Summary:
α-Defensins have been observed to have anti-HIV activity but have not been investigated in relation to mother-to-child HIV transmission. We measured the concentration of α-defensins in breast milk of HIV-positive mothers and tested whether the concentrations were associated with HIV transmission. A nested case-control study of 32 HIV-positive women who transmitted HIV to their infants and 52 randomly selected HIV-positive women who did not transmit HIV to their infants was conducted in Lusaka, Zambia. α-Defensins were detected in most (79%) of the milk samples tested. Concentrations of α-defensins increased as breast milk HIV RNA quantity increased, and breast milk HIV RNA quantity was, in turn, a strong and significant predictor of HIV transmission. After adjustment for milk HIV RNA quantity, however, α-defensin concentration was significantly associated with a decreased risk of intrapartum and postnatal HIV transmission (odds ratio = 0.3, 95% confidence interval: 0.09-0.93). Our data suggest that there may be a role for α-defensins in prevention of HIV transmission to breastfed infants.
Keywords: breast milk, HIV, defensins, transmission
Even in the absence of therapy, most infants of HIV-infected mothers do not become infected. Maternal viral load and immune status contribute to the rate of mother-to-child HIV transmission, but other as yet poorly defined factors, including HIV-specific T lymphocytes, IgA, and antiviral soluble factors,1-3 may be involved in determining whether perinatal and postnatal exposure to HIV results in infection.
Recently, α-defensins were discovered to account for some of the antiviral activity associated with lack of disease development among long-term nonprogressing adults.4 Although seemingly not the explanation for the still elusive CD8 cell antiviral factor (CAF), α-defensins have demonstrable anti-HIV activity.5,6 Defensins are a family of cationic peptides produced by a number of different immune cells, strictly conserved throughout phylogeny, and characterized by potent antimicrobial activity.7 In a study of repeatedly and recently exposed uninfected women who were sexual partners of HIV-infected men, α-defensin-expressing cells in peripheral blood and in cervicovaginal samples were found to be significantly elevated,7 supporting the notion that this innate immune factor may play a role in host resistance to HIV.
Although α-defensins have been detected in breast milk,8 their role in mother-to-child HIV transmission has not yet been investigated. In this study, we analyzed α-defensin concentrations in breast milk of HIV-infected mothers to investigate whether or not there was an association with transmission of HIV to infants.
METHODS
A nested case-control study was conducted within a cohort of mothers and children enrolled in the Zambia Exclusive Breastfeeding Study (ZEBS) underway in Lusaka, Zambia. In brief, the cohort consists of HIV-positive women recruited during pregnancy, who are given nevirapine for prevention of transmission and who are followed with their infants after delivery for up to 24 months at regular study visits.9 All women receive counseling to support exclusive breastfeeding to 4 months, and half are randomized to an intervention to encourage abrupt cessation of breastfeeding at 4 months. Heel-stick blood samples onto filter paper are collected at least monthly from children between birth and 6 months of age and at 3-month intervals thereafter. The presence of breast problems is ascertained by a standardized clinical examination and interview at each study visit. All participants signed informed consent forms for participation in the study, and the study was approved by the institutional review boards at the respective institutions of the investigators.
For the case-control study presented here, HIV-positive women with HIV-infected infants (n = 32) were selected as transmitting cases. A child with a confirmed positive HIV DNA polymerase chain reaction (PCR) test result at 4 months or earlier was defined as HIV infected. The patients were further stratified into (1) those who were presumed to have acquired HIV infection during the intrauterine period because they had a positive PCR test result on the sample collected on their day of birth (n = 9) and (2) those who were presumed to have acquired HIV infection intrapartum or postpartum because they had a negative PCR test result at birth but a positive PCR test result at 1 week of age or later (n = 23). For comparison, nontransmitting controls (n = 52) were randomly selected from among HIV-positive women with uninfected infants. A child with a negative PCR test result at 4 months or older and no positive PCR test results was defined as uninfected. Sixteen HIV-negative women from the same community were also included as negative controls.
