Abstract
Duchenne muscular dystrophy (DMD) is a fatal disease caused by mutation of the gene encoding the cytoskeletal protein dystrophin. Despite a wealth of recent information about the molecular basis of DMD, effective treatment for this disease does not exist because the mechanism by which dystrophin deficiency produces the clinical phenotype is unknown. In both mouse and human skeletal muscle, dystrophin deficiency results in loss of neuronal nitric oxide synthase, which normally is localized to the sarcolemma as part of the dystrophin–glycoprotein complex. Recent studies in mice suggest that skeletal muscle-derived nitric oxide may play a key role in the regulation of blood flow within exercising skeletal muscle by blunting the vasoconstrictor response to α-adrenergic receptor activation. Here we report that this protective mechanism is defective in children with DMD, because the vasoconstrictor response (measured as a decrease in muscle oxygenation) to reflex sympathetic activation was not blunted during exercise of the dystrophic muscles. In contrast, this protective mechanism is intact in healthy children and those with polymyositis or limb-girdle muscular dystrophy, muscle diseases that do not result in loss of neuronal nitric oxide synthase. This clinical investigation suggests that unopposed sympathetic vasoconstriction in exercising human skeletal muscle may constitute a heretofore unappreciated vascular mechanism contributing to the pathogenesis of DMD.
Duchenne muscular dystrophy (DMD) is a crippling, incurable disease caused by mutation of the gene encoding the sarcolemmal protein dystrophin (1). Since the discovery of dystrophin, there have been significant advances in our understanding of the molecular organization of the skeletal muscle cell membrane, including the identification of numerous dystrophin-associated proteins, mutation of some of which cause other forms of muscular dystrophy. Despite this abundance of new information, there currently is no effective treatment for DMD because the mechanism by which dystrophin deficiency produces the clinical phenotype is poorly understood.
Two principal theories, neither of which is mutually exclusive, have been proposed to explain the pathogenesis of DMD. The first is that dystrophin deficiency destabilizes the sarcolemma, thereby rendering the muscle fibers susceptible to damage during repetitive contractions (2). The second theory is that disruption of the dystrophin complex alters skeletal muscle cell signaling (3–5). In this regard, one of the dystrophin-associated proteins down-regulated in DMD is neuronal nitric oxide synthase (nNOS), the enzyme that produces the freely diffusible signaling molecule nitric oxide (NO) (6–8). In healthy muscle, NO derived from nNOS has been implicated in myofiber differentiation (9), modulation of contractile force (8), and exercise-induced glucose uptake (10). Whether changes in these or other NO-mediated processes are of functional significance in dystrophic muscle currently is unknown.
Recent in vivo mouse experiments suggest that skeletal muscle-derived NO also may play an important role in the regulation of blood flow in exercising skeletal muscle by modulating the vasoconstrictor response to activation of α-adrenergic receptors (11, 12). Such modulation was shown to be defective during contraction of nNOS-deficient skeletal muscles both of mdx mice, an animal model of DMD, and nNOS null mice (12). Although mdx mice and DMD patients have the same genetic defect, the clinical manifestation of the disease is substantially milder in the mice (13). We therefore sought to determine if we could extend the results of these previous mouse studies to DMD patients. We specifically hypothesized that (i) production of NO by contracting human skeletal muscle is a protective mechanism that blunts the α-adrenergic vasoconstrictor response to reflex sympathetic activation, thereby optimizing muscle perfusion, and (ii) this protective mechanism is defective in children with DMD, producing functional muscle ischemia when the dystrophic muscles are exercised.
To test these hypotheses in the clinical setting, we used near infrared (NIR) spectroscopy to measure changes in tissue oxygenation induced by reflex sympathetic activation in resting and exercising skeletal muscles of children with DMD, age-matched healthy controls, and disease controls. In healthy children, we found that the ability of sympathetic activation to decrease muscle tissue oxygenation was greatly attenuated when the muscles were exercised, indicating contraction-induced modulation of sympathetic vasoconstriction. Similar findings were observed in children with polymyositis (PM) or limb-girdle muscular dystrophy (LGMD), muscle diseases which do not result in loss of skeletal muscle nNOS (14). In contrast, in children with DMD and those with spinal muscular atrophy (SMA), sympathetically induced decreases in muscle oxygenation were abnormally preserved during exercise of nNOS-deficient skeletal muscle, resulting in functional muscle ischemia. Unopposed sympathetic vasoconstriction in exercising human skeletal muscle may constitute a heretofore unappreciated vascular mechanism contributing to the pathogenesis of DMD and other conditions accompanied by deficient nNOS expression in skeletal muscle.
Methods
Subjects.
