Abstract
SH2B1 (previously named SH2-B), a cytoplasmic adaptor protein, binds via its Src homology 2 (SH2) domain to a variety of protein tyrosine kinases, including JAK2 and the insulin receptor. SH2B1-deficient mice are obese and diabetic. Here we demonstrated that multiple isoforms of SH2B1 (α, β, γ, and/or δ) were expressed in numerous tissues, including the brain, hypothalamus, liver, muscle, adipose tissue, heart, and pancreas. Rat SH2B1β was specifically expressed in neural tissue in SH2B1-transgenic (SH2B1Tg) mice. SH2B1Tg mice were crossed with SH2B1-knockout (SH2B1KO) mice to generate SH2B1TgKO mice expressing SH2B1 only in neural tissue but not in other tissues. Systemic deletion of the SH2B1 gene resulted in metabolic disorders in SH2B1KO mice, including hyperlipidemia, leptin resistance, hyperphagia, obesity, hyperglycemia, insulin resistance, and glucose intolerance. Neuron-specific restoration of SH2B1β not only corrected the metabolic disorders in SH2B1TgKO mice, but also improved JAK2-mediated leptin signaling and leptin regulation of orexigenic neuropeptide expression in the hypothalamus. Moreover, neuron-specific overexpression of SH2B1 dose-dependently protected against high-fat diet–induced leptin resistance and obesity. These observations suggest that neuronal SH2B1 regulates energy balance, body weight, peripheral insulin sensitivity, and glucose homeostasis at least in part by enhancing hypothalamic leptin sensitivity.
Introduction
Body weight is controlled by a balance between energy intake and expenditure. Excess energy derived from a positive energy imbalance is stored as triglyceride (TG) in adipose tissue, resulting in obesity. Body weight is maintained within a narrow range by a homeostatic control system in which the brain, particularly the hypothalamus, senses and integrates various neuronal, hormonal, and nutrient-related signals, thereby coordinating food intake and energy expenditure. Recent findings provide a framework for understanding this homeostatic regulation of body weight. Leptin, which serves as an essential adiposity signal, is produced primarily by white adipose tissue to convey information about peripheral energy storage and availability to the hypothalamus (1–3). Genetic deficiency of either leptin or its receptor disrupts the communication between the peripheral energy stores and the central sensors/integrators, resulting in severe energy imbalance and morbid obesity (4–8). Leptin resistance plays a key role in the development of obesity, which is a primary risk factor for type 2 diabetes and various cardiovascular disorders.
Leptin binds to and activates its long form receptor (LEPRb) in the hypothalamus, initiating the activation of a variety of intracellular signaling pathways, including the STAT3 and PI3K pathways (8–12). Inhibition of either the STAT3 or PI3K pathways in the hypothalamus results in leptin resistance and obesity, demonstrating an essential role for these 2 pathways in mediating leptin regulation of energy metabolism and body weight (11–17). JAK2, a cytoplasmic tyrosine kinase, binds directly to LEPRb and is activated in response to leptin (9, 18). Activated JAK2 phosphorylates and activates downstream signaling molecules, including STAT3, insulin receptor substrate 1 (IRS1) and IRS2 (19). JAK2 is targeted by multiple negative regulators. PTP1B, a protein tyrosine phosphatase, binds to and dephosphorylates JAK2, thereby inhibiting JAK2 activation (20). Genetic deletion of PTP1B improves leptin sensitivity and protects against high-fat diet–induced (HFD-induced) obesity (21, 22). SOCS3 also inhibits JAK2 activation (23, 24). Both systemic SOCS3 haploinsufficiency and hypothalamus-specific deletion of SOCS3 improve leptin sensitivity (25, 26). Recent studies suggest that JAK2 may also be positively regulated by SH2-B, a JAK2-interacting protein (27).
SH2-B is one of 3 members of the SH2B family (SH2-B, APS, and Lnk), which have a conserved structure of a pleckstrin homology (PH) and Src homology 2 (SH2) domain (28–30). SH2-B, APS, and Lnk were recently renamed SH2B1, SH2B2, and SH2B3, respectively, by the HUGO Gene Nomenclature Committee. The SH2B1 (SH2-B) gene encodes, via alternative mRNA splicing, 4 isoforms (α, β, γ, and δ) that share an identical N-terminal region, including both PH and SH2 domains, but differ at their C termini following the SH2 domain (31). Since all forms contain conserved PH and SH2 domains, they are predicted to have similar functions. In cultured cells, SH2B1 binds via its SH2 domain to numerous protein tyrosine kinases, including both cytoplasmic tyrosine kinases (e.g., JAK1, JAK2, and JAK3) and receptor tyrosine kinases (e.g., the receptors for insulin, IGF-1, PDGF, FGF, nerve growth factor, brain-derived neurotrophic factor, and glial cell–derived neurotrophic factor) (29, 32–43). In animals, systemic deletion of SH2B1 results in morbid obesity and type 2 diabetes, demonstrating that SH2B1 is an essential player in the regulation of both body weight and glucose metabolism (44–46). In addition to obese and diabetic phenotypes, SH2B1-knockout mice also have defects in reproduction (47).
SH2B1 is abundantly expressed in both the central nervous system and peripheral tissues, including the brain, liver, muscle, and adipose tissue. However, relative contributions of SH2B1 in individual tissues to energy and/or glucose homeostasis remain unclear. This study investigated the potential role of central versus peripheral SH2B1 in regulating energy and glucose metabolism and provides convincing evidence that central rather than peripheral SH2B1 controls body weight, peripheral insulin sensitivity, and glucose metabolism at least partially by cell-autonomously enhancing leptin sensitivity in the hypothalamus.
Results
Neuron-specific restoration of SH2B1 rescues obesity and hyperlipidemia in SH2B1KO mice.
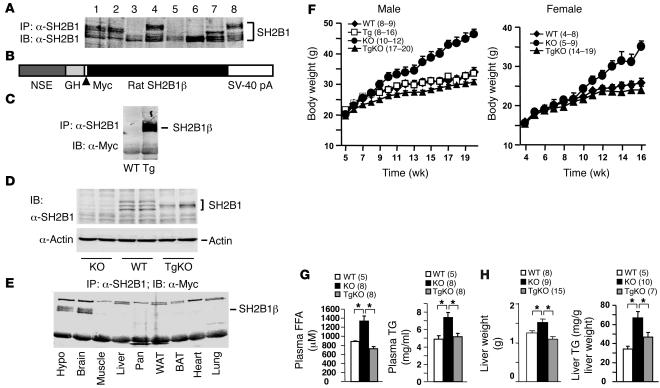
To determine tissue distribution of SH2B1, tissue extracts were prepared from spleen, pancreas, heart, hypothalamus, skeletal muscle, liver, white adipose tissue, and brain of WT mice. Tissue extracts were immunoprecipitated with anti-SH2B1 antibody (α-SH2B1) and immunoblotted. α-SH2B1, which was raised against a fusion protein of glutathione-S-transferase full-length rat SH2B1β, specifically recognizes all isoforms of SH2B1 (α, β, γ, and δ); in contrast, previously reported anti-SH2B1 antibodies preferentially recognize SH2B1β (44). Multiple forms of SH2B1 were detected in spleen, pancreas, hypothalamus, and brain (Figure 1A, lanes 1, 2, 4, and 8). In contrast, the heart, muscle, liver and adipose tissues predominantly expressed the fastest-migrating form of SH2B1 (Figure 1A, lanes 3 and 5–7).
