Abstract
The hair follicle is an epidermal derivative that undergoes cycles of growth, involution, and rest. The hair cycle has well-orchestrated kinetics regulated by interactions between mesenchymal and epithelial cells, although the intracellular signals remain unclear. We previously established keratinocyte-specific Stat3-disrupted mice, by which we demonstrated that signal transducer and activator of transcription 3 (Stat3) is required for wound healing and anagen progression in the hair cycle. Growth factor-dependent migration of Stat3-disrupted keratinocytes was severely impaired, suggesting that not only wound healing but also telogen-to-anagen progression required organized keratinocyte migration in response to mesenchymal stimuli. In the present study, to examine whether Stat3 activation in keratinocytes is a prerequisite for hair cycle progression, we applied methods for experimental anagen induction to Stat3-disrupted mice. It was demonstrated that anagen was successfully induced in Stat3-disrupted as well as wild-type mice by chemical or mechanical stimulation, i.e., by topical application of phorbol 12-myristate 13-acetate (PMA) or by hair plucking, respectively. This result indicated that anagen in these methods occurred in the absence of Stat3. Furthermore, PMA stimulated the migration of Stat3-disrupted keratinocytes in vitro, supporting a hypothesis that the protein kinase C (PKC) and Stat3 pathways occur independently in the postnatal anagen induction. Both Stat3-dependent and -independent migration of keratinocytes was inhibited by a phosphoinositide 3-kinase (PI3K) inhibitor, wortmannin. Therefore, we infer that entry into anagen is mediated by at least two distinct signaling pathways: Stat3-dependent pathway for spontaneous hair cycling and Stat3-independent (probably PKC-dependent) pathway for exogenously induced hair cycling, whereas both pathways require PI3K activation.
The hair cycle consists of three phases: growth (anagen), involution (catagen), and quiescence (telogen) phases. Although the mechanisms underlying hair cycling have not been fully elucidated, the core of the process is interactions between the mesenchymal and epithelial cell populations within the hair follicle unit (1). The most obvious force in the cycle is the follicular mesenchyme, in particular the dermal papilla. Factors from the papillary mesenchyme act as inductive signals for cycling of the follicular epithelium (2). It has been inferred that epithelial stem cells, which reside in the bulge area of the hair follicles, can respond to the inductive signal from the dermal papilla (3). This activation leads to proliferation of stem cells in the bulge area, and then the stem cell progeny forms a downgrowth into the deep dermis, followed by differentiative growth of matrix cells and generation of the complex follicular product, the shaft, and its housing sheath. Several growth factor families appear to play pivotal roles in follicle growth (2, 4, 5), namely fibroblast growth factor, epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-I, and transforming growth factor-β families, among others. The role of each growth factor, as well as signals downstream of it, in follicle growth has been determined in part by investigating transgenic or gene knockout mice (4, 6–10).
Signal transducer and activator of transcription 3 (Stat3) is activated by various growth factors and cytokines, including IL-6 family cytokines, EGF, HGF, platelet-derived growth factor, Granulocyte-colony stimulating factor (GCSF), and other factors (11, 12). To investigate the role of Stat3 in the skin, we previously established keratinocyte-specific Stat3 gene-disrupted mice by using the Cre-loxP system (10). The mutant mice whose Stat3 gene was disrupted under the control of the keratin 5 promoter were defective in Stat3 activation of both epidermal and follicular keratinocytes. Interestingly, the failure of Stat3 activation resulted in retardation of wound healing and a lack of second anagen progression, despite normal development in morphogenesis of the epidermis and hair follicles. Growth factor-dependent in vitro migration was compromised in primary cultured keratinocytes of mutant mice, indicating that Stat3 plays a crucial role in transducing a signal required for migration. Therefore, we suggested that the impairment of wound healing and of the hair cycle was because of compromised migration of Stat3-disrupted keratinocytes, which otherwise could migrate in response to the growth factors derived from the wound-associated mesenchyme or dermal papilla, respectively. Stat3-disrupted mice appeared to be normal in hair follicle development, suggesting that Stat3 is dispensable for the morphogenesis of hair follicles. On the other hand, the induction of the second anagen depends strictly on Stat3.