Thirty-three subjects, ages 7–15 years, were studied. Thirteen were normal healthy boys. The remainder had established diagnoses of DMD (10 males), LGMD (3 males and 1 female), SMA (2 males and 2 females), or PM (1 male and 1 female). The study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center, and informed written consent was obtained from each subject and their parent or legal guardian.
Hemodynamic Measurements.
Subjects were studied in the supine position. Heart rate was measured continuously by electrocardiography and blood pressure by automated oscillometric sphygmomanometry by using a child's cuff (Welch Allyn, Skaneateles Falls, NY). Resting forearm blood flow was measured by venous occlusion plethysmography and expressed as ml/min/100 ml forearm volume (15). Forearm vascular conductance was calculated as the quotient of forearm blood flow and mean arterial pressure.
Skeletal Muscle Oxygenation by NIR Spectroscopy.
The NIR method is based on the principle that laser light with wavelengths in the 700–900 nm range penetrates tissues with relative ease, and is absorbed by the iron-porphyrin moieties in hemoglobin and myoglobin. Changes in NIR light absorption are proportional to changes in the relative concentrations of oxygenated hemoglobin and myoglobin (HbO2 + MbO2). Because of their nearly identical absorption spectra, individual contributions of hemoglobin and myoglobin cannot be determined. The NIR signals reflect changes in oxygenation occurring mainly in the microvasculature, because vessels >1 mm in diameter are maximal absorbers of photons because of the high extinction coefficient of blood. Thus, the technique provides continuous measurement of the adequacy of tissue oxygen delivery relative to its use.
To monitor the tissue absorption of NIR light, two fiber optical bundles were placed on the skin over the left flexor digitorum profundus muscle, which is the main muscle recruited during handgrip exercise. NIR signals at four different wavelengths were sequentially sampled at a rate of 1 Hz, converted to optical densities by using known algorithms, displayed as the running average of 20 consecutive samples, and stored digitally for analysis (NIRO 500, Hamamatsu Photonics, Hamamatsu City, Japan). In each experiment, the total labile signal was defined as the difference in tissue oxygenation in the forearm at rest and during sustained circulatory arrest (inflation of a pneumatic cuff on the upper arm to 280 mmHg). Changes in the NIR signals in response to interventions are expressed as a percentage of the total labile signal.
Handgrip Exercise.
Handgrip was performed with a custom-made handgrip dynamometer connected to a force transducer (Interface MFG, Scottsdale, AZ). Force was displayed on an oscilloscope to provide the subject with visual feedback. Before each experiment, the subject's maximal voluntary contraction (MVC) was determined. Subjects performed intermittent, isometric handgrip (20 handgrips/min, 50% duty cycle) at 10, 20, or 33% MVC for 5 min. Previous studies have shown that these mild levels of handgrip exercise alone do not activate sympathetic outflow to skeletal muscle (16, 17).
Reflex Sympathetic Activation.
The subject's lower body was enclosed in a negative pressure chamber to the level of the iliac crest. Pressure inside the chamber was measured by a Statham transducer (Gould, Oxnard, CA). Lower body negative pressure (LBNP) at −20 to −25 mmHg simulates mild orthostatic stress (e.g., the transition from the supine to the seated position). This technique was used to selectively unload the cardiopulmonary mechanoreceptors, producing highly reproducible reflex increases in sympathetic nerve activity targeted to the skeletal muscle vasculature without concomitant changes in systemic arterial pressure (17, 18).
Experimental Protocol.
To measure exercise-induced attenuation of reflex vasoconstriction, LBNP was (i) applied at rest and then (ii) superimposed on the steady-state decreases in forearm muscle oxygenation during mild rhythmic handgrip.
Blood pressure, heart rate, respiration, handgrip force, and NIR signals were recorded in response to 2 min of LBNP applied at rest and during the 3rd and 4th min of each 5-min exercise period. Each exercise period was followed by at least 2 min of forearm circulatory arrest to establish the maximal decrease in muscle tissue oxygenation.
Western Blot Analysis.
Diagnostic skeletal muscle biopsies had been obtained previously from patients and stored at −80°C. Samples were individually homogenized in 20 vol of buffer containing 50 mM Tris⋅HCl (pH 7.5)/1 μg/ml aprotinin/2 μg/ml leupeptin/20 μM tetrahydrobiopterin/1 mM DTT/1 μg/ml pepstatin A/1 mM benzamidine/10 μg/ml soybean trypsin inhibitor/1 mM EDTA/0.5 mM PMSF/20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Muscle extracts (100 μg protein) were resolved by SDS/PAGE on a 6% gel and transferred to nitrocellulose. Membranes were incubated overnight at 4°C with a rabbit polyclonal antibody raised against the NH2 terminus of nNOS (1:4000) or inducible NOS (iNOS) (1:4000), or a mouse monoclonal endothelial NOS (eNOS) antibody (1:1000; Transduction Laboratories, Lexington, KY). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies and immunoreactivity was detected by enhanced chemiluminescence (Amersham International). Protein concentrations of extracts were determined by using a Bio-Rad DC Protein Assay kit.