Maternal blood collected during pregnancy was tested for CD4 T-cell counts (FACSCount system; BD Immunocytometry Systems, San Jose, CA) and HIV RNA levels (Roche Amplicor, version 1.5; Roche, Branchburg, NJ). Breast milk collected by manual expression at 1 week postpartum (mean = 8 days) was used in this analysis. Breast milk was processed within 4 hours of collection and was kept cold until processing. The milk was centrifuged at 400 g, and the cell pellet was removed. The supernatant and lipid portions of the milk were mixed together before aliquoting and were stored at 270°C until use. The fluid portions of breast milk samples were tested to quantitate HIV RNA using an ultrasensitive assay with a lower limit of detection of 50 copies/mL (Roche Amplicor). Breast milk concentration of α-defensins was assayed by a commercially available enzyme-linked immunosorbent assay (ELISA; Hbt Human HNP 1-3; HyCult Biotechnology, Uden, The Netherlands). Defensins were measured after the thawed stored samples had been centrifuged at 25,000 relative centrifugal force (RCF) 30 minutes at 4°C to remove the lipid layer.10 The supernatants were diluted 1:5000 in sterile saline solution or in phosphate-buffered saline (PBS) just before use. Processing was identical for all the samples, and α-defensin concentrations were measured blinded to all clinical data.
Continuous variables were compared between the groups (HIV-positive vs. HIV-negative and within the HIV-positive women by transmission status) using the nonpara-metric Wilcoxon test. Categorical variables were compared between groups using the x2 test. Log10 transformations of HIV RNA quantities and α-defensin plus 1 (to avoid taking the log of 0) were used to normalize skewed distributions. Descriptive statistics (means, medians, standard deviations [SDs], and interquartile ranges [IQRs; 25th to 75th percentile]) were calculated for each group. After transformations, Pearson correlation coefficients (r) were used for bivariate correlation of continuous variables. Multivariable analysis was done using logistic regression. The analysis was restricted to HIV-positive women, and those who had transmitted HIV infection during the intrauterine period were excluded. The outcome was dichotomous, comparing transmitters (via the intrapartum and postnatal routes) with nontransmitters, and maternal and breast milk parameters were entered as predictors. All statistical tests were 2-tailed, and P values,0.05 were considered statistically significant. Analyses were conducted using SAS statistical software (SAS Institute, Cary, NC).
RESULTS
α-Defensins were detected among 79 of 100 breast milk samples tested: specifically, among 69 (82%) of 84 breast milk samples from HIV-positive women compared with 10 (63%) of 16 samples from HIV-negative women (P = 0.08). The mean α-defensin concentration in breast milk from HIV-positive mothers was 316 ng/mL compared with 36 ng/mL in breast milk from HIV-negative mothers (P = 0.08), but the distribution of α-defensin concentrations in breast milk was noticeably skewed. There were no significant differences, using nonparametric tests, in α-defensin concentrations in breast milk from HIV-positive mothers by transmission outcome in unadjusted analyses (Table 1). HIV RNA quantity in breast milk was significantly higher (P, 0.0001) and CD4 cell count (P = 0.0001) was significantly lower among mothers who transmitted intrapartum or postnatally than among nontransmitting mothers (see Table 1).
TABLE 1.