Figure 1. Neuron-specific restoration of SH2B1β rescues obesity and hyperlipidemia in SH2B1KO mice.
(A) Tissue extracts (2 mg protein in hypothalamic and 3 mg protein in other tissue extracts) were prepared from WT mice, immunoprecipitated with α-SH2B1, and immunoblotted with α-SH2B1. Lanes 1–8 represent tissue extracts from spleen, pancreas, heart, hypothalamus, muscle, liver, white adipose tissue, and brain, respectively. (B) Schematic representation of the Myc-tagged SH2B1β transgene. (C) Brain extracts were prepared from an SH2B1Tg mouse and a WT littermate, immunoprecipitated with α-SH2B1, and immunoblotted with α-Myc. (D) Brain extracts were prepared from WT, SH2B1KO, and SH2B1TgKO-437 mice and immunoblotted with α-SH2B1. Each lane represents a sample from 1 mouse. (E) Tissue extracts were prepared from the hypothalamus (Hypo), brain, skeletal muscle, liver, pancreas (Pan), white adipose tissue (WAT), brown adipose tissue (BAT), heart, and lung from an SH2B1TgKO-437 female (16 weeks old); immunoprecipitated with α-SH2B1; and immunoblotted with α-Myc. (F) Growth curves for WT, SH2B1Tg, SH2B1KO, and SH2B1TgKO-437 mice. Number in parentheses indicates the number of mice per group. (G) Levels of plasma FFAs and TGs in males (17 weeks old) fasted overnight. (H) Liver weight and TG levels in mice (21–22 weeks old) fasted overnight. *P < 0.05.
To determine the role of SH2B1 in the brain, recombinant SH2B1β was specifically expressed in neural tissue under the control of the neuron-specific enolase (NSE) promoter/rat growth hormone (GH) enhancer. A similar NSE promoter/GH enhancer has been successfully used to drive the expression of LEPRb and proopiomelanocortin (POMC) transgenes in the hypothalamus, respectively, resulting in a reduction in both adiposity and body weight (48, 49). Rat SH2B1β cDNA was inserted into a transgenic vector 3′-end of the NSE promoter/GH enhancer sequences (Figure 1B). A Myc tag was engineered at the N terminus of SH2B1β to facilitate the detection of recombinant SH2B1β expression. The SH2B1β transgenic construct was used to generate heterozygous SH2B1-transgenic (SH2B1Tg) mice.
Eight independent SH2B1Tg founders were obtained. To examine the expression of the SH2B1 transgene, brain extracts were prepared from WT control and SH2B1Tg mice, immunoprecipitated with α-SH2B1 and immunoblotted with α-Myc. Recombinant SH2B1β was detected in SH2B1Tg but not WT mice (Figure 1C). All 8 SH2B1Tg lines expressed similar levels of recombinant SH2B1β (data not shown).
Two independent SH2B1Tg lines (SH2B1Tg-437 and SH2B1Tg-407) were crossed with heterozygous SH2B1-knockout mice to generate compound mutant mice (SH2B1TgKO-437 and SH2B1TgKO-407) that were heterozygous for the SH2B1β-transgenic allele and homozygous for the SH2B1KO allele. The expression of recombinant SH2B1β was similar in SH2B1Tg-437 and SH2B1Tg-407 mice and restricted to neural tissues in both SH2B1Tg-437 and SH2B1Tg-407 mice (data not shown). To compare the expression levels of the SH2B1 transgene and endogenous SH2B1 gene, brain extracts were immunoblotted with α-SH2B1. Multiple forms of endogenous SH2B1 were detected in WT but not homozygous SH2B1-knockout (SH2B1KO) mice (Figure 1D). Recombinant SH2B1β was the only form detected in SH2B1TgKO mice and was expressed at levels similar to those of endogenous SH2B1 in WT mice (Figure 1D). However, these data cannot exclude the possibility that the NSE promoter/GH enhancer may drive an overexpression of recombinant SH2Bβ in a subpopulation of neurons that either express extremely low levels of SH2B1 or do not express SH2B1 at all. To confirm specific expression of the SH2B1β transgene in neural tissue, multiple tissue extracts were prepared from SH2B1TgKO mice, immunoprecipitated with α-SH2B1, and immunoblotted with α-Myc. Recombinant SH2B1β was detected in both the hypothalamus and whole brain, but not in muscle, liver, pancreas, white adipose tissue, brown adipose tissue, heart, and lung (Figure 1E).
To determine the role of neuronal SH2B1 in regulating growth and body weight, SH2BKO, SH2BTgKO, SH2BTg, and WT mice were fed standard chow, and body weight was monitored. Systemic deletion of SH2B1 resulted in a marked increase in body weight in both male and female SH2B1KO mice, and neuron-specific restoration of SH2B1 (to endogenous levels) fully rescued the obese phenotype in SH2B1TgKO-437 mice (Figure 1F). Neuron-specific restoration of SH2B1 also markedly reduced body weight in another independent line (SH2BKO: 32.3 ± 1.6 g, n = 12; SH2B1TgKO-407: 25.5 ± 1.1 g, n = 5; 10 weeks). Neuron-specific restoration of recombinant SH2B1β alone was sufficient to rescue the obese phenotype observed in SH2BKO mice, suggesting that neuronal SH2B1 is required for maintaining normal body weight and that multiple isoforms of SH2B1 in the brain have similar functions in regulating body weight.
Obesity is commonly associated with hyperlipidemia. Systemic deletion of SH2B1 markedly increased both plasma FFA and triglyceride (TG) levels in SH2B1KO mice; neuron-specific restoration of SH2B1 completely corrected hyperlipidemia in SH2B1TgKO mice (Figure 1G). SH2B1KO mice had hepatic steatosis as revealed by an enlarged liver mass and significantly increased hepatic lipid content (Figure 1H). Neuron-specific restoration of SH2B1 largely reversed hepatic steatosis in SH2B1TgKO mice (Figure 1H).
Neuronal and adipose SH2B1 exert opposite effects on adiposity.
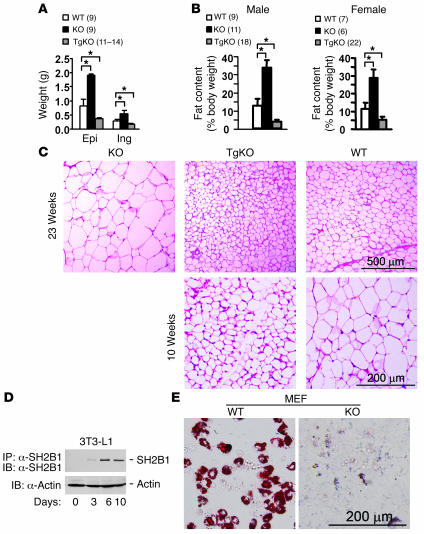
To further analyze adiposity, individual white adipose depots were weighed, and whole body fat content was measured by the dual-energy x-ray absorptiometry (DEXA) method. Systemic deletion of SH2B1 markedly increased the mass of both epididymal and inguinal fat depots in SH2B1KO mice (Figure 2A). Whole body fat content was increased by approximately 2.6-fold in male and approximately 2.5-fold in female SH2B1KO mice (Figure 2B). Neuron-specific restoration of SH2B1 (to endogenous levels) completely reversed the elevation in fat mass in SH2B1TgKO mice (Figure 2, A and B). Histological examination of epididymal fat depots revealed that systemic deletion of SH2B1 markedly increased the size of individual adipocytes in SH2B1KO mice; neuron-specific restoration of SH2B1 completely reversed adipocyte hypertrophy in SH2B1TgKO mice (Figure 2C, upper panels). These data demonstrated that neuronal SH2B1 negatively controls adiposity in animals.