Statistics.
Data are expressed as means ± SEM. Comparisons were made by using paired and unpaired t tests as relevant. A P value of <0.05 was considered significant and adjusted by the method of Bonferroni when appropriate.
Results
DMD vs. Healthy Controls.
Baseline values.
Blood pressures (Table 1) were similar in DMD and healthy controls, whereas heart rates were significantly higher in DMD as reported (19, 20). Resting forearm blood flows also were similar between groups. As expected, the boys with DMD were much weaker than controls.
Table 1.
Baseline characteristics of the two main study groups
| Group | n | Age | SBP, mmHg | DBP, mmHg | HR, beats per min | FBF, ml/min per 100 ml of tissue | MVC, kg |
|---|---|---|---|---|---|---|---|
| Control | 13 | 10.9 ± 0.5 | 107 ± 2 | 57 ± 1 | 73 ± 3 | 4.7 ± 0.5 | 15.1 ± 1.7 |
| DMD | 10 | 10.8 ± 0.5 | 107 ± 2 | 61 ± 2 | 91 ± 3* | 4.8 ± 0.4 | 4.5 ± 0.5* |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; FBF, forearm blood flow (controls, n = 8; DMD, n = 5); MVC, maximal voluntary contraction.
*, P < 0.05 versus controls.
Comparative effects of reflex sympathetic activation on blood flow and tissue oxygenation in resting forearm muscle.
NIR spectroscopy has been used to measure tissue oxygenation as an indication of microvascular perfusion in resting and exercising human forearm muscle (17, 21–23). In adults, decreases in forearm muscle oxygenation evoked by reflex sympathetic activation during simulated orthostatic stress (by LBNP) provide a valid measure of sympathetic vasoconstriction (17). In the present study, we first sought to confirm that NIR responses also could be used to estimate sympathetic vasoconstriction in children. As expected, blood flow and muscle oxygenation measured in the resting forearms decreased in parallel during LBNP. In healthy boys, forearm blood flow decreased by 24 ± 7% and muscle oxygenation by 23 ± 2%, whereas in boys with DMD, forearm blood flow and muscle oxygenation decreased by 42 ± 9 and 30 ± 2%, respectively.
Exercise-induced attenuation of sympathetic vasoconstriction.
We performed several variations of our experimental protocol to provide a detailed comparison of vasoconstrictor responses in boys with DMD vs. healthy controls. First, we compared NIR responses to the same level of LBNP (−20 mmHg) in patients and controls during handgrip at the same relative force (20% MVC). In resting forearm, LBNP alone elicited similar decreases in muscle oxygenation in both groups (Fig. 1). During the increased oxygen consumption accompanying handgrip exercise alone, muscle oxygenation decreased in both groups, reaching a new steady-state level within the first minute of exercise. LBNP was then applied during handgrip, and when compared with responses in resting forearm, LBNP-induced decreases in muscle oxygenation were attenuated by 74% in controls (Δ HbO2 + MbO2: rest, −23 ± 2% vs. exercise, −6 ± 3%), whereas no such attenuation was observed in DMD patients (Δ HbO2 + MbO2: rest, −27 ± 3% vs. exercise, −29 ± 5%) (Fig. 1).
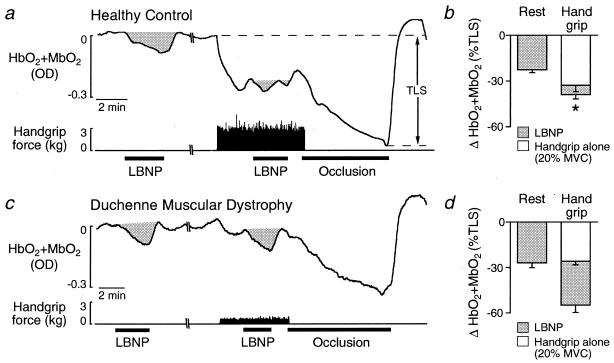
Figure 1.
Effect of handgrip at the same relative workload on the decreases in muscle oxygenation (HbO2 + MbO2) elicited by reflex sympathetic activation by using LBNP. (a and c) In the healthy boy, the robust LBNP-induced decrease in muscle oxygenation in resting forearm was greatly diminished during forearm exercise. In contrast, in the boy with DMD, LBNP produced similar decreases in muscle oxygenation in resting and exercising forearm. Complete forearm vascular occlusion was used to maximally decrease tissue oxygenation to determine the total labile signal (TLS). OD, optical density. (b and d) Summary data showing LBNP-induced decreases in muscle oxygenation in resting and exercising forearm in (b) 12 healthy controls and (d) 9 boys with DMD. *, P < 0.05, LBNP at rest vs. handgrip.
