Abstract
The present study examined the thesis that positive affect may serve to broaden the scope of attentional filters, reducing their selectivity. The effect of positive mood states was measured in two different cognitive domains: semantic search (remote associates task) and visual selective attention (Eriksen flanker task). In the conceptual domain, positive affect enhanced access to remote associates, suggesting an increase in the scope of semantic access. In the visuospatial domain, positive affect impaired visual selective attention by increasing processing of spatially adjacent flanking distractors, suggesting an increase in the scope of visuospatial attention. During positive states, individual differences in enhanced semantic access were correlated with the degree of impaired visual selective attention. These findings demonstrate that positive states, by loosening the reins on inhibitory control, result in a fundamental change in the breadth of attentional allocation to both external visual and internal conceptual space.
Keywords: attention, emotion, creativity, inhibition, problem solving
Viewing the world “through rose-colored glasses” may be less proverb and more empirical fact. Converging evidence suggests that affective states are associated with changes in attention that may affect differentially perception and cognition (e.g., refs. 1–7). Attentional processes are those aspects of cognition that allow the control of perception, thought, and behavior and are generally acknowledged to depend on inhibitory control, such as suppression of irrelevant information and response inhibition (8). It has been proposed that executive control (5) and, thereby, the focus of attention may be influenced by the current affective state of the observer (6). For instance, it has been long hypothesized that arousal during negative affective states is associated with a constriction of attentional focus (7). Evidence for this narrowing of attention (9) sometimes is referred to as “weapon focus,” where attention is narrowed at the expense of encoding peripheral details (10). Although the interaction between negative affect and attention is an active focus of research, including attentional biases in affective disorders such as anxiety or depression (e.g., ref. 4), much less is appreciated regarding the role positive affect and well-being may have on attention.
Within the emerging field of positive psychology, the “broaden-and-build” theory suggests that a primary function of positive emotions is to broaden people's thought-action repertoires (11, 12), increasing their flexibility and enhancing their global scope. Consistent with such views, a robust and widely confirmed finding is that positive affect is linked to a creative and more generative mindset that results in greater cognitive flexibility across diverse situations, including medical diagnosis (13, 14), industrial negotiations (15), intuitive judgments (16), decision making (17), and creative problem-solving tasks (18, 19). For example, on the remote associates task (RAT; ref. 20), a test of creative problem solving, people are more likely to solve unusual word associations when they are in a positive, compared with negative or neutral, mood (16, 18, 19, 21). Similarly, positive mood generates more solutions to the Duncker (22) candle task (18, 19), which can be solved only by using the elements in an unconventional way. Thus, positive affect has been significantly linked with an increased capacity for creativity and novel thinking.
The present study examined the hypothesis that the increased cognitive flexibility and creative thinking associated with positive mood reflects a fundamental change in selective attention (23–25). In contrast with the proposed tunnel vision of negative affective states (9), positive affect may serve the opposite function: to enhance the scope of attention (6). Preliminary evidence for this assertion comes from studies examining global precedence, whereby positive mood is associated with greater global or holistic processing (i.e., seeing the forest before the trees) versus local processing (i.e., the trees before the forest) (23, 24). Under positive mood, individuals indicate a square made of triangles is more similar to a square than a triangle. Rather than a genuine change in the manner or breadth of how attention may be allocated, such cognitive biases have been interpreted within the affect-as-information framework (26), whereby happy moods increase access to what is in mind during the task at hand. In the case of global precedence, positive affect will accentuate further a bias toward global configurations (27).
Consistent with attention as having a measurable “breadth” or scope, research has suggested attentional focus can vary in its spatial extent, which has led to the use of different metaphors in characterizing the nature of attention. For example, attention has been compared with the beam from a spotlight (28, 29) or to the zoom lens of a camera (30, 31), suggesting that attention can be either narrowly focused or more widely distributed to include surrounding stimuli. Although attention can act in ways unlike a spotlight (e.g., refs. 32–34), more than just a metaphor, convergent physiological evidence from the extent of activation in primary visual cortex suggests that attention does have a measurable spatial scope (35). We hypothesize that positive affect may result in a relaxation of attentional selection, thus increasing the breadth of the proverbial spotlight of spatial attention.