Figure 2. Neuronal and adipose SH2B1 have opposite effects on adiposity.
(A) Weight of epididymal (Epi) and inguinal fat depots (Ing) from SH2B1KO, SH2B1TgKO-437, and WT males at age 23–24 weeks. (B) Whole body fat content in SH2B1KO, SH2B1TgKO-437, and WT mice. (C) Representative H&E staining of epididymal fat depots from SH2B1KO, SH2B1TgKO-437, and WT males at age 23 weeks (upper panels) or from SH2B1TgKO-437 and WT males at age 10 weeks (lower panels). (D) 3T3-L1 preadipocytes were differentiated into adipocytes for 0, 3, 6, or 10 days. Cell extracts were immunoprecipitated with α-SH2B1 and immunoblotted with α-SH2B1 (upper panel). Cell extracts were also immunoblotted with anti–β-actin antibodies (lower panel). (E) WT and SH2B1KO MEF primary cultures were subjected to adipocyte differentiation for 10 days. Differentiated cells were stained with oil red O. *P < 0.05.
Compared with those in age-matched WT control mice, both total fat mass and the size of individual white adipocytes were significantly reduced in SH2B1TgKO mice, which lacked SH2B1 in adipose tissue (Figure 2, A–C). These results suggest that adipose SH2B1 may be involved in adipocyte growth and/or differentiation. To obtain additional evidence, we examined SH2B1 expression during 3T3-L1 adipocyte differentiation. Cell extracts were immunoprecipitated with α-SH2B1 and immunoblotted with α-SH2B1. 3T3-L1 preadipocytes expressed low levels of endogenous SH2B1. SH2B1 expression increased progressively during adipocyte differentiation, reaching maximal levels within 6 days after the induction of differentiation (Figure 2D). The effect of SH2B1 deficiency on adipocyte differentiation was examined using mouse embryonic fibroblasts (MEFs). Both WT and SH2B1KO MEF primary cultures were subjected to an in vitro adipocyte differentiation treatment and then stained with oil red O to identify differentiated adipocytes. Deletion of SH2B1 impaired the ability of MEFs to differentiate into adipocytes (Figure 2E). These results suggest that adipose SH2B1 is involved in adipogenesis in a cell-autonomous fashion. However, neuronal SH2B1 may play a dominant role over adipose SH2B1 in controlling adiposity in vivo; therefore, deletion of SH2B1 in both the brain and adipose tissue results in adipocyte hypertrophy and obesity in SH2B1KO mice.
Neuron-specific restoration of SH2B1 reverses energy imbalance in SH2B1KO mice.
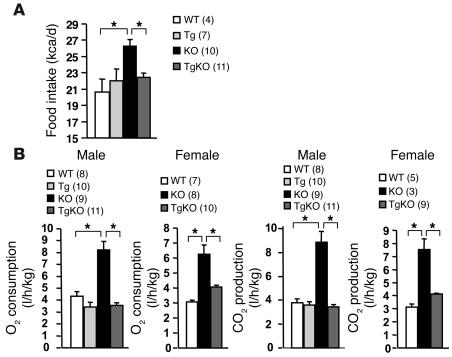
Systemic deletion of SH2B1 resulted in hyperphagia, markedly increasing food intake in SH2B1KO mice (Figure 3A). SH2B1KO mice also had significantly elevated energy expenditure, as revealed by significantly increased O2 consumption and CO2 production (Figure 3B). A previous report showed that energy intake still exceeds energy expenditure in this setting, resulting in obesity in SH2B1KO mice (45). Neuron-specific restoration of SH2B1 largely corrected hyperphagia and markedly reduced energy expenditure in SH2B1TgKO mice (Figure 3, A and B). These results suggest that SH2B1 in the brain controls body weight and adiposity by inhibiting both energy intake and expenditure.
Figure 3. Neuron-specific restoration of SH2B1 corrects energy imbalance in SH2B1KO mice.
(A) Food intake in WT, SH2B1Tg, SH2B1KO, and SH2B1TgKO-437 mice at age 14–15 weeks. (B) O2 consumption and CO2 production in WT, SH2B1Tg, SH2B1KO, and SH2B1TgKO-437 mice at age 16–17 weeks. *P < 0.05.
Neuron-specific restoration of SH2B1 corrects leptin resistance and hypothalamic neuropeptide expression in SH2B1KO mice.
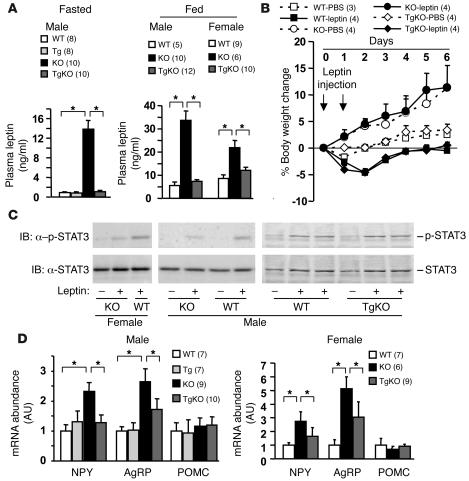
Systemic deletion of SH2B1 dramatically increased plasma leptin levels (hyperleptinemia) in both fasted and fed SH2B1KO mice, a hallmark of leptin resistance (Figure 4A). Neuron-specific restoration of SH2B1 expression completely reversed hyperleptinemia in SH2B1TgKO mice (Figure 4A). Acute exogenous leptin treatment markedly reduced body weight and food intake in WT mice, as predicted (Figure 4B and data not shown). Systemic deletion of SH2B1 abolished these physiological responses to leptin in SH2B1KO mice, including leptin-induced reduction in body weight (Figure 4B). Neuron-specific restoration of SH2B1 fully rescued the ability of leptin to reduce body weight in SH2B1TgKO mice (Figure 4B). These data indicate that neuron-specific restoration of SH2B1 (to endogenous levels) is sufficient to rescue leptin resistance in SH2B1KO mice.
Figure 4. Neuron-specific restoration of SH2B1 reverses leptin resistance in SH2B1KO mice.
(A) Plasma leptin levels in fasted (10 weeks old) and fed ad libitum (13 weeks old) mice. (B) Male mice (9 weeks old) were housed individually and injected intraperitoneally with leptin (2 mg/kg body weight) or PBS (control) twice a day (6:00 pm and 12:00 am). Body weight was monitored both before and after the injection. Changes in body weight were calculated as a percentage of the initial values prior to the injection. (C) Female (right panel: 6 weeks old) and male (middle panel: 12 weeks old; right panel: 9 weeks old) mice were fasted for 24 hours and injected intraperitoneally with leptin (1 mg/kg of body weight) or PBS as control. Hypothalamic extracts were prepared 45 minutes after injection and immunoblotted with α–p-STAT3 or α-STAT3. Each lane represents a combination of 2 hypothalami. (D) Hypothalamic RNA was prepared from males (22 weeks old, fasted overnight). NPY, AgRP, and POMC mRNA levels were measured using quantitative real-time PCR and normalized to the expression of β-actin. *P < 0.05.