Breast Milk a-Defensin Concentrations and Other Characteristics of 84 HIV-Positive and 16 HIV-Negative Women in Lusaka, Zambia
| Mother HIV-Positive |
||||
|---|---|---|---|---|
| Transmitters HIV-Infected Child |
||||
| Intrauterine | Intrapartum/Postnatal | Nontransmitters Uninfected Child | Mother HIV-Negative |
|
| Controls Uninfected Child | ||||
| N | 9 | 23 | 52 | 16 |
| Age in years, mean (SD) | 26.9 (6.29) | 26.4 (4.41) | 24.9 (4.84) | 24.9 (5.37) |
| Maternal CD4 count, mean (SD) | 296 (219) | 197 (102) | 383 (203) | 963 (258) |
| Plasma HIV RNA (log10) copies/mL, mean (SD) | 4.84 (0.69) | 4.83 (0.85) | 4.55 (0.83) | Not applicable |
| Breast milk HIV RNA (log10) copies/mL, mean (SD) | 2.80 (1.23) | 3.00 (0.95) | 2.01 (0.61) | Not applicable |
| Number of (%) breast problems | 0 | 2 (8.7%) | 1 (1.9%) | 0 |
| Breast milk α-defensins (ng/mL) | ||||
| N (%) detectable | 6 (66.7) | 19 (82.6) | 44 (84.6) | 10 (62.5) |
| Range | 0-779 | 0-376 | 0-5286 | 0-300 |
| Mean (SD) | 241 (350) | 45.4 (87.5) | 448 (1153) | 35.6 (78.5) |
| Median (IQR) | 20.1 (0-556) | 10.5 (0.7-52.2) | 8.47 (2.1-127.3) | 2.6 (0-35.2) |
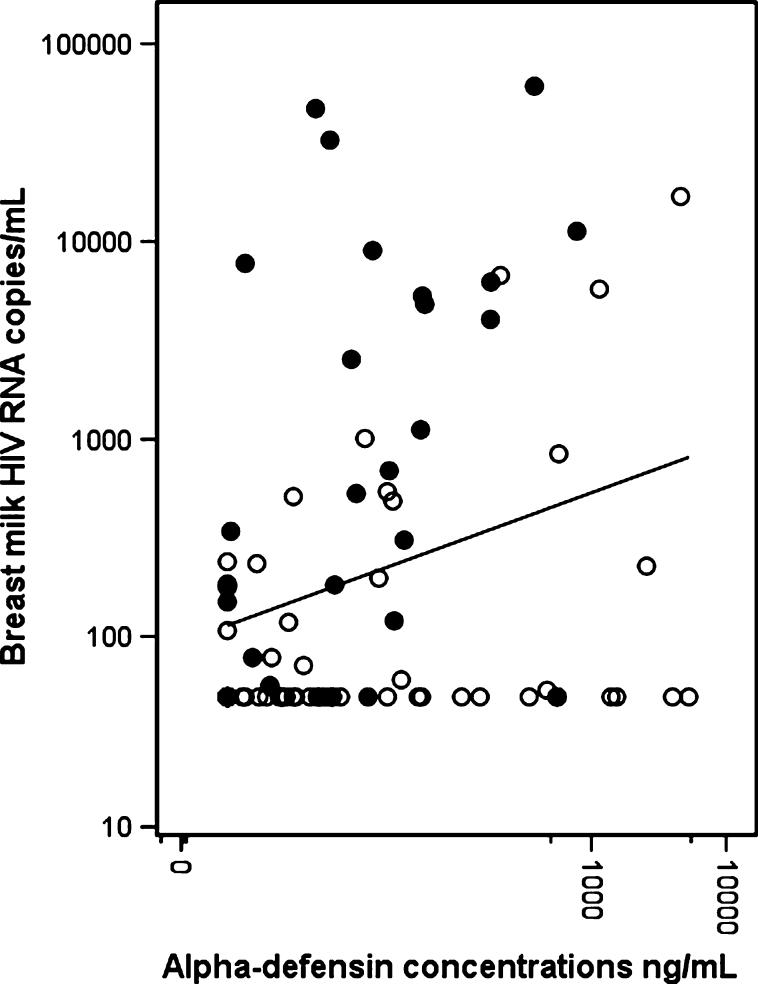
Among HIV-positive mothers, concentrations of α-defensins in breast milk were significantly correlated with HIV RNA quantity in breast milk (r = 0.23, P = 0.04; Fig. 1) but were not significantly correlated with maternal CD4 cell count or plasma viral load. Breast milk viral load was, on average, 2 log lower than plasma viral load and was also significantly correlated with plasma viral load (r = 0.27, P = 0.02) and lower CD4 cell counts (r = 20.39, P = 0.0003).