Because the boys with DMD were considerably weaker than healthy controls, handgrip at the same relative force (20% MVC) represented a smaller absolute force and caused a smaller decrease in muscle oxygenation than in controls (Figs. 1 and 2). We therefore compared NIR responses to LBNP at −20 mmHg in patients and controls during handgrip at the same absolute force (1.5 kg), a level of exercise that produced comparable decreases in muscle oxygenation in the two groups (Figs. 2 and 3). Even under these conditions, there was little or no handgrip-induced attenuation of the decrease in muscle oxygenation caused by LBNP in patients compared with a 54% attenuation in controls (Fig. 3). As a result, muscle oxygenation was decreased from resting levels by 53 ± 7% during combined handgrip plus LBNP in the patients but only by 34 ± 4% in controls (P < 0.05).
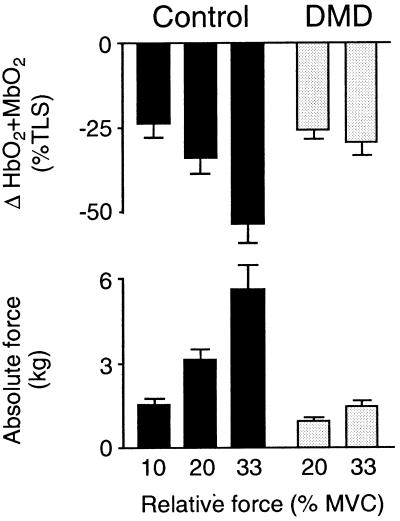
Figure 2.
Decreases in forearm muscle oxygenation (HbO2 + MbO2) in response to handgrip alone. In both healthy controls and boys with DMD, handgrip produced intensity-dependent decreases in forearm muscle oxygenation. Because the controls were much stronger than the DMD patients, handgrip at the same relative workload decreased muscle oxygenation to a greater extent in the controls than in patients. However, handgrip at the same absolute workload produced similar decreases in muscle oxygenation in the two groups. Control, n = 12 at 10 and 20% MVC, n = 7 at 33% MVC; DMD, n = 9.
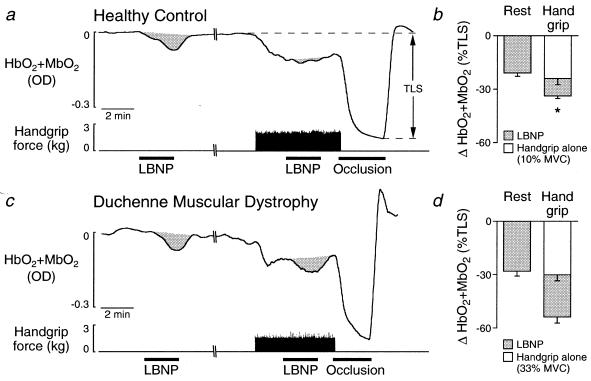
Figure 3.
Effect of handgrip at similar absolute workloads on the decreases in muscle oxygenation (HbO2 + MbO2) elicited by reflex sympathetic activation by using LBNP. (a and c) In the healthy boy, the decrease in muscle oxygenation elicited by LBNP in resting forearm was greatly attenuated during forearm exercise at 10% MVC. In the boy with DMD, LBNP elicited similar decreases in muscle oxygenation in the forearm at rest and during exercise at 33% MVC. TLS, total labile signal. (b and d) Summary data showing LBNP-induced decreases in muscle oxygenation in resting and exercising forearm in (b) 12 healthy controls and (d) 9 boys with DMD. *, P < 0.05, LBNP at rest vs. handgrip.
Because LBNP at −20 mmHg produced a somewhat greater reflex decrease in both vascular conductance and muscle oxygenation in resting forearm muscle of DMD patients than controls, we performed additional experiments in which we increased the level of LBNP to −25 mmHg in controls to match the decreases in muscle oxygenation produced by LBNP at −20 mmHg in the patients (Fig. 4a). Under these conditions as well, there was little or no handgrip-induced attenuation of the decrease in muscle oxygenation caused by LBNP in patients compared with virtual abrogation of the response in controls (Fig. 4 b and c). As a result, muscle oxygenation decreased by 53 ± 5% during handgrip plus LBNP in the patients, but only by 34 ± 4% in controls (P < 0.05).