To examine leptin signaling in the hypothalamus, mice were fasted overnight and injected intraperitoneally with leptin (1 mg/kg body weight). Hypothalamic extracts were immunoblotted with anti–phospho-STAT3 antibodies that specifically recognize phosphorylated and active STAT3. Systemic deletion of SH2B1 significantly impaired leptin-stimulated phosphorylation of STAT3 (Figure 4C). Neuron-specific restoration of SH2B1 increased leptin-stimulated phosphorylation of hypothalamic STAT3 in SH2B1TgKO mice to levels similar to those in age-matched WT controls, suggesting that neuronal SH2B1 may cell-autonomously enhance leptin signaling in the hypothalamus (Figure 4C).
Leptin inhibits the expression of orexigenic neuropeptide Y (NPY) and agouti-related protein (AgRP) and stimulates the expression of anorexigenic POMC in the arcuate nucleus of the hypothalamus (1, 3, 50). NPY and AgRP promote positive energy imbalance, whereas α–melanocyte-stimulating hormone (α-MSH), a proteolytic product of POMC, promotes negative energy imbalance (2, 3, 51). The abundance of hypothalamic NPY, AgRP, and POMC mRNA was measured using quantitative real-time PCR assays and normalized to the expression of β-actin. Systemic deletion of SH2B1 markedly increased NPY and AgRP but not POMC expression in SH2B1KO mice (Figure 4D). Neuron-specific restoration of SH2B1 dramatically reduced NPY expression in SH2B1TgKO mice to levels similar to those in WT controls (Figure 4D). AgRP expression was also significantly reduced in SH2B1TgKO mice, but to a lesser extent (Figure 4D). Neuronal SH2B1 may inhibit the expression of orexigenic NPY and AgRP presumably by enhancing leptin signaling in the hypothalamus.
Neuron-specific overexpression of SH2B1 protects against HFD-induced leptin resistance and obesity.
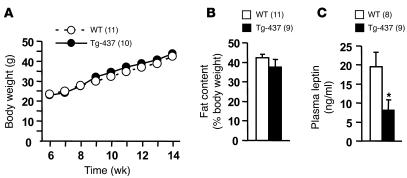
To determine whether a modest increase in neuronal SH2B1 expression protects against leptin resistance and obesity, SH2B1Tg and WT littermates were fed an HFD. Body weight and fat content were similar in SH2B1Tg and WT littermates (Figure 5, A and B). However, blood leptin levels were significantly reduced, by 58%, in SH2B1Tg mice, suggesting an increase in leptin sensitivity in SH2B1Tg mice (Figure 5C). These results suggest that while a modest increase in neuronal SH2B1 expression mildly increases leptin sensitivity, this is insufficient to protect against HFD-induced obesity in SH2B1Tg mice.
Figure 5. Neuronal SH2B1 increases leptin sensitivity.
(A) SH2B1Tg-437 males and WT littermates were fed an HFD at age 7 weeks, and body weight was monitored weekly. (B) Fat content of SH2B1Tg-437 males and WT littermates fed an HFD for 9 weeks. (C) Plasma leptin levels in SH2B1Tg-437 males and WT littermates fed an HFD for 10 weeks. *P < 0.05.
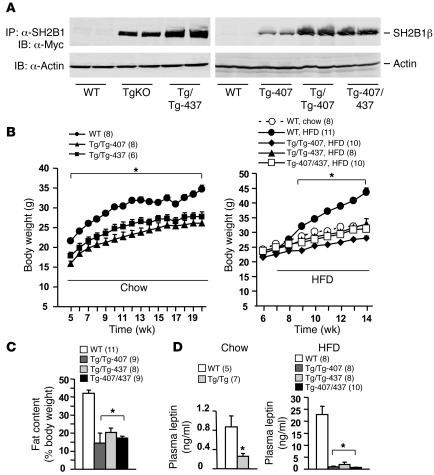
To increase neuronal SH2B1 expression, SH2B1Tg mice were bred to generate homozygous SH2B1-transgenic mice (SH2B1Tg/Tg). Two independent SH2B1Tg/Tg lines (SH2B1Tg/Tg-437 and SH2B1Tg/Tg-407) were obtained. To examine the SH2B1 transgene expression, brain extracts were immunoprecipitated with α-SH2B1 and immunoblotted with α-Myc to detect Myc-tagged recombinant SH2B1β. The expression of the SH2B1β transgene was significantly higher in SH2B1Tg/Tg than in SH2B1TgKO-437 and SH2B1Tg-407 mice (heterozygous for the SH2B1β transgene) (Figure 6A). Neuron-specific overexpression of SH2B1 did not have an obviously deleterious effect on the overall health of SH2B1Tg/Tg mice.
Figure 6. Neuron-specific overexpression of SH2B1 dose-dependently reduces HFD-induced leptin resistance and obesity.
(A) Brain extracts were prepared from WT, SH2B1TgKO-437, SH2B1Tg/Tg-437, SH2B1Tg-407, SH2B1Tg/Tg-407, and SH2B1Tg-407/437 males, immunoprecipitated with α-SH2B1, and immunoblotted with α-Myc. Extracts were also immunoblotted with anti–β-actin. Each lane represents a sample from 1 mouse. (B) Growth curves for male mice (WT, SH2B1Tg-407, SH2B1Tg/Tg-407, and SH2B1Tg-407/437) fed normal chow or HFD as indicated. (C) Fat content in male mice (16 weeks old) fed an HFD for 9 weeks. (D) Fasting plasma leptin levels in male mice fed standard chow (WT and SH2B1Tg/Tg-437; 17 weeks old) or HFD for 7 weeks (WT, SH2B1Tg/Tg-407, SH2B1Tg/Tg-437, and SH2B1Tg-407/437; 14 weeks old). *P < 0.05.
To determine the dosage effect of neuronal SH2B1 on leptin sensitivity and adiposity, body weight and blood leptin levels were measured. Body weight markedly decreased in both SH2B1Tg/Tg-437 and SH2B1Tg/Tg-407 compared with WT control mice fed normal chow (Figure 6B, left panel). Fasting plasma leptin levels were significantly lower in SH2B1Tg/Tg than in WT control mice (Figure 6D).
Mice were fed an HFD at 7 weeks of age. HFD markedly increased both body weight and fat content in WT mice, whereas both SH2B1Tg/Tg-407 and SH2B1Tg/Tg-437 mice were resistant to HFD-induced obesity (Figure 6, B, right panel, and C). HFD induced severe hyperleptinemia (a hallmark of leptin resistance) in WT mice, increasing fasting blood leptin levels by 26-fold; in contrast, plasma leptin levels were only mildly elevated in HFD-fed SH2B1Tg/Tg mice. Neuronal overexpression of SH2B1 reduced blood leptin levels by 98% in SH2B1Tg/Tg-407 and 92% in SH2B1Tg/Tg-437 compared with WT control mice (Figure 6D, right panel). Since the 2 lines of SH2B1Tg/Tg mice were similarly protected against HFD-induced leptin resistance and obesity, overexpression of neuronal SH2B1, rather than other mutations derived from the random insertion of the SH2B1 transgene, enhances leptin sensitivity in SH2B1Tg/Tg mice.
To avoid the complete disruption of a gene by the transgenic insertion, SH2B1Tg/Tg-437 and SH2B1Tg/Tg-407 mice were crossed to generate SH2B1Tg-407/437 mice heterozygous for both SH2B1Tg-437 and SH2B1Tg-407 alleles. The expression levels of recombinant SH2B1β were similar in SH2B1Tg-407/437 and SH2B1Tg/Tg-437 or SH2B1Tg/Tg-407 mice but higher in SH2B1Tg-407/437 than in heterozygous SH2B1Tg mice (Figure 6A). Importantly, SH2B1Tg-407/437 mice were protected against HFD-induced obesity to a similar extent as were SH2B1Tg/Tg-407 and SH2B1Tg/Tg-437 mice (Figure 6, B, right panel, and C). Blood leptin levels were also reduced by 97% in SH2B1Tg-407/437 mice compared with WT mice (Figure 6D, right panel).