As selective attention is associated with the inhibitory filtering of task-irrelevant distraction (36), increased attentional breadth would be reflected in a decreased capacity to inhibit processing of spatially adjacent irrelevant information. Our operational definition of broader attention in the visuospatial domain is thus an impairment of spatial selective attention, resulting in a more leaky filtering of unattended information, whereby ignored information is more fully processed (37, 38). To this end, we used the Eriksen flanker task (39) in which observers are asked to selectively attend to a central target and ignore irrelevant flanking distractors. Failure of selective attention is demonstrated when the to-be-ignored flankers influence performance, indicated by a slowing in response to the central target when flanked by response-incompatible letters. Also consistent with the spatially limited scope of attentional focus, previous research has found that flanker interference decreases with increasing distance from the central target, despite response competition demands remaining constant (39, 40). The present study manipulated distractor eccentricity to allow a more fine-grained analysis of the influence of positive affective state on the scope of visual selective attention.
If positive affect results in a more fundamental broadening of the scope of attentional selection, then it may have a common influence on processing of external visual stimulation and internal conceptual representations. Attentional state has been shown to influence the scope of semantic access (41, 42). Reduced capacity for attentional selection during positive affect then may facilitate access to a greater diversity of semantic information (21). To index increased scope of semantic access, we used the RAT (20), where participants are asked to override typical semantic associations to find semantically distant or remote associations. If positive mood is associated with an underlying broadening of attention, from perceptual to conceptual processing, then impaired selective attention, as indexed by the Eriksen flanker task, would be associated with facilitated access to remote semantic associations, as indexed by performance on the RAT.
Results
Mood Induction.
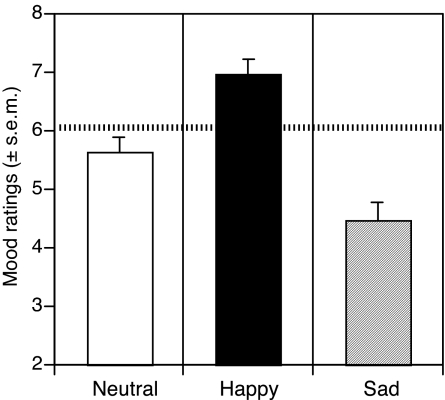
Positive and sad music induction increased and decreased positive affect, respectively (Fig. 1). Mood valence and overall arousal ratings were submitted to separate one-way ANOVAs with four levels of the mood induction factor: initial/preinduction and after neutral, positive, and negative mood inductions. Valence ratings differed significantly depending on induction phase [F(3, 66) = 28.22, P < 0.001]. The initial mood of participants before mood induction and task performance was slightly positive and was nonsignificantly decreased during neutral mood induction, t (66) = 1.57, P > 0.12. Positive [t (66) = 4.81, P < 0.001] and negative [t (66) = −4.23, P < 0.001] mood inductions resulted in a similar magnitude of increased and decreased positive affect relative to neutral conditions. By contrast, the overall level of subjective arousal of participants was consistent with a moderate level of alertness that did not differ across mood induction phase [F(3, 66) < 1].
Fig. 1.
Effect of mood manipulations. Participants rated degree of mood valence after neutral, happy, and sad mood manipulations (from 1 extremely unpleasant to 9 extremely pleasant). Dotted line, initial mood.
RAT.
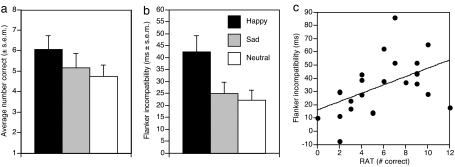
Consistent with a broader spread of semantic activation, positive mood was associated with increased access to remote semantic associations (Fig. 2a). The number of completed remote associate items was submitted to a repeated measures ANOVA with mood (happy, sad, and neutral) as a within-subject variable. A main effect of mood [F(2, 46) = 3.56, P = 0.04] revealed that RAT performance depended on mood. Significantly more RAT problems were correctly solved when participants were in a happy [mean (M) = 6.08, SD = 3.16] compared with sad (M = 5.17, SD = 3.40, t = 2.16, P = 0.04) or neutral (M = 4.75, SD = 2.64, t = 2.30, P = 0.031) mood. The sad vs. neutral difference was not reliable (P = 0.50).
