000127824.42507.68. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Meadows GG. J Leukocyte Biol. 2005;78:1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 21.Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- 22.St Leger AS, Cochrane AL, Moore F. Lancet. 1979;i:1017–1020. doi: 10.1016/s0140-6736(79)92765-x. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 24.Ellison RC. Epidemiology. 1990;1:337–339. [PubMed] [Google Scholar]
- 25.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 26.Skoldstam L, Hagfors L, Johansson G. Ann Rheum Dis. 2003;62:208–214. doi: 10.1136/ard.62.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagfors L, Nilsson I, Skoldstam L, Johansson G. Nutr Metab (London) 2005;2:26. doi: 10.1186/1743-7075-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Nutrition. 2005;21:131–136. doi: 10.1016/j.nut.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Pattison DJ, Symmons DP, Young A. Proc Nutr Soc. 2004;63:137–143. doi: 10.1079/pns2003319. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Mendoza S, Davis-Gorman G, Cohen Z, Gonzales R, Tuttle H, McDonagh PF, Watson RR. Alcohol Alcohol. 2003;38:109–114. doi: 10.1093/alcalc/agg049. [DOI] [PubMed] [Google Scholar]
- 31.Steward A, Bayley DL. Agents Actions. 1992;35:268–272. doi: 10.1007/BF01997510. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Kreutz M, Loffler G, Scholmerich J, Straub RH. J Endocrinol. 2000;164:161–169. doi: 10.1677/joe.0.1640161. [DOI] [PubMed] [Google Scholar]
- 33.Les Dees W, Hiney JK, Srivastava V. Alcohol Health Res World. 1998;22:165–169. [PMC free article] [PubMed] [Google Scholar]
- 34.Lang CH, Fan J, Lipton BP, Potter BJ, McDonough KH. Alcohol Clin Exp Res. 1998;22:823–829. [PubMed] [Google Scholar]
- 35.Colao A, De Rosa M, Pivonello R, Balestrieri A, Cappabianca P, Di Sarno A, Rochira V, Carani C, Lombardi G. J Clin Endocrinol Metab. 2002;87:4193–4197. doi: 10.1210/jc.2002-020453. [DOI] [PubMed] [Google Scholar]
- 36.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TV, Eisman JA, Kelly PJ, Sambrook PN. Am J Epidemiol. 1996;144:255–263. doi: 10.1093/oxfordjournals.aje.a008920. [DOI] [PubMed] [Google Scholar]
- 38.Jochems C, Islander U, Erlandsson M, Verdrengh M, Ohlsson C, Carlsten H. Arthritis Res Ther. 2005;7:R837–R843. doi: 10.1186/ar1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zare F, Magnusson M, Bergstrom T, Brisslert M, Josefsson E, Karlsson A, Tarkowski A. J Leukocyte Biol. 2006;79:482–488. doi: 10.1189/jlb.0805426. [DOI] [PubMed] [Google Scholar]
- 40.Carlsten H, Nilsson L, Tarkowski A. Int Arch Allergy Appl Immunol. 1986;81:322–325. doi: 10.1159/000234156. [DOI] [PubMed] [Google Scholar]
- 41.Josefsson E, Carlsten H, Tarkowski A. Autoimmunity. 1993;14:251–257. doi: 10.3109/08916939309077373. [DOI] [PubMed] [Google Scholar]
- 42.Smith NB. Clin Chem. 1984;30:1672–1674. [PubMed] [Google Scholar]
- 43.Penton Z. Clin Chem. 1987;33:2094–2095. [PubMed] [Google Scholar]
- 44.Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Inflamm Res. 2003;52:341–346. doi: 10.1007/s00011-003-1182-8. [DOI] [PubMed] [Google Scholar]
- 45.Bremell T, Abdelnour A, Tarkowski A. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]