To determine whether Stat3 is required for anagen induction in response to exogenous stimuli, we examined the effect of phorbol 12-myristate 13-acetate (PMA) in vivo and hair plucking, well known experimental anagen initiators (13). We found that PMA treatment or hair plucking activated the telogen follicles to initiate a new anagen in Stat3-disrupted as well as wild-type mice. Further analysis of in vitro migration revealed that there are at least two distinct signaling pathways underlying anagen induction: Stat3-dependent and -independent pathways.
Materials and Methods
Mice.
Generation of keratinocyte-specific Stat3-disrupted mice was previously described (10). Briefly, mice carrying a keratin-5 promoter-driven (K5)-Cre transgene and a Stat3-null allele (K5-Cre: Stat3+/−) were mated with Stat3flox/flox mice. Offspring carrying a floxed Stat3 allele and/or K5-Cre transgene (K5-Cre: Stat3flox/+, K5-Cre: Stat3flox/−, Stat3flox/+, Stat3flox/−) were used for further analyses. Allele-specific PCR was carried out to determine the genotypes of the mice, and the expression of a truncated Stat3 was verified by Western blotting with anti-Stat3 (C-20; Santa Cruz Biotechnology) as previously described. In each experiment, controls were from littermates of Stat3flox/+ mice as Stat3+/+ (wild type), or Stat3flox/−, and K5-Cre: Stat3flox/+ as Stat3+/−. Because Stat3-heterozygous mice are devoid of any alterations, therefore, they were used as controls in some experiments. Care of mice was in accordance with our institutional guidelines.
BrdUrd Pulsing.
Mice were given four rounds of BrdUrd (Nacalai Tesque, Kyoto, 0.25 mg in 0.2 ml saline/head) by i.p. injection every 12 h (1.0 mg/head in total) on postnatal day (PND) 10 and 11. Saline was used as the control.
Preparation and in Vitro Culture of Keratinocytes.
Full-thickness skin taken from newborn to 5-day-old mice was treated with 250 units/ml of dispase (Godo Shusei, Tokyo) overnight at 4°C, and the epidermis was peeled off from the dermis and trypsinized to prepare single cells. They were suspended in MCDB153 medium (Kyokuto Pharmaceutical, Tokyo) supplemented with 0.1 mM monoethanolamine/0.1 mM phosphoryl ethanolamine/0.5 μM hydrocortisone/60 μg/ml kanamycin monosulfate at 37°C under an atmosphere of 5% CO2/95% air. After being cultured in dishes precoated with collagen type I (Iwaki Glass, Tokyo) for 5 h until cells were attached and spread, unattached cells were then removed by washing with PBS, and the attached cells were cultured in MCDB medium supplemented as described for 2 to 3 days.
In Vitro Migration Assay.
Keratinocytes cultured to confluency were treated with 10 μg/ml mitomycin C for 2 h to prevent subsequent proliferation of the cells and then studied by using an in vitro wound closure assay. A cell-free area was introduced by scraping the monolayer with a plastic pipette tip. Cell migration to the cell-free area during the next 24 to 48 h was evaluated in the absence or presence of 30 ng/ml epidermal growth factor (Upstate Biotechnology, Lake Placid, NY), 30 ng/ml HGF (Collaborative Biomedical Products, Bedford, MA), 30 ng/ml IGF-I (Austral Biological), 5 μg/ml insulin (Sigma), and/or 30 ng/ml PMA (Sigma). Photographs were taken by using a phase-contrast microscope (DIAPHOT 300; Nikon). The number of migrating keratinocytes was counted after taking photographs of four nonoverlapping fields. Values represent the mean numbers ± SEM of migrating cells per square millimeters beyond the frontlines of the introduced wound edge. In some experiments, keratinocytes were pretreated with a PI3K inhibitor, wortmannin (Nacalai Tesque) or a protein kinase C (PKC) inhibitor, GF109203X (Tocris Cookson, Bristol, UK) at the indicated concentrations for 30 or 45 min, respectively, before stimulation with growth factors. Subsequently, they were cultured for 24–48 h in the presence of inhibitors.
Western Blot Analysis.