Neuron-specific restoration of SH2B1 reverses peripheral insulin resistance and glucose intolerance in SH2B1KO mice.
SH2B1 binds directly to the insulin receptor, thereby enhancing the activation of the insulin receptor and multiple downstream pathways in cultured cells (44, 52). Systemic deletion of SH2B1 results in severe insulin resistance and type 2 diabetes (44–46). However, it is unclear whether SH2B1 enhances insulin sensitivity directly by promoting insulin signaling in the liver, skeletal muscle, and/or adipose tissue or indirectly by reducing adiposity through its action in the brain.
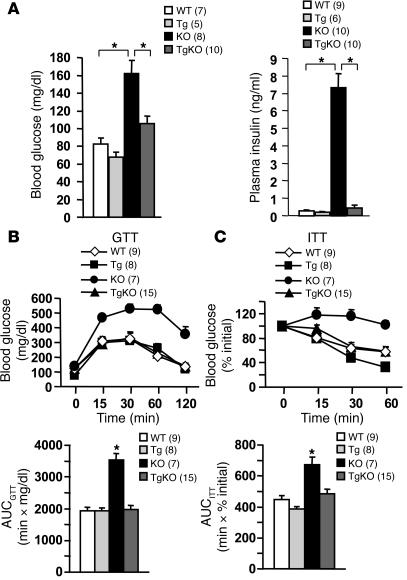
Insulin sensitivity and glucose metabolism were compared in SH2B1KO mice (which completely lack SH2B1 in all tissues) and SH2B1TgKO mice (which only express SH2B1 in neural tissue). SH2B1KO mice developed severe hyperglycemia and hyperinsulinemia, hallmarks of insulin resistance (Figure 7A). Fasting plasma insulin levels increased by more than 26-fold in SH2B1KO compared with age-matched WT controls. Neuron-specific restoration of SH2B1 corrected both hyperglycemia and hyperinsulinemia in SH2B1TgKO mice (Figure 7A).
Figure 7. Neuron-specific restoration of SH2B1 rescues insulin resistance and glucose intolerance in SH2B1KO mice.
(A) Blood glucose in fasted males at age 17 weeks and plasma insulin in fasted males at age 21 weeks. GTTs (B) and ITTs (C) in males at age of 21 weeks. The area under the curve (AUC) was calculated in both GTTs and ITTs using the trapezoidal rule. *P < 0.05.
To further examine peripheral insulin sensitivity, glucose and insulin tolerance tests (GTTs and ITTs) were performed. In GTTs, mice were fasted overnight and injected intraperitoneally with d-glucose (2 g/kg body weight), and blood glucose levels were measured at various time points after glucose injection. Compared with WT controls, SH2B1KO mice were severely intolerant to exogenous glucose as revealed by a marked increase in both the magnitude and duration of the elevation of blood glucose in response to glucose injection (Figure 7B). Neuron-specific restoration of SH2B1 (to endogenous levels) fully rescued glucose intolerance in SH2B1TgKO mice (Figure 7B). In ITTs, exogenous insulin (1 U/kg body weight) failed to decrease blood glucose levels in SH2B1KO mice; in contrast, insulin-induced reductions in blood glucose levels were comparable in SH2B1TgKO and WT mice (Figure 7C). The area under the curve (AUC) significantly increased in SH2BKO mice in both GTTs and ITTs, and this was reversed by neuron-specific restoration of SH2B1 in SH2B1TgKO mice (Figure 7, B and C). Blood glucose and plasma insulin levels and glucose tolerance were similar in SH2B1Tg and WT mice (Figure 7, A and B). Therefore, neuronal SH2B1 rather than SH2B1 expressed in the liver, muscle, and adipose tissue controls peripheral insulin sensitivity and glucose metabolism under these experimental conditions.
Discussion
The SH2B family contains 3 members (SH2B1, SH2B2, and SH2B3) that contain a conserved PH and SH2 domain. SH2B1 is believed to mediate cell signaling in response to multiple hormones, growth factors, and cytokines, including GH, leptin, insulin, IGF-1, PDGF, FGF, nerve growth factor, brain-derived neurotrophic factor, and glial cell–derived neurotrophic factor (29, 32–42). Systemic deletion of SH2B1 resulted in energy imbalance, morbid obesity, and severe glucose intolerance, suggesting that SH2B1 has a unique function in the regulation of body weight and glucose metabolism, which cannot be compensated for by SH2B2 and SH2B3. SH2B1 is ubiquitously expressed in both neuronal and non-neuronal tissues, including the brain, liver, skeletal muscle, and adipose tissue. All these tissues are involved in the regulation of adiposity and glucose metabolism. Interestingly, neuron-specific restoration of recombinant SH2B1 in SH2B1TgKO mice corrected the hyperphagia, obesity, hyperglycemia, and glucose intolerance observed in SH2B1KO mice. Moreover, neuron-specific overexpression of SH2B1 dose-dependently protected against HFD-induced obesity. These observations suggest that endogenous SH2B1 in the brain plays a key role in controlling body weight and glucose homeostasis. Multiple forms of SH2B1 were expressed in the brain; however, neuron-specific restoration of recombinant SH2B1β alone was sufficient to reverse obese and glucose-intolerant phenotypes observed in SH2B1KO mice, suggesting that SH2B1β and other isoforms of SH2B1 in the brain similarly regulate energy and glucose metabolism.
Neuronal SH2B1 regulates energy and glucose metabolism at least in part by enhancing leptin sensitivity in the brain, particularly in the hypothalamus. Systemic deletion of SH2B1 resulted in severe leptin resistance as demonstrated by marked hyperleptinemia, significantly reduced physiological responses to leptin (e.g., leptin-induced anorexia and inhibition of hypothalamic NPY and AgRP expression), and impaired leptin signaling in the hypothalamus. Leptin resistance precedes the onset of obesity in SH2B1KO mice (45). Leptin regulates energy metabolism and body weight mainly by activating LEPRb in the brain. Neuron-specific restoration of SH2B1 fully rescued not only leptin resistance but also obesity in SH2B1TgKO mice, which do not express SH2B1 in peripheral tissues (e.g., muscle, adipose tissue, and liver). A modest increase in neuronal SH2B1 expression reduced blood leptin levels by 58% in SH2B1Tg mice, which were heterozygous for the SH2B1 transgene, compared with WT littermates fed an HFD. Further increases in neuronal SH2B1 expression reduced blood leptin levels to a much higher extent in SH2B1Tg/Tg-407 (by 98%), SH2B1Tg/Tg-437 (by 92%), and SH2B1Tg-407/437 (by 97%) mice. After normalization to total fat mass, blood leptin levels were still significantly reduced in SH2B1Tg/Tg-407 (by 93%), SH2B1Tg/Tg-437 (by 93%), and SH2B1Tg-407/437 (by 92%) mice. Therefore, neuronal SH2B1 improves leptin sensitivity in a dose-dependent manner. More importantly, SH2B1Tg/Tg-407, SH2B1Tg/Tg-437, and SH2B1Tg-407/437 mice were protected against HFD-induced obesity, supporting the idea that neuronal SH2B1 may negatively regulate adiposity at least in part by enhancing leptin sensitivity. However, these studies do not exclude the possibility that neuronal SH2B1 may regulate energy and glucose metabolism by additional leptin-independent mechanisms. SH2B1 directly enhances insulin signaling (44, 52). Insulin regulates energy metabolism and body weight by activating its receptor in the brain (53, 54). SH2B1 may also regulate energy and glucose metabolism by enhancing insulin sensitivity in the brain.