Fig. 2.
Effect of mood manipulation on task performance. (a) Correct RAT responses. (b) Magnitude of flanker task incompatibility effects in milliseconds (incompatible minus compatible). (c) Correlation between RAT (number correctly identified) and flanker compatibility (incompatible minus compatible) under positive mood.
Flanker Task.
Response time data were submitted to a repeated measures ANOVA, with mood (positive, negative, and neutral), flanker compatibility (compatible vs. incompatible), and spacing (near, medium, and far levels) as within subject variables. Trials with response times >1,000 ms were considered incorrect and excluded from response time analysis. Analysis of response times was restricted to correct trials only. There was a high level of task accuracy (93.6%).
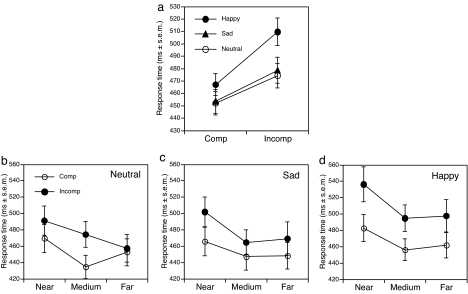
A highly reliable main effect of flanker compatibility [F(1, 23) = 410.91, P < 0.0001] revealed that responses to incompatible trials (M = 487.56, SD = 15.75) were slower than compatible (M = 457.68, SD = 14.62) flanker trials. There was also a main effect of spacing [F(2, 46) = 54.75, P < 0.0001] with response times at near (M = 491.25, SD = 16.12) significantly slower than both medium [M = 462.07, SD = 13.92, F(1, 23) = 89.00, P < 0.0001] and far [M = 464.54, SD = 15.61, F(1, 23) = 74.60, P < 0.0001] levels of spacing. The effect of flanker spacing interacted with flanker compatibility [F(2, 46) = 4.69, P < 0.02] such that compatibility effects were reduced with greater flanker distance, with near versus far spacing revealing a 45% reduction in the effect of flanker compatibility.
Consistent with the hypothesized influence of positive affect on visual selective attention, positive moods resulted in greater flanker interference relative to both sad and neutral moods (Fig. 2b). There was a marginal overall slowing on positive relative to both sad and neutral moods [F(2, 23) = 3.09, P < 0.06], which was largely due to an interaction between mood and compatibility [F(2, 46) = 3.86, P < 0.03], with a disproportionate slowing to incompatible flankers under positive mood (Fig. 3a). A focused test on the interaction revealed that positive moods resulted in greater incompatibility effects relative to neutral [F(1, 46) = 6.56, P < 0.02] and sad moods [F(1, 46) = 4.90, P < 0.04]. Sad and neutral moods did not differ (F < 1). Focused analyses on incompatible distractor trials demonstrated that positive moods resulted in pronounced slowing relative to both sad [F(1, 46) = 30.37, P < 0.0001] and neutral mood [F(1, 46) = 39.41, P < 0.0001]. Sad mood did not result in additional slowing relative to neutral moods on incompatible trials (F < 1).
Fig. 3.
Effect of mood manipulation and flanker distance on compatibility effects. (a) Compatibility collapsed across flanker distances. (b–d) Neutral (b), sad (c), and happy (d) mood at near, medium, and far flanker eccentricities. Comp, compatible flanking distractors; Incomp, incompatible flanking distractors.