Keratinocytes were stimulated for 20 min with 50 ng/ml HGFs or 25 μg/ml insulin, then lysed directly with 2× sample buffer (4% SDS/20% glycerol/12.5 mM Tris (pH 6.8)/0.004% bromophenol blue/10% 2-mercaptoethanol). The lysates were resolved by SDS/PAGE, transferred to a nitrocellulose membrane, and blotted with anti-Akt (1:1,000, New England Biolabs), or antiphospho-Akt (1:1000, Ser-473; New England Biolabs). After treatment with horseradish peroxidase-conjugated anti-rabbit Ig (Amersham Pharmacia), bands were visualized with an enhanced chemiluminescence system (Amersham Pharmacia).
Statistical Analysis.
Data were expressed as the mean ± SEM for cell migration. Student's t test was used to test for differences between the study groups; P < 0.05 was considered significant.
Results
Stat3 Ablation in Keratinocytes Results in Impairment of the Second Anagen Entry and Subsequent Hair Growth.
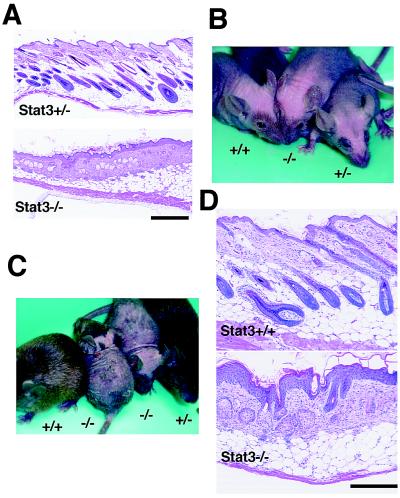
In mice, the first and second hair cycles are highly synchronized (13). Follicular morphogenesis in the first 16 days of postnatal life is followed by a short catagen/telogen period lasting 3–4 days. The following anagen phase begins around PND 21–22 and lasts about 9 days (Fig. 1A Upper). Meanwhile, it was shown that keratinocyte-specific Stat3-disrupted mice were arrested in the first telogen phase (Fig. 1A Lower), as previously described (10). To verify the impairment of hair growth in the second anagen, we shed the first anagen-derived hair by pulsing with BrdUrd. BrdUrd is an analogue of thymidine and is incorporated in cells synthesizing DNA, so it is widely used to detect proliferating cells with anti-BrdUrd antibody (14). However, treatment with an excessive dose of BrdUrd leads to cell damage, especially in highly proliferative cells. Here we treated mice with BrdUrd at doses about 10 times higher than routinely used for labeling in vivo (14). When BrdUrd was used for pulsed labeling on PND 10–11, which is around the late anagen of the first hair cycle, it was rapidly incorporated in the highly proliferative cells in the bulb, i.e., hair matrix cells (data not shown). This resulted in the shedding of hair, probably because of cytotoxic effects of BrdUrd on matrix cells, the “rapid labeling cells” (3). Mice shed their hair about 5 days after the injection and suffered alopecia regardless of their genotypes (Fig. 1B). Stem cells in the bulge region, however, escaped from the cytotoxicity of BrdUrd because they are “slow labeling cells” (3) and were later responsive to the signals from the dermal papilla for initiation of the second anagen. In fact, the regrowth of hair was demonstrated when the second anagen began in the control mice (Fig. 1C). Histological examination revealed synchronized growing anagen follicles of the second hair cycle (Fig. 1D Upper). In contrast, Stat3-disrupted mice remained alopecic (Fig. 1C) and harbored telogen follicles (Fig. 1D Lower). These results well verified that Stat3-disrupted mice were impaired in the second anagen progression of hair cycle. It should be noted that Stat3-disrupted mice at this time showed a histological phenotype including hyperplasia of epidermis, with a marked cellularity and fibrosis in the dermis. None of these features were present in the wild-type mice (Fig. 1 A and D). Further investigation will be required to elucidate the mechanistic relation between these changes and the absence of Stat3 in keratinocytes.
Figure 1.