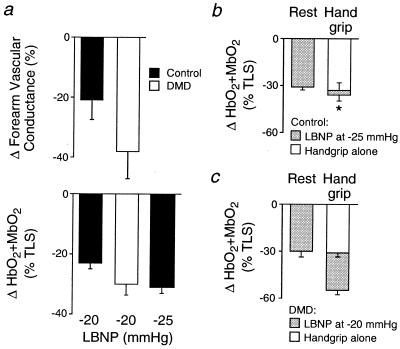
Figure 4.
Decreases in forearm vascular conductance and muscle oxygenation (HbO2 + MbO2) elicited by reflex sympathetic activation by using LBNP. (a) The same level of LBNP produced a slightly greater vasoconstrictor response in the DMD patients than in controls. Controls, n = 8; DMD, n = 5. (b and c) In resting forearm, LBNP at −25 mmHg in the controls (b) produced decreases in muscle oxygenation comparable to LBNP at −20 mmHg in the DMD patients (c). In contracting forearm, the LBNP-induced decreases in muscle oxygenation were greatly attenuated in the controls, but not in the DMD patients. Controls, n = 7; DMD, n = 8; *, P < 0.05, LBNP at rest vs. handgrip.
DMD vs. Disease Controls.
Table 2 shows the baseline characteristics of the patients used as disease controls. Compared with DMD patients, maximal grip strength was greater in patients with LGMD or PM and weaker in those with SMA.
Table 2.
Baseline characteristics of the disease controls
| Characteristics | n | Age, years | SBP, mmHg | DBP, mmHg | HR, beats per min | MVC, kg |
|---|---|---|---|---|---|---|
| SMA | 4 | 9.1 ± 0.7 | 103 ± 4 | 64 ± 4 | 77 ± 3 | 1.2 ± 0.5 |
| LGMD | 4 | 12.6 ± 0.9 | 112 ± 2 | 60 ± 5 | 77 ± 4 | 9.1 ± 2.9 |
| PM | 2 | 11.5, 11.7 | 108, 105 | 69, 62 | 87, 78 | 10.9, 13.2 |
SMA, spinal muscular atrophy; LGMD, limb-girdle muscular dystrophy; PM, polymyositis; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; MVC, maximal voluntary contraction.
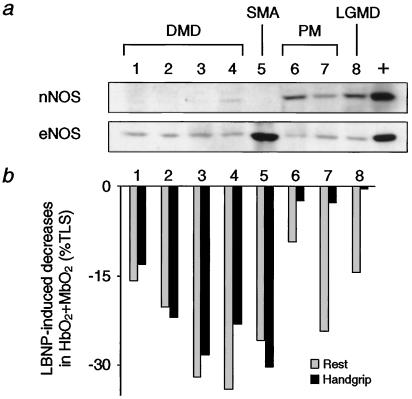
Fig. 5 juxtaposes NOS expression in skeletal muscle biopsies of these different patients with their NIR responses to LBNP during handgrip at 33% MVC. Although eNOS expression was comparable in all patients, nNOS expression was greatly decreased in the patients with DMD or SMA compared with the abundant expression in the patients with PM or LGMD. There was no detectable iNOS immunoreactivity in any of the biopsy samples (data not shown). There was little or no handgrip-induced attenuation of the decreases in muscle oxygenation caused by LBNP in the five patients with nNOS-deficient skeletal muscles, compared with the marked attenuation seen in the three patients in whom skeletal muscle nNOS was plentiful. These results from a subset of the disease control patients were confirmed when we performed NIR studies on all of the disease controls. LBNP-induced decreases in muscle oxygenation were not attenuated during handgrip in the SMA patients (Δ HbO2 + MbO2: rest, −35 ± 9% vs. exercise, −39 ± 8%; n = 4), in contrast to the diminished responses observed in the LGMD patients (Δ HbO2 + MbO2: rest, −24 ± 7% vs. exercise, −8 ± 5%, P < 0.05; n = 4) and in the PM patients (Δ HbO2 + MbO2: rest, −24% and −9% vs. exercise, −3% and −3%; n = 2).
Figure 5.
Correspondence between skeletal muscle nNOS and blunted vasoconstriction in exercising forearm. (a) Immunoblots of skeletal muscle biopsy samples from children with DMD, SMA, PM, or LGMD. Blots were probed for nNOS, eNOS, and iNOS (not detected in any sample). +, positive control. (b) Sympathetic vasoconstriction, as assessed by LBNP-induced decreases in muscle oxygenation, in resting and exercising forearm measured in the same children whose biopsies were analyzed in a. Vasoconstrictor responses were not attenuated in exercising forearm in children with DMD or SMA, but were greatly attenuated in those with PM or LGMD.