FIGURE 1.
Scatterplot of α-defensin concentrations versus HIV RNA copy numbers in breast milk from 84 HIV-positive women. Solid dots represent the transmitting mothers and open dots represent the nontransmitting mothers. The line indicates the slope of the linear relation between α-defensin concentrations and HIV RNA copy numbers (on a log10 scale) in all the HIV-positive women.
In multivariable analysis, the concentration of α-defensins in breast milk (log10 scale) was significantly associated with a decreased odds of intrapartum or postnatal HIV transmission (odds ratio = 0.30, 95% confidence interval [CI]: 0.09-0.93) after adjusting statistically for HIV RNA in breast milk and maternal CD4 cell counts (Table 2). Maternal plasma viral load was not significantly associated with transmission once maternal CD4 cell count or breast milk viral load was included in the model. The results were almost unchanged if those with breast problems were excluded. The concentration of α-defensins was not significantly associated with intrauterine transmission regardless of which other variables were included in the model (data not shown).
TABLE 2.
Predictors of Intrapartum/Postnatal HIV Transmission Versus Nontransmitters Among 75 Infants of HIV-Positive Mothers (intrauterine infections were excluded)
| Unadjusted* Odds Ratio | 95% CI | Adjusted† Odds Ratio | 95% CI | |
|---|---|---|---|---|
| α-defensin concentration in breast milk (per each log10 (ng/mL)) | 0.76 | 0.46-1.26 | 0.30 | 0.09-0.93 |
| Breast milk HIV RNA (per each log10(copies/mL)) | 4.33 | 2.09-8.99 | 8.25 | 2.21-30.79 |
| Maternal CD4 count (per 10 counts) | 0.93 | 0.89-0.97 | 0.95 | 0.90-0.99 |
Unadjusted odds ratios calculated from 3 separate logistic regression models, including each predictor individually.
Adjusted odds ratios calculated from 1 multivariable logistic regression model, including the 3 predictors simultaneously.
DISCUSSION
α-Defensins were detectable among most (79%) of the 1-week breast milk samples tested, and HIV-positive women with higher concentrations in their breast milk were less likely to transmit HIV to their infants via the intrapartum or postnatal route than women with lower concentrations, even after adjusting for the quantity of cell-free virus in the milk and for maternal CD4 cell count. A recent study among uninfected women in the United States also observed that α-defensins are detectable in breast milk from most women.8 Thus, α-defensins seem to be yet another anti-infective factor, among the plethora of immunologically active components,11 produced in human milk under certain circumstances. Concentrations were higher in the US study8 than in ours, possibly because of population differences, differences in postnatal ages at which the samples were collected, or differences in laboratory methodologies. Nevertheless, within our study population, higher concentrations of α-defensins distinguished the nontransmitting from the transmitting HIV-positive women.
α-Defensins tended to be detected more often and at higher levels in the breast milk from HIV-positive mothers compared with milk from HIV-negative control mothers. Furthermore, among HIV-positive mothers, α-defensin concentrations were correlated with cell-free breast milk viral load (ie, as HIV RNA quantity in milk increased, so did α-defensin concentrations). Thus elevated α-defensin concentrations may be, at least in part, stimulated by active HIV infection. α-defensins may be produced to augment mucosal immune defenses in the breast when physiologically needed.