Requirement for Stat3 in keratinocytes for anagen entry in the second hair cycle and subsequent hair growth. (A) Histological examination of the back skin revealed that the hair follicles of Stat3−/− were arrested in telogen (Lower) at PND 30, whereas anagen follicles of the second cycle were demonstrated in wild-type (not shown) or heterozygous litter mates (Stat3+/−, Upper). Hematoxylin–eosin stain. (Bar = 400 μm.) (B) Pulsing with BrdUrd at a total dose of 1 mg per head during late-anagen of the first hair cycle (PND 10 and 11) resulted in alopecia regardless of the genotypes of mice. Photograph was taken at PND 24. (C and D) Hair regrew in control littermates (C, Stat3+/+ and +/−, PND 37) as their hair follicles entered the second anagen (D Top, PND 30), whereas Stat3−/− mice remained alopecia (C, Stat3−/−) with their follicles arrested in telogen (D Lower). (Bar = 200 μm.)
Activation of the Hair Follicles in Stat3-Disrupted Mice by Hair Plucking.
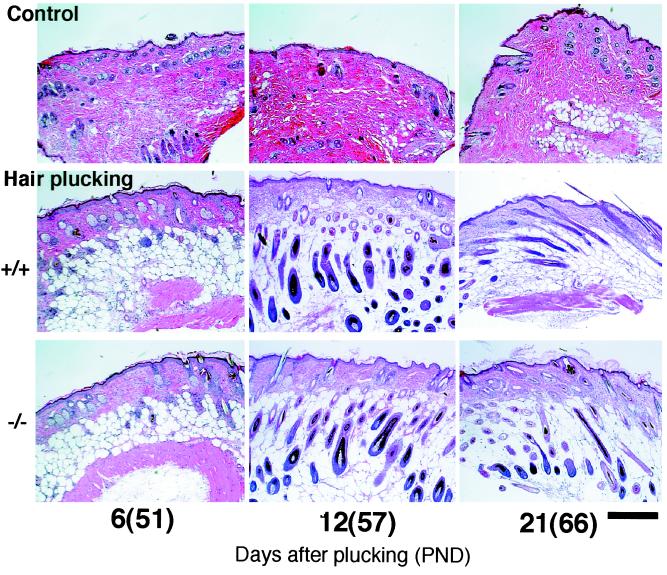
Follicles enter the second telogen phase around PND 30 and then remain in this resting phase for at least 40 days. Taking advantage of this long telogen period, “the third anagen” was experimentally induced (13). Plucking of the telogen hair in this phase is a well-established method for induction of new hair formation. We found that around 10 days after plucking hairs from the back of mice, new hair fibers emerged from the skin surface in Stat3-disrupted as well as wild-type mice, and morphologically normal anagen follicles were formed (Fig. 2) with no difference between the two types of mice. This result implied that the plucking-induced anagen occurred independently of Stat3.
Figure 2.
Induction of the anagen progression by hair plucking in Stat3−/− mice as well as controls. Hair in the back was plucked at PND 45, which is during the second telogen. Approximately 10 days later, hair emerged from the skin surface in both Stat3+ /+ and Stat3−/− mice (data not shown). Histological anagen stage, characterized by the appearance of thick and elongated follicles in deep adipose tissue, was demonstrated in both Stat3+/+ and Stat3−/− mice at day 12 after plucking (Middle and Bottom of PND 57). Follicles of Stat3+/+ and Stat3−/− mice were equally in late anagen at PND 66 (day 21 after plucking), as shown by their shortening and partial shrinkage in the dermis. Meanwhile, untreated control mice (Stat3+/+) remained in telogen (Top), in which untreated Stat3−/− mice likewise remained in telogen (data not shown for simplicity). (Bar = 300 μm.)
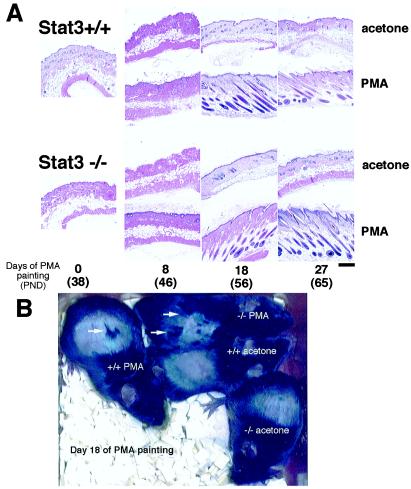
Activation of the Hair Follicles by Topical Application of PMA in Stat3-Disrupted Mice.