Discussion
Contraction-induced attenuation of α-adrenergic vasoconstriction previously was shown to be defective in nNOS-deficient skeletal muscle of both the nNOS null mouse and the mdx mouse, which is an animal model of DMD (12). The major new conclusion of the present study is that contraction-induced modulation of reflex sympathetic vasoconstriction also is defective in the nNOS-deficient skeletal muscle of children with DMD, resulting in functional muscle ischemia. Similar responses were observed in children with SMA, in which skeletal muscle nNOS expression has been reported to be decreased (14). However, no such defect was observed in age-matched healthy controls or children with LGMD or PM, muscle diseases in which skeletal muscle nNOS is plentiful (14). Taken together, these data suggest that unopposed sympathetic vasoconstriction in exercising human skeletal muscle may constitute a novel vascular mechanism contributing to the pathogenesis of DMD and other conditions characterized by deficient nNOS expression in skeletal muscle.
This interpretation is based on the measurement of muscle tissue oxygenation with NIR spectroscopy. As indicated by our previous study in healthy adults (17), this technique provides several advantages over more conventional hemodynamic approaches to study contraction-induced modulation of sympathetic vasoconstriction. NIR spectroscopy provides a continuous measurement of oxygen availability within the microcirculation (24, 25), the part of the vascular tree most accessible to metabolic products released from contracting skeletal muscle such as NO. Measurements can be acquired during exercise, which is a critical advantage over techniques (e.g., venous occlusion plethysmography) that permit accurate blood flow measurements only in resting limbs. Finally, the spatial resolution of NIR spectroscopy is sufficient to reflect changes in oxygenation in the truly active, rather than the adjacent inactive, small muscle groups of the forearm.
In healthy adults, we previously showed that decreases in forearm muscle oxygenation evoked by reflex sympathetic activation during simulated orthostatic stress (LBNP) provide a valid measure of sympathetic vasoconstriction, because these decreases are accompanied by parallel decreases in forearm blood flow and are abolished by local sympathetic blockade (17). Similarly, in the present study, blood flow and muscle oxygenation measured in the resting forearms of healthy children and those with DMD also decreased in parallel during LBNP, suggesting that this experimental paradigm is a valid approach to study sympathetic vasoconstrictor responses in children as well as adults.
In healthy children, our finding that reflex activation of sympathetic nerves consistently decreased oxygenation in resting forearm muscle, but had little or no effect on oxygenation when the muscles were exercised, provided evidence for the contraction-induced modulation of sympathetic vasoconstriction. Such modulation was impaired in children with DMD because reflex sympathetic activation produced comparable decreases in oxygenation in resting and exercising forearm muscle.
The extent of contraction-induced modulation of sympathetic vasoconstriction normally reflects an interplay between the intensity of the exercise and the strength of the sympathetic vasoconstrictor stimulus (17, 26). Initially, we used a standard experimental approach in the patients and controls by comparing NIR responses to the same level of LBNP during handgrip at the same relative workload. However, we also performed additional protocols to address several important differences between the groups. First, DMD patients were considerably weaker than healthy controls, so handgrip at the same relative intensity (20% MVC) represented a smaller absolute force in patients versus controls. When we had the children perform the same absolute workload by increasing handgrip intensity to 33% MVC in the patients while decreasing it to 10% in controls, handgrip still caused greater attenuation of sympathetically mediated decreases in muscle oxygenation in healthy controls than in DMD patients. Second, in resting muscle, LBNP at −20 mmHg produced a slightly greater reflex decrease both in vascular conductance and muscle oxygenation in DMD patients than in healthy controls. When we matched the LBNP-induced NIR responses in resting muscle by increasing LBNP to −25 mmHg in the controls, handgrip still caused greater attenuation of the LBNP-induced decrease in muscle oxygenation in controls than in DMD patients. Thus, these additional experiments demonstrated that defective contraction-induced modulation of sympathetic vasoconstriction observed in our initial experiments in the DMD patients could not be attributed either to a lower intensity of exercise or a stronger vasoconstrictor stimulus than in controls.
Taken together, these data indicate that in DMD, defective modulation of sympathetic vasoconstriction in exercising muscle can produce functional muscle ischemia. To show that this abnormal phenotype was related specifically to nNOS deficiency and not to some other feature of dystrophic muscle, we studied three additional groups of children with muscle weakness caused by neuromuscular diseases that do not involve dystrophin: PM and LGMD in which muscle nNOS is plentiful (14), and SMA in which nNOS has been reported to be reduced in intermediate-sized muscle fibers and absent in small atrophic fibers (14). Our findings that responses in children with PM or LGMD resembled those in healthy controls, whereas responses in children with SMA resembled those with DMD, suggest that contraction-induced attenuation of sympathetic vasoconstriction is markedly impaired only in those diseases that display reduced skeletal muscle nNOS expression. In contrast, eNOS expression did not differ among the children with muscle diseases, suggesting a specific relationship between skeletal muscle nNOS expression and the modulation of sympathetic vasoconstriction during exercise.