The amount of HIV RNA quantified in breast milk was a strong predictor of mother-to-child HIV transmission in our data, as has been previously observed in other studies.12,13 Paradoxically, despite the correlation between breast milk viral load and α-defensins, once breast milk viral load was controlled for, α-defensin concentrations were strongly and significantly associated with lack of HIV transmission. In other words, at comparable breast milk viral load levels, elevated α-defensin concentrations were associated with a reduced risk of HIV transmission among breastfed infants. A limitation of our study is that we did not measure cellassociated virus in breast milk, which has been suggested to be a more important predictor of postnatal transmission than cellfree virus.14 Failure to adjust for this parameter is unlikely to explain our observed association between α-defensin concentrations and lack of transmission, however, because the quantity of cell-associated virus is significantly correlated with the quantity of cell-free virus14; adjustment for cell-free virus in our data strengthened rather than diminished the magnitude of the association. It is interesting to note that in the study of cell-associated virus, levels in breast milk samples collected soon after birth were not associated with postnatal transmission14 and that, in general, innate immune factors, including α-defensins, tend to decline from high levels in colostrum to lower levels in samples collected at later postnatal ages.8 The significance of this is unclear and needs to be further investigated.
Lower CD4 cell counts were independently associated with transmission in our data possibly because of more robust maternal adaptive immunity. Declining CD4 cell counts tend to correlate with increasing deficits in antigen-specific cell-mediated immunity.15 In our data, however, immunosuppression (measured by a low CD4 cell count) was not associated with lower levels of α-defensin concentrations in breast milk and thus did not explain the association we observed with transmission.
These results are consistent with the studies among HIV-infected adults that have observed that high concentrations of α-defensins are correlated with long-term nonprogression and are observed among HIV-exposed high-risk but uninfected individuals.4,7 Our data suggest that α-defensins may also have some role in prevention of HIV transmission to breastfed infants. Because all infants in our study were breastfed, a limitation is that we cannot distinguish whether the association pertains only to postnatal transmission through breastfeeding or also to intrapartum transmission during delivery. Both of these routes of transmission involve exposure to maternal mucosal fluids,16 and it would be interesting to investigate whether α-defensin concentrations are similarly elevated in cervicovaginal secretions among nontransmitting HIV-positive mothers.
It is not a novel suggestion that maternal breast milk factors may influence infant susceptibility to infection. Breast milk is known to contain several immunologically active components that augment the newborn’s immune defenses.11 Several of these factors, including secretory leukocyte inhibitor (SLPI), lactoferrin, lysozyme, human b-defensin, and regulated upon activation, normal T-cell expressed and secreted (RANTES) among others, also have anti-HIV activity under certain conditions. A limitation of our study is that we cannot ascertain whether the elevated α-defensin concentrations are simply correlates of other factors in breast milk with anti-HIV activity that might be present under the same conditions that promote local α-defensin production. Studies have not necessarily observed significant associations between concentrations of these factors in breast milk and reduced HIV transmission17,18; thus the relative importance of these factors in reducing the risk of postnatal transmission remains unknown.
Elevated α-defensins or other innate immune factors in breast milk may influence transmission by processes involved in reducing the infectivity of virus in breast milk and/or in increasing infant resistance, possibly via mechanisms analogous to passive immunization. They may also support adaptive immune responses operating locally in the breast or in the child. Our study is unable to determine whether the association between elevated α-defensin concentration and reduced transmission is simply a result of the correlation between this innate immune factor and other immune responses in breast milk, such as neutralizing antibody responses, HIV-specific cytotoxic or helper cell responses, or natural killer cells. Some but not all studies have observed associations between anti-HIV antibodies in breast milk and reduced transmission.19,20 HIV-specific cytotoxic cells also seem to be quite common in milk from HIV-positive women.21 α-defensins may be one of perhaps several mucosal immune factors or responses that are stimulated in the breast and may help to explain why, despite the prolonged and extensive exposure of breastfed infants to virus, transmission through this route is relatively inefficient.