Even as flanker eccentricity increased, positive mood resulted in pronounced slowing relative to negative and neutral moods (Fig. 3 b–d). A significant three-way interaction revealed that the effect of spacing on flanker compatibility was influenced by mood [F(4, 92) = 3.43, P < 0.02]. Critically, under neutral mood, the effect of flanker compatibility at the far distance was no longer significant [F(1, 92) < 1], consistent with abolished processing of flanker content (Fig. 3b). By contrast, under positive mood, far incompatible flankers maintained pronounced and greatest interference relative to compatible flankers [F(1, 92) = 31.12, P < 0.0001; Fig. 3d]. Although flanker compatibility effects still were found during negative mood [Fig. 3c; F(1, 92) = 10.93, P < 0.002], focused analyses of incompatible flanker trials demonstrated that positive mood resulted in highly robust interference relative to both negative [F(1, 46) = 31.12, P < 0.0001] and neutral moods [F(1, 46) = 40.07, P < 0.0001]. Further illustrative of the importance of mood relative to flanker distance, during positive mood, incompatible distractors at far distances (M = 498 ms) resulted in statistically equivalent response times to near distractors during neutral (M = 491) and negative (M = 501) mood (P > 0.3).
Relation Between RAT and Flanker Performance.
We next examined individual differences in enhanced access to remote associates and impaired visuospatial selective attention associated with positive mood. Although these tasks tap putatively different domains of cognitive function, individual differences in performance during positive mood may reveal a common underlying influence on information processing. After removal of 2 response time outliers (>4 SDs), a significant correlation was found between the number of remote associates correctly identified and slowed response times associated with flanker incompatibility (incompatible minus compatible) (r = 0.49, P < 0.02; Fig. 2c). As such, during positive mood, individuals with the greatest breadth in semantic access (indexed by number of remote associates accessed) demonstrated the most pronounced visuospatial attentional breadth (indexed by increased flanker incompatibility effect). This finding did not reflect a more general association between RAT and flanker performance, or generalization to negative affective states, as indicated by the lack of significant correlation under neutral (r = −0.09) and negative moods (r = 0.10).
Discussion
Relative to both neutral and sad mood, positive mood was associated with increased capacity to generate remote associates for familiar words (16, 18). This finding is consistent with a proposed broadening function of positive affect (11, 12). We show here this broadening in informational access is not restricted to its beneficial search for semantically distant associations. It extends to a detrimental influence on visuospatial selective attention. Positive affect impaired the ability to selectively focus on a target and thereby increased processing of spatially distant flanking distractors, consistent with expanded scope of the proverbial attentional “spotlight.” Positive moods thus facilitate tasks requiring a more global (24) and encompassing style of information processing, such as in the RAT, but impair those calling for a narrow, focused style, such as selective visual attention. A buoyant mood may represent a fundamental shift in the breadth of information processing, the result of which would be to cultivate a more open and exploratory mode of attention to both exteroceptive and interoceptive sources of information. We did not, however, observe that sad mood resulted in the opposite influence of positive mood (6). This may reflect that mild melancholic states evoked by music are not exclusively aversive, reflecting mixed-feeling states (43). The evocation of anxiety or fear-related states may be necessary for the proposed attentional narrowing of negative affect (9, 10).
In addition to the hypothesized increased breadth of attentional selection during positive mood, it has been suggested that affective states selectively modulate task-related neural activity within the prefrontal cortex (e.g., ref. 44), paralleling the anterior hemispheric asymmetries thought to support positive and negative affect (45). Gray et al. (44) found negative states facilitated tasks supported by the right hemisphere, such as visual working memory, whereas positive states benefited verbal working memory, supported by the left hemisphere. Evidence for enhanced semantic search and impaired visuospatial selective attention shown here may be interpreted within this hemispheric lateralization framework. However, positive affect has been linked with facilitating a broader, more generative mindset (11, 12, 18, 46) across diverse situations that include verbal and visual materials (13, 14). As such, the pattern of facilitation and interference in the present results is unlikely related to material specificity (verbal vs. visual) but rather is indicative of a shift in mode of attentional selection that operates on these visual and verbal materials.