Percutanous treatment with PMA is another well-known method of activating hair follicles in telogen phase to commence anagen (13). At PND 38, the hair was shaved, and PMA was applied onto the back skin five times in the first week and then twice weekly. After almost 2 weeks of treatment with PMA, new hair regrew from the skin surface of both types of mice, appearing in islands or patches (Fig. 3B, arrows). This pattern is characteristic of the third hair cycle, in which hair growth distributes somewhat randomly over the body, less synchronized than the first and second hair cycles (13). There was no difference in the histological appearance between the mutant and wild-type mice (Fig. 3A). Taken collectively, these findings show that follicle growth occurs in response to either mechanical or chemical stimuli whose downstream signals bypass the Stat3-dependent pathway, whereas the spontaneously occurring second anagen is strictly Stat3 dependent (Fig. 1A).
Figure 3.
Induction of the anagen progression by topical application of PMA in Stat3+/+ and Stat3−/− mice. Percutaneous treatment with PMA was made from PND 38. PMA (13.4 μg) in acetone was applied on the back skin five times in the first week and then twice weekly. Approximately 2 weeks later, newly formed hair emerged in both Stat3+/+ and Stat−/− mice, appearing in islands or patches (B, arrows), whose histology showed the anagen stage (A, 18 days of PMA application, PND 56). After 27 days of application (PND 65), follicles in both Stat3+/+ and Stat3−/− mice were at late anagen stage. During the observation period, Stat3+/+ and Stat3−/− mice in the acetone control group remained in telogen (A Top and third row), and hence no hair emerged (B). (Bar = 300 μm.)
PMA-Stimulated Migration of Stat3-Disrupted Keratinocytes.
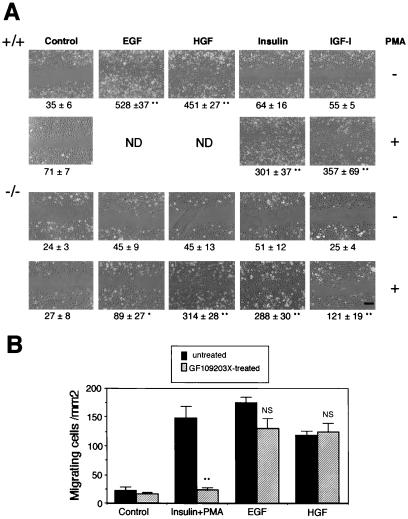
We previously demonstrated that Stat3-disrupted keratinocytes were impaired in in vitro migration in response to growth factors such as EGF, HGF, and IL-6, all of which activate Stat3 (10). Follicular keratinocytes including bulge stem cell progeny migrate downward during anagen. We previously suggested that this is why the failure of keratinocyte migration in Stat3-disrupted mice resulted not only in retardation of wound healing but also in impairment of the hair cycle. As we have previously reported (10), wild-type keratinocytes exhibited strong migration in response to EGF or HGF (Fig. 4A Top), both of which inherently activate Stat3, whereas Stat3-disrupted keratinocytes did not respond to them (Fig. 4A, third row). This result implied that EGF and HGF are ligands of Stat3-dependent signaling for keratinocytes to migrate. Although PMA per se did not stimulate migration of the wild-type keratinocytes, it promoted appreciable migration when insulin or IGF-I was also present (Fig. 4A, second row). Neither insulin nor IGF-I alone stimulated migration (Fig. 4A Top). The synergistic effect of PMA and insulin or IGF-I was also seen in Stat3-disrupted keratinocytes (Fig. 4A Bottom), suggesting that this coordinated signaling for cell migration was independent of Stat3. Interestingly, EGF and HGF, the ligands of Stat3-dependent signaling, promoted the migration of Stat3-disrupted keratinocytes to some degree when PMA was added simultaneously (Fig. 4A Bottom). One hypothesis is that PKC acts downstream of the Stat3-signaling pathway, which could be bypassed by treatment with PMA, and therefore Stat3-disrupted cells could migrate in response to synergistic stimulation with PMA and growth factors. To clarify this, we treated wild-type cells with GF109203X, a specific PKC inhibitor. As shown in Fig. 4B, cell migration induced by insulin plus PMA was completely abolished by GF109203X, whereas EGF- or HGF-induced migration was insensitive to this inhibitor. This finding clearly indicated that Stat3-dependent migration was independent of PKC activation. These results further reveal the existence of an alternative signaling pathway that does not involve Stat3 and requires PKC activation for keratinocyte migration.