These data raise the question of why some mothers produce high levels of breast milk α-defensins when their breast milk viral load is high, whereas others apparently do not. Genetic polymorphins that facilitate production of these antiviral factors may be involved and have been identified for b-defensins but not for α-defensins.22 Our data suggest that there may be a role for α-defensins in prevention of mother-to-child HIV transmission. Further investigation of the dynamics and source of this mucosal antiviral factor and its association with other local and systemic immune factors and with HIV transmission through different routes is warranted.
Footnotes
Supported in part by the National Institute of Child Health and Human Development (grants 39611 and 40777) and by grants from the Instituto Superiore di Sanita’ “Programma Nazionale di Ricerca sull’ AIDS”, Centro di Eccellenza CISI, the EMPRO and AVIP EC WP6 Projects, and the Tuscany Region, General Direction, Right to Health and Solidarity Policy.
REFERENCES
- 1.Kuhn L, Meddows-Taylor S, Gray G, et al. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin Infect Dis. 2002;34:267–276. doi: 10.1086/338153. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar C, Van Cott TC, Mbori-Ngacha DA, et al. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J Infect Dis. 2002;186:1173–1176. doi: 10.1086/343805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzoli S, Trabattoni D, Lo Caputo S, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Yu W, He T, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 5.Mackewicz CE, Yuan J, Tran P, et al. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17(Suppl):F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Owen SM, Rudolph DL, et al. Activity of alpha-and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173:515–520. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- 7.Trabattoni D, Lo Caputo S, Maffeis G, et al. Human a-defensin in HIV-exposed but uninfected individuals. J Acquire Immun Defic Syndr. 2004;35:455–463. doi: 10.1097/00126334-200404150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Armogida SA, Yannaras NM, Melton AL, et al. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004;25:297–304. [PubMed] [Google Scholar]
- 9.Thea DM, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention—the Zambia Exclusive Breast-Feeding Study. Control Clin Trials. 2004;25:353–365. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Bottcher MF, Jenmalm MC, Garofalo RP, et al. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:157–162. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12:664–671. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Pillay K, Coutsoudis A, York D, et al. Cell-free virus in breast milk of HIV-1 seropositive women. J Acquir Immune Defic Syndr. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau CM, Nduati RW, Richardson BA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousseau CM, Nduati R, Richardson B, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan MJ, Clerici M, Blatt SP, et al. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Van de Perre P. Mother-to-child transmission of HIV-1: the “all mucosal” hypothesis as a predominant mechanism of transmission. AIDS. 1999;13:1133–1138. doi: 10.1097/00002030-199906180-00018. [DOI] [PubMed] [Google Scholar]
- 17.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of human immunodeficiency virus-infected women. J Hum Lact. 1999;15:301–306. doi: 10.1177/089033449901500407. [DOI] [PubMed] [Google Scholar]
- 18.Becquart P, Gresenguet G, Hocini H, et al. Secretory leukocyte protease inhibitor in colostrum and breast milk is not a major determinant of the protection of early postnatal transmission of HIV. AIDS. 1999;13:2599–2602. doi: 10.1097/00002030-199912240-00018. [DOI] [PubMed] [Google Scholar]
- 19.Van de Perre P, Simonon A, Hitimana DG, et al. Infective and anti-infective properties of breastmilk from HIV-1 infected women. Lancet. 1993;341:914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 20.Becquart P, Hocini H, Levy M, et al. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. J Infect Dis. 2000;181:532–539. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 21.Sabbaj S, Edwards BH, Ghosh MK, et al. Human immunodeficiency virus-specific CD8(+) T cells in human breast milk. J Virol. 2002;76:7365–7373. doi: 10.1128/JVI.76.15.7365-7373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braida L, Boniotto M, Pontillo A, et al. A single-nucleotide poly-morphism in the human beta-defensin 1 gene is associated with HIV-1 infection in Italian children. AIDS. 2004;18:1598–1600. doi: 10.1097/01.aids.0000131363.82951.fb. [DOI] [PubMed] [Google Scholar]