An analysis of individual differences revealed that Flanker interference during positive mood was correlated with enhanced RAT performance, suggesting a common origin. Such an underlying increase in breadth of processing, extending from external perceptual to internal conceptual processing, may be derived from a central origin in cognitive or inhibitory control. Cognitive control is thought to limit the amount of information entering the focus of attention (8, 36) and, thereby, influences the capacity for selective attention (47). Similar to the effect of positive mood, individual differences in cognitive control are associated with the capacity for selective attention, as indexed by susceptibility to the “cocktail party effect,” where observers report hearing their own name on an unattended ear during dichotic listening (47). We suggest that positive mood reflects a global relaxation of inhibitory control (8, 36), resulting in an altered capacity for selective attention from early perceptual to later postsemantic levels of analysis.
How altered inhibitory control may alter the extent of visuospatial and semantic processing may be best understood with respect to the “attentional-load” theory of selective attention (37), which attempts to integrate early (perceptual) and late (postsemantic) models of attention. Attentional-load theory suggests that inhibitory suppression of ignored events is related to the degree (48) and type (37) of cognitive resources allocated to a primary task. To the extent that attentional resources are more available, inhibition of ignored events will be decreased, resulting in leaky and ineffective filtering of unattended information (37, 48). In addition, compared with perceptual load (e.g., difficult perceptual discrimination), manipulating cognitive load (e.g., working memory) results in late relative to early attentional filtering (37, 38). With regard to performance on the Flanker task, central target processing difficulty was manipulated through perceptual load via perceptual crowding (i.e., near versus far flankers), which is known to impair perceptual encoding (49–51). Paralleling the present results, such perceptual interference effects are suppressed by focal attention (52, 53) and exacerbated by more diffuse spatial attention (50). It is important to note that this attentional-load account need not suggest that decreased effort is applied under positive mood. Enhanced RAT performance indicates positive affect did not decrease effort or primary task engagement. Rather, we suggest the easing of inhibitory control alters the quality of attention, resulting in a shift from a narrow focused state to a more broad and diffuse attentional focus.
In parallel with influences on perceptual resources, positive affect may influence the allocation of cognitive resources (5, 21) to postsemantic levels of analysis. As such, attentional-load theory also may account for the effect of positive mood on semantic processing. Similar to studies examining the role of attention on perceptual encoding, examinations of semantic memory have demonstrated that attention plays a prominent role in semantic encoding. Attention to specific semantic features has been shown to bias semantic retrieval toward the attended dimension, inhibiting access to unattended associations (42). Also, studies examining semantic priming under different arousal-related attentional states show that focal attention increases priming for strong associates, whereas diffuse attentional focus results in increased priming for weak associates (41). With regard to performance on the RAT, greater attention toward a narrow set of semantic features will inhibit retrieval of more remote/weak semantic associations. For example, biased attention to the most strongly associated semantic features of the cue “widow” (i.e., the death of one's husband) would inhibit access to more remote associations (i.e., spider) in common with the cue's “bite” and “monkey.” More diffuse attentional focus under positive affect would attenuate such biases, broadening the scope of cognitive resources to include more semantically remote associations on which creative solutions depend.
In summation, the present results suggest a unifying framework in which to understand the interaction between positive affect, attention, and creativity. Mirroring the broad attentional focus and enhanced creative problem solving during positive mood, individual differences in creative intelligence have been associated with decreased attentional filtering. For instance, creative individuals have been shown to exhibit less latent inhibition, a measurement of attentional decrements to stimuli deemed irrelevant (54). As such, positive affect may represent a fundamental shift in information processing style, reflecting a relaxation of inhibitory control and, thereby, reducing the tendency to narrowly focus attention across disparate informational domains. The result of this altered capacity for attentional selection is a broadening of thought-action routines (11), engendering a broad exploratory (11, 18, 55) rather than narrow vigilant processing mode (25, 56). Donning the proverbial rose-colored glasses of positive mood then may be less about the color and more the expansiveness of the view.
Materials and Methods
Participants and Design.
Twenty-four university students (12 female) participated in a multipart experiment for either course credit or monetary remuneration. All participants were tested between 11 a.m. and 1 p.m., in the middle of the peak and off-peak periods of their circadian cycle (for a review of circadian arousal patterns, see ref. 57).