Figure 4.
PMA promotes in vitro keratinocyte migration independent of Stat3. (A) Primary cultured keratinocytes were subjected to in vitro migration assays in the presence of the indicated growth factors. Every assay was performed after treating keratinocytes with mitomycin C to prevent their proliferation. Stat3+/+ keratinocytes showed strong migration in response to EGF or HGF (Top) as previously described (10) but not in response to insulin or IGF-I (Top). In contrast, Stat3−/− keratinocytes did not migrate in response to any growth factor tested (third row). PMA synergistically stimulated the migration of Stat3+/+ (second row) and Stat3−/− (Bottom) keratinocytes in the presence of insulin or IGF-I. In addition, Stat3−/− keratinocytes slightly but significantly migrated in response to PMA plus EGF (P < 0.05) and considerably to PMA plus HGF (Bottom). Note that treatment with PMA alone did not stimulate migration of Stat3+/+ or Stat3−/− keratinocytes (second and third rows). ND, not done. (Bar = 200 μm.) Quantitative evaluation of cell migration (see Materials and Methods) is shown below each panel. Significantly different from the control (*, P < 0.05; **, <0.01) as determined according to Student's t test. (B) Although PMA plus insulin-induced migration of wild-type keratinocytes was completely canceled by GF109203X (5 μM), a specific PKC inhibitor, Stat3-dependent (EGF- or HGF-induced) migration was insensitive to this inhibitor, indicating that Stat3 signaling is independent of PKC activation. Black bars, hatched bars, untreated and GF109203X-treated, respectively. Significantly different from inhibitor-free control (**, P < 0.01) according to Student's t test. NS, not significantly different from inhibitor-free controls.
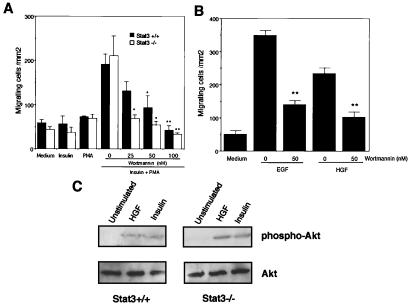
Involvement of PI3K Activation in Keratinocyte Migration.
Activation of PI3K leads to a variety of cellular events, including cell proliferation, antiapoptosis, and migration (15). The involvement of PI3K in keratinocyte migration was tested by inclusion of wortmannin, a PI3K inhibitor. Migration in response to synergistic stimulation with insulin and PMA of either Stat3-wild-type or -mutant keratinocytes was attenuated by treatment with wortmannin in a dose-dependent manner (Fig. 5A), indicative of the involvement of the PI3K pathway in Stat3-independent migration. In addition, EGF- and HGF-induced migration of wild-type keratinocytes was also impaired by wortmannin (Fig. 5B), suggesting that PI3K activation is also required for Stat3-dependent migration. The synergistic effect of PMA and EGF or HGF on the migration of Stat3-disrupted keratinocytes was also abrogated by wortmannin (data not shown). Taken together, these results suggest that both Stat3-dependent and -independent signaling pathways for migration require PI3K activation.
Figure 5.
Involvement of PI3K in Stat3-dependent and -independent migration of keratinocytes. (A) Costimulation with insulin and PMA promoted appreciable migration of either wild-type (+/+, black bars) or Stat3-disrupted (−/−, white bars) keratinocytes, either of which did not migrate by insulin or PMA alone. Migration of both types of cells was blocked by a PI3K inhibitor, wortmannin, in a dose-dependent manner. *, P < 0.05; **, P < 0.01 by Student's t test. (B) Wortmannin (50 nM) blocked 60%, 56% of the migration of wild-type cells promoted by EGF, HGF, respectively. **, P < 0.01 by Student's t test. (C) Western blot analyses revealed that stimulation not only of Stat3+/+ but also of Stat3−/− keratinocytes with HGF (50 ng/ml) or insulin (25 μg/ml) resulted in phosphorylation of Akt, a direct target of PI3K (16), indicating that PI3K activation in response to HGF or insulin was not affected by Stat3 ablation.