Neuronal SH2B1 increases leptin sensitivity at least in part by directly enhancing leptin signaling in LEPRb-expressing neurons in a cell-autonomous manner. SH2B1 directly binds via its SH2 domain to phosphorylated Tyr813 in JAK2, enhancing JAK2 activation in cultured cells (27, 29, 55–57). Deletion of SH2B1 impairs leptin-stimulated activation of hypothalamic JAK2 in SH2B1KO mice (45). Systemic deletion of SH2B1 markedly reduced leptin-stimulated phosphorylation of hypothalamic STAT3 (a main substrate of JAK2), which was fully rescued by neuron-specific restoration of SH2B1 in SH2B1TgKO mice. Moreover, SH2B1 binds simultaneously to both JAK2 and IRS2, thereby promoting leptin-stimulated tyrosine phosphorylation of IRS2, presumably by both enhancing JAK2 activation and recruiting IRS2 to JAK2 (58). Disruption of SH2B1 blocks leptin-stimulated tyrosine phosphorylation of hypothalamic IRS2 (45). IRS2 is an upstream activator of the PI3K pathway, which is required for leptin regulation of energy metabolism (16, 17, 59). Interestingly, PTP1B inhibits leptin signaling by inhibiting JAK2 activation and JAK2-mediated pathways, which is reversed by SH2B1 (27, 45, 56). Therefore, leptin sensitivity appears to be regulated by a balance between positive (e.g., SH2B1) and negative regulators (e.g., PTP1B and SOCS3) in LEPRb-expressing cells. JAK2 is likely to integrate signals from both intracellular positive (e.g., SH2B1) and negative modulators (e.g., PTP1B and SOCS3) in LEPRb-expressing neurons.
Leptin reduces body weight by both decreasing energy intake and increasing energy expenditure. Surprisingly, SH2B1KO mice have a marked increase in both energy intake and expenditure in the presence of severe systemic leptin resistance. Neuron-specific restoration of SH2B1 reversed both leptin resistance and energy imbalance in SH2B1TgKO mice. These results suggest that leptin regulates energy intake and expenditure by 2 distinct pathways in the brain. Consistent with this idea, melanocortin-4 receptor–expressing (MC4R-expressing) neurons in the paraventricular hypothalamus and/or the amygdala, which are directly innervated by hypothalamic LEPRb-neurons, control energy intake, whereas the MC4R-expressing neurons in other areas control energy expenditure (60). Energy intake and expenditure may be controlled by 2 distinct subpopulations of LEPRb-expressing neurons. SH2B1 may cell-autonomously enhance leptin signaling to a much higher degree in LEPRb-neurons controlling energy intake than in LEPRb-neurons controlling energy expenditure. Therefore, systemic deletion of SH2B1 may impair leptin sensitivity more severely in the LEPRb-neurons controlling energy intake than in the LEPRb-neurons controlling energy expenditure. Compensatory hyperleptinemia may not overcome severe leptin resistance in the LEPRb-neurons controlling energy intake, resulting in hyperphagia in SH2B1KO mice. Conversely, hyperleptinemia may be sufficient to overcome mild leptin resistance in the LEPRb-neurons controlling energy expenditure, resulting in increased energy expenditure in SH2B1KO mice. However, neuronal SH2B1 may also regulate energy intake and expenditure by additional leptin-independent mechanisms.
Adipose SH2B1 may also be involved in the regulation of adiposity. SH2B1 expression was upregulated during adipocyte differentiation of 3T3-L1 cells. Deletion of SH2B1 impaired the ability of MEFs to differentiate into adipocytes in vitro. Moreover, SH2B1TgKO mice, which do not express SH2B1 in adipose tissue, had a significant reduction in both fat content and the size of individual white adipocytes. These results suggest that adipose SH2B1 may cell-autonomously regulate adipocyte growth, differentiation, and/or function. SH2B1 binds to the insulin receptor, enhancing insulin signaling (44, 52). Insulin promotes adipogenesis; therefore, adipose SH2B1 may regulate adipogenesis by enhancing insulin signaling. However, disruption of both neuronal and adipose SH2B1 resulted in adipocyte hypertrophy and massive obesity in SH2B1KO mice, suggesting that neuronal SH2B1 play a dominant role in controlling adipocyte differentiation and/or growth in vivo.
SH2B1 was abundantly expressed in peripheral insulin target tissues, including the liver, muscle, and adipose tissues. In cultured cells, SH2B1 binds via its SH2 domain to the insulin receptor, enhancing insulin signaling (32, 33, 35, 40, 44, 52). Systemic deletion of SH2B1 resulted in marked hyperglycemia, hyperinsulinemia, and glucose and insulin intolerance in SH2B1KO mice, as expected. Surprisingly, neuron-specific restoration of SH2B1 fully rescued insulin resistance and glucose intolerance in SH2B1TgKO mice, even though these mice still lack SH2B1 in the liver, muscle, and adipose tissue. These results suggest that central rather than peripheral SH2B1 is essential for regulating systemic insulin sensitivity and glucose homeostasis in mice fed normal chow. However, it is unclear whether peripheral SH2B1 deficiency in the liver, muscle, and adipose tissue exacerbates insulin resistance and glucose intolerance induced by an HFD and/or other cellular stress. The mechanisms of neuronal SH2B1 regulation of systemic insulin sensitivity and glucose metabolism remain largely unknown. First, neuronal SH2B1 negatively regulates adiposity, thus enhancing peripheral insulin sensitivity. Second, central leptin action increases peripheral insulin sensitivity by an adiposity-independent mechanism (61, 62). The PI3K pathway mediates leptin-induced and adiposity-independent enhancement of peripheral insulin sensitivity (62). SH2B1 promotes leptin-stimulated activation of the PI3K pathway (45, 58); therefore, neuronal SH2B1 may promote peripheral insulin sensitivity via a leptin-dependent and adiposity-independent mechanism. Third, central insulin action improves hepatic insulin sensitivity (63–66). Neuronal SH2B1 may promote peripheral insulin sensitivity and glucose metabolism by cell-autonomously enhancing insulin signaling in the hypothalamus.
In summary, systemic deletion of SH2B1 resulted in severe leptin resistance, insulin resistance, morbid obesity, and glucose intolerance in SH2B1KO mice, all of which were largely reversed by neuron-specific restoration of SH2B1 in SH2B1TgKO mice. Neuron-specific overexpression of SH2B1 protected against HFD-induced leptin resistance and obesity in a dose-dependent manner. Therefore, neuronal SH2B1 may serve as a potential target for therapeutic treatment of both obesity and type 2 diabetes.
Methods
Animal experiments.