Mood Induction and Assessment.
For the happy mood induction, participants listened to a jazzed-up version of Bach's Brandenberg Concerto No. 3 (played by Hubert Laws). The sad mood was induced by listening to Prokofiev's Alexander Nevsky: Russia under the Mongolian Yoke played at half speed. These selections have been validated in previous mood research (e.g., refs. 58 and 59). The neutral mood was induced by reading a collection of basic facts about Canada, e.g., population size, land mass, gross national product, etc. Participants rated the valence of their moods and overall arousal at four time points throughout the experiment by using a nine-point scale, anchored by end points “extremely unpleasant” and “extremely pleasant.” The first time point was before mood induction and task performance. The remaining time points followed each of the neutral, positive, and negative mood induction phases.
RAT.
Three lists of 16 moderately difficult word problems were chosen from Mednick et al.'s (20) RAT task and counterbalanced between mood manipulations. Each problem consisted of a word triad (e.g., MOWER, ATOMIC, and FOREIGN), and a one-word solution related to all of the words (the solution to the above example is POWER).
Flanker Task.
This task was based on Eriksen and Eriksen's (39) classic flanker task, in which centrally presented targets are flanked on either side by response-compatible or response-incompatible letters. Processing of irrelevant flankers is typically indicated by a slowing in response to the central target when flanked by incompatible distractors. Because previous research has found that flanker interference decreases with increasing distance from the central target (39, 40), we also manipulated target/distractor distance to allow a more fine-grained analysis of attentional scope/breadth. We expected that if positive affect impairs visual selective attention through increasing the scope of attention, distant flankers would have a more pronounced effect, reflecting greater processing of unattended information (37, 38). Spacing between the central and flanker letters was manipulated, divided equally between near, medium, and far distances (0, 1, or 2 letter widths) for each of the compatible and incompatible target/flanker combinations. Examples of a compatible “near” space trial and incompatible “far” space trial are NNNNN and H H N H H, respectively. Each letter appeared in uppercase in Times New Roman 12-point font, measuring 0.5 cm high on the screen. Participants viewed the stimuli from ≈60 cm.
Procedure Overview.
The experiment was conducted in one session, and participants were tested individually on a computer by using E-Prime stimulus presentation software. All participants performed both tasks during each of the three induced affective states. Thus, an example of one experimental session would be the following: Sad mood induction was followed by the RAT and Flanker tasks, happy mood induction was followed by the RAT and Flanker tasks, and neutral mood induction was followed by the RAT and Flanker tasks. The order of task presentation (RAT first or Flanker first) was counterbalanced between moods that, in turn, were counterbalanced between participants. Participants rated their mood at the beginning of the experiment and after each mood manipulation.
Mood Induction.
Participants were instructed to listen to the designated music and generate matching thoughts for a period of 10 min; e.g., think about something happy while listening to the happy music. A shorter version of this procedure was repeated for a 2-min “booster” between the two tasks in each mood condition. The relevant music was played softly during the remainder of the testing period.
RAT Procedure.
For each of the three mood manipulations, word triads from one of the lists were presented individually on a computer screen for a maximum of 30 sec, and participants were instructed to provide the answer out loud. If they generated a solution (correct or incorrect) during that time, the experimenter pressed the spacebar to continue to the next problem, otherwise the computer automatically proceeded to the next trial after 30 sec. Responses were recorded by the experimenter. There were 16 word triad trials after each mood manipulation. All words were neutral.
Flanker Procedure.
Participants were given 36 practice trials on the first of the three flanker blocks. Other than these practice trials, each task block consisted of 96 randomly presented trials, presented at varying interstimulus intervals (500, 700, and 800 ms). Participants were instructed to identify the central letter in the display as quickly as possible by making an assigned key press. The computer then proceeded to the next trial. At the end of the testing session, participants were debriefed and completed a basic health questionnaire and a vocabulary test (60).