Although little is known about the regulation of skeletal muscle nNOS, its localization at the sarcolemma may provide a Ca2+-dependent mechanism that links activation of nNOS to the contractile activity of skeletal muscle. This localization also may facilitate diffusion of skeletal muscle-derived NO to the adjacent microvasculature where it interferes with α-adrenergic receptor signaling, possibly by opening ATP-sensitive potassium channels (27). Indeed, recent studies have shown that NO diffusion from skeletal muscle fibers stimulates a signaling cascade in vascular smooth muscle cells associated with increased cGMP formation, inhibition of myosin light chain phosphorylation, and relaxation (11, 28, 29).
The present human data strengthen the emerging hypothesis that NO produced by skeletal muscle nNOS may play an important role in regulating blood flow and oxygen delivery in exercising skeletal muscle by blunting the vasoconstrictor response to an otherwise potentially adverse sympathetic activation. We suggest that this protective mechanism is defective in DMD, resulting in enhanced sympathetic vasoconstriction and thus functional ischemia of the exercising dystrophic muscles. Our experimental model of mild simulated orthostatic stress applied during rhythmic handgrip approximates the common clinical situation of a patient with DMD seated in a wheelchair performing mild arm exercise.
Interestingly, mechanical and/or functional obstruction of the skeletal muscle microcirculation was one of the earlier theories proposed to explain the pathogenesis of DMD. This vascular theory was based mainly on the histological observation in DMD patients of small random foci of muscle fibers in the same stage of degeneration or regeneration, and on the experimental reproduction in animals of these characteristic lesions by maneuvers that disrupted the microvasculature (30, 31). However, subsequent morphological (32–34) and clinical studies (35, 36) provided no definitive evidence in support of either structural or functional vascular abnormalities, although the clinical studies generally measured blood flows only under conditions of rest or during maximal vasodilation and interpretation of the results often was clouded by the lack of age- and strength-matched control subjects. Our present data together with recent data from three different dystrophic mouse models demonstrating vascular abnormalities (12, 37, 38) suggest that this vascular theory should be revisited. Although nNOS deficiency alone clearly is not sufficient to produce the dystrophic phenotype in mouse models (39), in children with DMD, progressive muscle fibrosis may be accelerated when the dystrophin-deficient, nNOS-deficient muscles are subjected to repeated bouts of ischemic exercise.
Acknowledgments
We thank K. Lau for kindly providing the nNOS and iNOS antibodies. This work was supported by National Institutes of Health Grant HL06296 (R.G.V. and J.T.S.). M.S. was supported by the Danish Heart Foundation and the Michaelsen Foundation. B.C. was supported by an American College of Cardiology Merck Research Fellowship Award and National Institutes of Health Training Grant HL07360.
Abbreviations
- nNOS
neuronal nitric oxide synthase
- DMD
Duchenne muscular dystrophy
- NIR
near infrared
- MVC
maximal voluntary contraction
- LBNP
lower body negative pressure
- NO
nitric oxide
- LGMD
limb-girdle muscular dystrophy
- SMA
spinal muscular atrophy
- PM
polymyositis
- iNOS
inducible NOS
- eNOS
endothelial NOS
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 13464.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250379497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250379497
References
- 1.Hoffman E P, Brown R H, Jr, Kunkel L M. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Petrof B J, Shrager J B, Stedman H H, Kelly A M, Sweeney H L. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafael J A, Townsend E R, Squire S E, Potter A C, Chamberlain J S, Davies K E. Hum Mol Genet. 2000;9:1357–1367. doi: 10.1093/hmg/9.9.1357. [DOI] [PubMed] [Google Scholar]