SH2B1-knockout mice (129Sv/C57BL/6 genetic background) were generated by homologous recombination as described previously (44). An SH2B1 transgene construct was prepared by inserting Myc-tagged full-length rat SH2B1β cDNA 3′-prime of an NSE promoter/GH enhancer sequence. The SH2B1 transgene construct was microinjected into F2 mouse oocytes (C57BL/6 × SJL) and surgically transferred to recipients in the University of Michigan Transgenic Animal Model Core to generate heterozygous SH2B1-transgenic (SH2B1Tg) animals. Genotyping was performed by PCR-based assays. Two independent SH2B1Tg lines (407 and 437) were inbred to generate homozygous SH2B1-transgenic animals (SH2B1Tg/Tg). In parallel experiments, these 2 lines were crossed with SH2B1-knockout mice to generate SH2B1-transgenic and -knockout compound mutants (SH2B1TgKO). Mice were housed on a 12-hour light/12-hour dark cycle in the Unit for Laboratory Animal Medicine (ULAM) at the University of Michigan, with free access to water and standard mouse chow (21% kcal from fat) or an HFD (45% kcal from fat). Animal experiments were conducted following protocols approved by the University Committee on the Use and Care of Animals (UCUCA).
Blood samples were collected from the tail vein and assayed for plasma insulin and leptin using rat insulin or mouse leptin ELISA kits (Crystal Chem Inc.), respectively. FFAs and TGs were measured using Wako NEFAC and Free Glycerol Reagent (Sigma-Aldrich), respectively.
For GTTs, mice were fasted overnight (approximately 16 hours), and d-glucose (2 g/kg of body weight) was injected intraperitoneally. Blood glucose was monitored at 0, 15, 30, 60, and 120 minutes after glucose injection. For ITTs, mice were fasted for 6 hours, and human insulin (1 IU/kg of body weight) was injected intraperitoneally. Blood glucose was monitored at 0, 15, 30, and 60 minutes after insulin injection.
For histological analysis, adipose tissues were isolated and fixed in Bouins’ solution (Sigma-Aldrich). Paraffin sections were prepared and stained with H&E. Images were visualized using a BX51 microscope (Olympus) and captured using a DP70 Digital Camera (Olympus).
To examine leptin inhibition of food intake and weight gain, mice were housed individually with free access to food and water. Mice were injected with leptin (2 mg/kg body weight) or PBS (as control) twice daily (6:00 pm and 12:00 am) for 2 days. Food intake and body weight were monitored both before and after leptin injection.
To measure tissue TG, tissues were homogenized in chloroform/methanol (2:1) and incubated at room temperature for 4 hours. Tissue extracts were air dried, resuspended in KOH (3 M), incubated at 70°C for 1 hour, neutralized with MgCl2, and subjected to a TG assay as described above.
Measurements of energy expenditure.
Metabolic rates were measured by indirect calorimetry (Windows Oxymax Equal Flow system; Columbus Instruments). Mice were housed individually in air-tight respiratory cages through which room air was passed at a flow rate of 0.5 l/min. Exhaust air was sampled at 27-minute intervals for a period of 1 minute; O2 and CO2 content of the exhaust air was determined by comparison with the O2 and CO2 content of standardized sample air. Mice were acclimatized to the cages for 48 hours before measurements were taken. Lean body mass and fat content were determined using the DEXA method (Dexa Sabre Bone Densitometry; Norland). VO2, VCO2, and heat production were normalized to lean body mass.
Quantitative real-time PCR analysis.
Mice were fasted for 8 hours (from 9:00 am to 5:00 pm) and sacrificed by decapitation. The hypothalamus was isolated immediately, and total hypothalamic RNA was prepared using TRI zol reagent (Invitrogen). The first-strand cDNAs were synthesized using oligo-dT(12-18) and M-MLV reverse transcriptase (Promega). NPY, POMC, AgRP, and β-actin mRNA levels were measured using the Brilliant SYBR Green QPCR Kit and Mx3000P Real-Time PCR System (Stratagene). The expression of NPY, AgRP, and POMC was normalized to the expression of β-actin. Primers for real-time RT-PCR were: NPY sense 5′-TCAGACCTCTTAATGAAGGAAAGCA-3′, NPY antisense 5′-GAGAACAAGTTTCATTTCCCATCA-3′; AgRP sense 5′-GGCCTCAAGAAGACAACTGC, AgRP antisense 5′-GACTCGTGCAGCCTTACACA-3′; POMC sense 5′-CTGCTTCAGACCTCCATAGATGTG-3, POMC antisense CAGCGAGAGGTCGAGTTTGC; β-actin sense 5′-AAATCGTGCGTGACATCAAA-3′, β-actin antisense 5′-AAGGAAGGCTGGAAAAGAGC.
Adipocyte differentiation.
Confluent 3T3-L1 preadipocytes were grown for 2 additional days in DMEM–high glucose supplemented with 8% calf serum at 5% CO2 and 37°C. Cells were then cultured for 3 days in a differentiation medium (DMEM–high glucose supplemented with 10% FCS, 0.1 μM insulin, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine) and 3 days in DMEM–high glucose supplemented with 10% FCS, 0.1 μM insulin. Differentiated adipocytes were maintained in DMEM–high glucose supplemented with 8% fetal calf serum. MEF primary cultures were differentiated similarly, except that 0.1 μM rosiglitazone was added into the differentiation medium.
Adipocytes were washed with PBS, fixed in 10% formalin for 5 minutes, stained in oil red O working solution for 2 hours, and washed extensively with water. Oil red O working solution was prepared by diluting oil red O stock solution (0.5% in isopropanol) with water to a 6:4 ratio. Adipocytes were visualized using a BX51 microscope, and images were captured using a DP70 Digital Camera.
Immunoprecipitation and immunoblotting.
Mice were fasted for 24 hours and injected intraperitoneally with leptin (1 mg/kg body weight) or PBS (as control). Forty-five minutes later, mice were sacrificed by decapitation, and the hypothalamus was isolated and homogenized in lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 250 mM sucrose, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.2 mM benzamidine, 2 mM DTT, 1% Nonidet P-40). The same amount of protein in hypothalamic extracts was immunoblotted with anti–phospho-STAT3 (pTyr705) antibody (Santa Cruz Biotechnology Inc.). The same blots were reprobed with anti-STAT3 antibody (Santa Cruz Biotechnology Inc.) to estimate total STAT3 protein.
Statistics.
The data are presented as mean ± SEM. Two-tailed Student’s t tests were used for comparisons between 2 groups. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Hongyan Yang and Chaojun Duan for helpful discussions. We thank Streamson Chua Jr. (Albert Einstein College of Medicine) for providing the NSE promoter/GH enhancer. This study was supported by a Career Development Award (7-03-CD-11) from the American Diabetes Association and grants R01 DK 065122 and R01 DK073601 from the NIH (all to L. Rui). D. Morris was supported by a Cellular and Molecular Approaches to Systems and Integrative Biology Training Grant (NIH T32 GM008322). This work utilized the cores supported by the Michigan Diabetes Research and Training Center (funded by NIH grant 5P60 DK20572), University of Michigan Comprehensive Cancer Center (funded by NIH grant 5P30 CA46592), University of Michigan Nathan Shock Center for Excellence in the Basic Biology of Aging, Michigan Gastrointestinal Peptide Research Center, University of Michigan Center for Organogenesis, and University of Michigan Center for Integrative Genomics.
Footnotes
Nonstandard abbreviations used: AgRP, agouti-related protein; GH, growth hormone; GTT, glucose tolerance test; HFD, high-fat diet; IRS1, insulin receptor substrate 1; ITT, insulin tolerance test; LEPRb, long form of the leptin receptor; MEF, mouse embryonic fibroblast; NPY, neuropeptide Y; NSE, neuron-specific enolase; PH, pleckstrin homology; POMC, proopiomelanocortin; SH2, Src homology 2; TG, triglyceride; TgKO, transgenic/knockout compound mutation.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:397–406 (2007). doi:10.1172/JCI29417
Decheng Ren and Yingjiang Zhou contributed equally to this work.