Acknowledgments
We would like to thank Itria Ma, Stephanie Tsicos, and Angela Romano for assisting with data collection and Lynn Hasher for generously providing testing space for pilot data collection. This work was funded by a Natural Sciences and Engineering Research Council discovery grant (A.K.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Anderson AK. J Exp Psychol Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. J Neurosci. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JR. Curr Dir Psychol Sci. 2004;13:46–48. [Google Scholar]
- 4.MacLeod C, Mathews A, Tata P. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Oaksford M, Morris F, Grainger B, Williams JMG. J Exper Psychol Learn Mem Cogn. 1996;22:476–492. [Google Scholar]
- 6.Derryberry D, Tucker DM. In: The Heart's Eye: Emotional Influences in Perception and Attention. Niedenthal PM, Kitayama S, editors. San Diego: Academic; 1994. pp. 167–196. [Google Scholar]
- 7.Easterbrook JA. Psychol Rev. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- 8.Hasher L, Lustig C, Zacks RT. In: Variation in Working Memory. Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. New York: Oxford Univ Press; in press. [Google Scholar]
- 9.Derryberry D, Reed MA. Pers Individ Dif. 1988;25:745–761. [Google Scholar]
- 10.Christianson S, Loftus EF. Bull Psychon Soc. 1990;28:195–198. [Google Scholar]
- 11.Fredrickson BL. Am Psychol. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredrickson BL. Am Sci. 2003;91:330–335. [Google Scholar]
- 13.Estrada C, Isen AM, Young MJ. Motiv Emot. 1994;18:285–299. [Google Scholar]
- 14.Estrada C, Isen AM, Young MJ. Organ Behav Hum Decis Process. 1997;72:117–135. [Google Scholar]
- 15.Carnevale PJ, Isen AM. Organ Behav Hum Decis Process. 1986;37:1–13. [Google Scholar]
- 16.Bolte A, Goschke T, Kuhl J. Psychol Sci. 2003;14:416–421. doi: 10.1111/1467-9280.01456. [DOI] [PubMed] [Google Scholar]
- 17.Isen AM. J Consum Psychol. 2001;11:75–85. [Google Scholar]
- 18.Isen AM, Daubman KA, Nowicki GP. J Pers Soc Psychol. 1987;52:1122–1131. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- 19.Isen AM, Johnson MM, Mertz E, Robinson GF. J Pers Soc Psychol. 1985;48:1413–1426. doi: 10.1037//0022-3514.48.6.1413. [DOI] [PubMed] [Google Scholar]
- 20.Mednick MT, Mednick SA, Mednick EV. J Abnorm Psychol. 1964;69:84–88. doi: 10.1037/h0045994. [DOI] [PubMed] [Google Scholar]
- 21.Ashby FG, Isen AM, Turken AU. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 22.Duncker K. Psychol Monogr. 1945;58:1–110. [Google Scholar]
- 23.Basso MR, Schefft BK, Ris MD, Dember WN. J Int Neuropsychol Soc. 1996;2:249–255. doi: 10.1017/s1355617700001193. [DOI] [PubMed] [Google Scholar]
- 24.Gasper K, Clore GL. Psychol Sci. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- 25.Fenske MJ, Eastwood JD. Emotion. 2003;3:327–343. doi: 10.1037/1528-3542.3.4.327. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz N, Clore GL. J Pers Soc Psychol. 1983;45:513–523. [Google Scholar]
- 27.Navon D. Cognit Psychol. 1977;9:353–383. [Google Scholar]
- 28.LaBerge D. J Exp Psychol Hum Percept Perform. 1983;9:371–379. doi: 10.1037//0096-1523.9.3.371. [DOI] [PubMed] [Google Scholar]
- 29.Posner MI, Snyder CR, Davidson BJ. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- 30.Eriksen CW, St James JD. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 31.Eriksen CW, Yeh YY. J Exp Psychol Hum Percept Perform. 1985;11:583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- 32.Awh E, Pashler H. J Exp Psychol Hum Percept Perform. 2000;26:834–846. doi: 10.1037//0096-1523.26.2.834. [DOI] [PubMed] [Google Scholar]