- 4.Hack A A, Cordier L, Shoturma D I, Lam M Y, Sweeney H L, McNally E M. Proc Natl Acad Sci USA. 1999;96:10723–10728. doi: 10.1073/pnas.96.19.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grady D, Rubin S M, Petitti D B, Fox C S, Cummings V L. Ann Intern Med. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 6.Brenman J E, Chao D S, Xia H, Aldape K, Bredt D S. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 7.Chang W J, Iannaccone S T, Lau K S, Masters B S S, McCabe T J, McMillan K, Padre R C, Spencer M J, Tidball J G, Stull J T. Proc Natl Acad Sci USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobzik L, Reid M B, Bredt D S, Stamler J S. Nature (London) 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 9.Lee K H, Baek M Y, Moon K Y, Song W K, Chung C H, Ha D B, Kang M S. J Biol Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- 10.Roberts C K, Barnard R J, Scheck S H, Balon T W. Am J Physiol. 1997;273:E220–E225. doi: 10.1152/ajpendo.1997.273.1.E220. [DOI] [PubMed] [Google Scholar]
- 11.Lau K S, Grange R W, Chang W-J, Kamm K E, Sarelius I, Stull J T. FEBS Lett. 1998;431:71–74. doi: 10.1016/s0014-5793(98)00728-5. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G D, Sander M, Lau K S, Huang P L, Stull J T, Victor R G. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dangain J, Vrbova G. Muscle Nerve. 1984;7:700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- 14.Grozdanovic Z, Christova T, Gosztonyi G, Mellerowicz H, Blottner D, Gossrau R. Histochem J. 1997;29:97–104. doi: 10.1023/a:1026425120156. [DOI] [PubMed] [Google Scholar]
- 15.Siggaard-Anderson O. Dan Med Bull. 1970;17:1–68. [PubMed] [Google Scholar]
- 16.Victor R G, Seals D R, Mark A L. J Clin Invest. 1987;79:508–516. doi: 10.1172/JCI112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen J, Thomas G D, Harris S A, Parsons W J, Victor R G. J Clin Invest. 1996;98:584–596. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vissing S F, Scherrer U, Victor R G. Circ Res. 1989;65:1710–1717. doi: 10.1161/01.res.65.6.1710. [DOI] [PubMed] [Google Scholar]
- 19.Perloff K, Roberts W C, DeLeon A C, O'Doherty D. Am J Med. 1967;42:179–188. doi: 10.1016/0002-9343(67)90017-4. [DOI] [PubMed] [Google Scholar]
- 20.Sockolov R, Irwin B, Dressendorfer R H, Bernauer E M. Arch Phys Med Rehab. 1977;58:195–201. [PubMed] [Google Scholar]
- 21.Hampson N B, Piantadosi C A. J Appl Physiol. 1988;64:2449–2457. doi: 10.1152/jappl.1988.64.6.2449. [DOI] [PubMed] [Google Scholar]
- 22.De Blasi R A, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. J Appl Physiol. 1994;76:1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyasu T, Tan N, Kondo N, Nishiyasu M, Ikegami H. Acta Physiol Scand. 1999;166:123–130. doi: 10.1046/j.1365-201x.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 24.Piantadosi C A. J Crit Care. 1989;4:308–318. [Google Scholar]
- 25.Mancini D M, Bolinger L, Li H, Kendrick B, Chance B, Wilson J R. J Appl Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 26.Thomas G D, Hansen J, Victor R G. Am J Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- 27.Thomas G D, Hansen J, Victor R G. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau K S, Grange R W, Isotani E, Sarelius I H, Kamm K E, Huang P L, Stull J T. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Grady R M, Grange R W, Lau K S, Maimone M M, Nichol M C, Stull J T, Sanes J R. Nat Cell Biol. 1999;1:215–220. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 30.Demos J, Ecoiffier J. Rev Franc Etudes Clin Biol. 1957;2:489–494. [PubMed] [Google Scholar]
- 31.Mendell J R, Engel W K, Derrer E C. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- 32.Jerusalem F, Engel A G, Gomez M R. Brain. 1974;97:115–122. doi: 10.1093/brain/97.1.115. [DOI] [PubMed] [Google Scholar]
- 33.Koehler J. Neurology. 1977;27:861–868. doi: 10.1212/wnl.27.9.861. [DOI] [PubMed] [Google Scholar]
- 34.Musch B C, Papapetropoulos T A, McQueen D A, Hudgson P, Weightman D. J Neurol Sci. 1975;26:221–234. doi: 10.1016/0022-510x(75)90034-9. [DOI] [PubMed] [Google Scholar]
- 35.Bradley W G, O'Brien M D, Walder D N, Murchison D, Johnson M, Newell D J. Arch Neurol (Chicago) 1975;32:466–473. doi: 10.1001/archneur.1975.00490490070007. [DOI] [PubMed] [Google Scholar]
- 36.Paulson O B, Engel A G, Gomez M R. J Neurol Neurosurg Psychiatr. 1974;37:685–690. doi: 10.1136/jnnp.37.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coral-Vazquez R, Cohn R D, Moore S A, Hill J A, Weiss R M, Davisson R L, Straub V, Barresi R, Bansai D, Hrstka R F, et al. Cell. 1999;98:465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 38.Durbeej M, Cohn R D, Hrstka R F, Moore S A, Allamand V, Davidson B L, Williamson R A, Campbell K P. Mol Cell. 2000;5:141–151. doi: 10.1016/s1097-2765(00)80410-4. [DOI] [PubMed] [Google Scholar]
- 39.Chao D S, Silvagno F, Bredt D S. J Neurochem. 1998;71:784–789. doi: 10.1046/j.1471-4159.1998.71020784.x. [DOI] [PubMed] [Google Scholar]