References
- 1.Flier J.S. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M.W., Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Chua S.C., Jr., et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee G.H., et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 8.Vaisse C., et al. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 9.Bjorbaek C., Uotani S., da Silva B., Flier J.S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 10.Baumann H., et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao A.Z., Huan J.N., Gupta S., Pal R., Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat. Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 12.Niswender K.D., et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 13.Bates S.H., et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y., et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol. Cell. Biol. 2004;24:258–269. doi: 10.1128/MCB.24.1.258-269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q., et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki R., et al. Both insulin signaling defects in the liver and obesity contribute to insulin resistance and cause diabetes in Irs2(–/–) mice. J. Biol. Chem. 2004;279:25039–25049. doi: 10.1074/jbc.M311956200. [DOI] [PubMed] [Google Scholar]
- 17.Lin X., et al. Dysregulation of insulin receptor substrate 2 in β cells and brain causes obesity and diabetes. J. Clin. Invest. 2004;114:908–916. doi: 10.1172/JCI200422217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks A.S., Davis S.M., Bates S.H., Myers M.G., Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 19.Bjorbaek C., Kahn B.B. Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 20.Myers M.P., et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A., et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 22.Zabolotny J.M., et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 23.Bjorbaek C., Elmquist J.K., Frantz J.D., Shoelson S.E., Flier J.S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 24.Ungureanu D., Saharinen P., Junttila I., Hilton D.J., Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol. Cell. Biol. 2002;22:3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard J.K., et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 26.Mori H., et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 27.Rui L., Carter-Su C. Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7172–7177. doi: 10.1073/pnas.96.13.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne M.A., Dalton S., Kochan J.P. The yeast tribrid system — genetic detection of trans-phosphorylated ITAM-SH2-interactions. Biotechnology (N. Y.). 1995;13:1474–1478. doi: 10.1038/nbt1295-1474. [DOI] [PubMed] [Google Scholar]
- 29.Rui L., Mathews L.S., Hotta K., Gustafson T.A., Carter-Su C. Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 1997;17:6633–6644. doi: 10.1128/mcb.17.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokouchi M., et al. Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene. 1997;15:7–15. doi: 10.1038/sj.onc.1201163. [DOI] [PubMed] [Google Scholar]
- 31.Yousaf N., Deng Y., Kang Y., Riedel H. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 2001;276:40940–40948. doi: 10.1074/jbc.M104191200. [DOI] [PubMed] [Google Scholar]
- 32.Kotani K., Wilden P., Pillay T.S. SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 1998;335:103–109. doi: 10.1042/bj3350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelms K., et al. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm. Genome. 1999;10:1160–1167. doi: 10.1007/s003359901183. [DOI] [PubMed] [Google Scholar]
- 34.Qian X., Riccio A., Zhang Y., Ginty D.D. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017–1029. doi: 10.1016/s0896-6273(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 35.Riedel H., Wang J., Hansen H., Yousaf N. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J. Biochem. (Tokyo). 1997;122:1105–1113. doi: 10.1093/oxfordjournals.jbchem.a021868. [DOI] [PubMed] [Google Scholar]
- 36.Rui L., Carter-Su C. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bbeta with PDGF receptor and phosphorylation of SH2-Bbeta. J. Biol. Chem. 1998;273:21239–21245. doi: 10.1074/jbc.273.33.21239. [DOI] [PubMed] [Google Scholar]
- 37.Rui L., Herrington J., Carter-Su C. SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 1999;274:26485–26492. doi: 10.1074/jbc.274.37.26485. [DOI] [PubMed] [Google Scholar]
- 38.Rui L., Herrington J., Carter-Su C. SH2-B is required for nerve growth factor-induced neuronal differentiation. J. Biol. Chem. 1999;274:10590–10594. doi: 10.1074/jbc.274.15.10590. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K., et al. Association of SH2-B to phosphorylated tyrosine residues in the activation loop of TrkB. Res. Commun. Mol. Pathol. Pharmacol. 2002;111:27–39. [PubMed] [Google Scholar]
- 40.Wang J., Riedel H. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 1998;273:3136–3139. doi: 10.1074/jbc.273.6.3136. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y., et al. Interaction of SH2-Bbeta with RET is involved in signaling of GDNF-induced neurite outgrowth. J. Cell Sci. 2006;119:1666–1676. doi: 10.1242/jcs.02845. [DOI] [PubMed] [Google Scholar]
- 42.Kong M., Wang C.S., Donoghue D.J. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. A role in STAT5 activation. J. Biol. Chem. 2002;277:15962–15970. doi: 10.1074/jbc.M102777200. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien K.B., O’Shea J.J., Carter-Su C. SH2-B family members differentially regulate JAK family tyrosine kinases. J. Biol. Chem. 2002;277:8673–8681. doi: 10.1074/jbc.M109165200. [DOI] [PubMed] [Google Scholar]
- 44.Duan C., Yang H., White M.F., Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol. Cell. Biol. 2004;24:7435–7443. doi: 10.1128/MCB.24.17.7435-7443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren D., Li M., Duan C., Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Ren D., Iseki M., Takaki S., Rui L. Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology. 2006;147:2163–2170. doi: 10.1210/en.2005-1313. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsuka S., et al. SH2-B is required for both male and female reproduction. Mol. Cell. Biol. 2002;22:3066–3077. doi: 10.1128/MCB.22.9.3066-3077.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowalski T.J., Liu S.-M., Leibel R.L., Chua S.C., Jr. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno T.M., Kelley K.A., Pasinetti G.M., Roberts J.L., Mobbs C.V. Transgenic neuronal expression of proopiomelanocortin attenuates hyperphagic response to fasting and reverses metabolic impairments in leptin-deficient obese mice. Diabetes. 2003;52:2675–2683. doi: 10.2337/diabetes.52.11.2675. [DOI] [PubMed] [Google Scholar]
- 50.Cone R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 51.Seeley R.J., Drazen D.L., Clegg D.J. The critical role of the melanocortin system in the control of energy balance. Annu. Rev. Nutr. 2004;24:133–149. doi: 10.1146/annurev.nutr.24.012003.132428. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed Z., Pillay T.S. Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem. J. 2003;371:405–412. doi: 10.1042/BJ20021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruning J.C., et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 54.Benoit S.C., Clegg D.J., Seeley R.J., Woods S.C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 55.Hu J., Hubbard S.R. Structural basis for phosphotyrosine recognition by the Src homology-2 domains of the adapter proteins SH2-B and APS. J. Mol. Biol. 2006;361:69–79. doi: 10.1016/j.jmb.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 56.Kurzer J.H., et al. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol. Cell. Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishi M., et al. Kinase activation through dimerization by human SH2-B. Mol. Cell. Biol. 2005;25:2607–2621. doi: 10.1128/MCB.25.7.2607-2621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan C., Li M., Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J. Biol. Chem. 2004;279:43684–43691. doi: 10.1074/jbc.M408495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White M.F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 60.Balthasar N., et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 61.Pocai A., et al. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 62.Morton G.J., et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Inoue H., et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Gelling R.W., et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Obici S., Feng Z., Karkanias G., Baskin D.G., Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 66.Obici S., Zhang B.B., Karkanias G., Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]