- 33.Driver J, Baylis GC. J Exp Psychol Hum Percept Perform. 1989;15:448–456. doi: 10.1037//0096-1523.15.3.448. [DOI] [PubMed] [Google Scholar]
- 34.O'Craven KM, Downing PE, Kanwisher N. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- 35.Brefczynski JA, DeYoe EA. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 36.Friedman NP, Miyake A. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- 37.Lavie N, Hirst A, de Fockert JW, Viding E. J Exp Psychol Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- 38.Yi DJ, Woodman GF, Widders D, Marois R, Chun MM. Nat Neurosci. 2004;7:992–996. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]
- 39.Eriksen BA, Eriksen CW. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 40.Zeef EJ, Sonke CJ, Kok A, Buiten MM, Kenemans JL. Psychophysiology. 1996;33:555–565. doi: 10.1111/j.1469-8986.1996.tb02432.x. [DOI] [PubMed] [Google Scholar]
- 41.Stickgold R, Scott L, Rittenhouse C, Hobson JA. J Cogn Neurosci. 1999;11:182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 42.Sohn MH, Anderson JR, Reder LM, Goode A. Psychon Bull Rev. 2004;11:729–734. doi: 10.3758/bf03196627. [DOI] [PubMed] [Google Scholar]
- 43.Larsen JT, McGraw AP, Mellers BA, Cacioppo JT. Psychol Sci. 2004;15:325–330. doi: 10.1111/j.0956-7976.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- 44.Gray JR, Braver TS, Raichle ME. Proc Natl Acad Sci USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RJ. In: Brain Asymmetry. Davidson RJ, Hughdahl K, editors. Cambridge, MA: MIT Press; 1995. pp. 361–387. [Google Scholar]
- 46.Fredrickson BL. Rev Gen Psychol. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway AR, Cowan N, Bunting MF. Psychon Bull Rev. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- 48.Rees G, Frith CD, Lavie N. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- 49.Bouma H. Vision Res. 1973;13:767–782. doi: 10.1016/0042-6989(73)90041-2. [DOI] [PubMed] [Google Scholar]
- 50.Strasburger H. J Vis. 2005;5:1024–1037. doi: 10.1167/5.11.8. [DOI] [PubMed] [Google Scholar]
- 51.Wolford G, Chambers L. Percept Psychophys. 1983;33:129–138. doi: 10.3758/bf03202830. [DOI] [PubMed] [Google Scholar]
- 52.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 53.Kastner S, De Weerd P, Pinsk MA, Elizondo MI, Desimone R, Ungerleider LG. J Neurophysiol. 2001;86:1398–1411. doi: 10.1152/jn.2001.86.3.1398. [DOI] [PubMed] [Google Scholar]
- 54.Carson SH, Peterson JB, Higgins DM. J Pers Soc Psychol. 2003;85:499–506. doi: 10.1037/0022-3514.85.3.499. [DOI] [PubMed] [Google Scholar]
- 55.Fredrickson BL, Joiner T. Psychol Sci. 2002;13:172–175. doi: 10.1111/1467-9280.00431. [DOI] [PubMed] [Google Scholar]
- 56.Forster J, Tory Higgins E. Psychol Sci. 2005;16:631–636. doi: 10.1111/j.1467-9280.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 57.Hasher L, Goldstein D, May CP. In: Human Learning and Memory: Advances in Theory and Application. Izawa C, Ohta N, editors. Mahwah, NJ: Erlbaum; 2005. pp. 119–217. [Google Scholar]
- 58.Green JD, Sedikides C, Saltzberg JA, Wood JV, Forzano LA. Br J Soc Psychol. 2003;42:147–157. doi: 10.1348/014466603763276171. [DOI] [PubMed] [Google Scholar]
- 59.Wood JV, Saltzberg JA, Goldsamt LA. J Pers Soc Psychol. 1990;58:899–908. doi: 10.1037//0022-3514.58.5.899. [DOI] [PubMed] [Google Scholar]
- 60.Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]