Impairment of migration of the Stat3-disrupted cells in the presence of EGF/HGF might be because of their inability to activate PI3K. To examine whether Stat3 disruption may influence the PI3K pathway, we assessed the phosphorylation of Akt, a downstream target of PI3K (16). Western blot analysis by using lysates from Stat3-disrupted keratinocytes revealed that the treatment with HGF, EGF (data not shown), as well as insulin or IGF-I (data not shown), resulted in phosphorylation of Akt, which was comparable to those from the wild-type cells (Fig. 5C). Phosphorylation of Akt did not occur in response to PMA alone (data not shown). These findings indicated that (i) EGF/HGF per se did not promote migration of Stat3-disrupted cells irrespective of their impairment of PI3K activation, and (ii) HGF, EGF, insulin, or IGF-I caused PI3K activation in Stat3-disrupted cells, and they need simultaneous PKC activation to migrate (Figs. 4 and 5). Thus, induction of anagen in Stat3-disrupted mice by topical PMA application appeared to be consistent with the results of the in vitro migration assay.
Discussion
The anagen phase, a process of follicle downgrowth, is regulated by mesenchymal signals (1, 4, 17), which are intrinsically derived from dermal papilla. Analyses of the skin phenotypes of a considerable number of transgenic or gene knockout mice have shed light on the mesenchymal (dermal papilla) and epithelial (keratinocyte) interactions involved in the morphogenesis of hair follicles in the fetus (18–20). However, the mechanisms underlying the biological switch in postnatal follicles occurring between telogen and anagen have remained unclear, although it has been believed that the postnatal anagen progression is analogous to the embryonic folliculogenesis (17). We previously reported that Stat3 activation of keratinocytes is indispensable for the progression of the second hair cycle onwards (Fig. 1), which represents one of the remodeling events of the skin, although Stat3 is dispensable for the morphogenesis of follicles (10). Stat3-independent processes should, therefore, be involved in the formation of epidermis and follicles during morphogenesis. Novel technology used in temporal and spatial gene disruption, such as the tamoxifen application-Cre ERtam system (21), will serve as a powerful tool for determination of the specific window of Stat3 dependency for the hair cycle. During late telogen, a slow-cycling stem cell of the bulge area is activated and divides to generate progenitor cells by dermal papilla cells through growth factors in a paracrine fashion (2, 4). Progenitor cells become transient amplifying cells that undergo continuous proliferation and migrate downwards to form matrix cells. Therefore, we assessed the migration activity of keratinocytes in vitro to evaluate their anagen potential. The growth factors involved in the onset of the second anagen should be activators of Stat3. The most likely candidate for such factors is HGF, because HGF is found in the dermal papilla fibroblasts and its receptor Met, in the neighboring keratinocytes (22). A highly critical fact is that expression of both HGF and Met peaks during the initial phase of the second anagen (22). By contrast, during the early stages of folliculogenesis, dermal papilla fibroblasts in close vicinity to the proximal hair peg showed no HGF signal (22). This finding appears consistent with the finding that Stat3 is dispensable for the first hair cycle, which in turn requires Stat3-independent signaling for downward migration of keratinocytes.
In the present study, we found that PMA treatment in vivo promoted anagen progression in Stat3-disrupted mice as well as wild-type controls (Fig. 3), implying that their bulge stem cells and successive transient amplifying cells were activated by the stimuli to migrate in the absence of Stat3 activation. This finding appeared to be consistent with the results that Stat3-disrupted keratinocytes migrated in vitro in response to the combinational stimuli of PMA and growth factors that elicit PI3K activation (Fig. 5C). Inhibition assay by using GF109203X revealed that there is no crosstalk between Stat3- and PKC-dependent signaling pathways regarding cell migration (Fig. 4B). EGF promoted in vitro migration of wild-type, but not Stat3-disrupted, cells (Fig. 4A), indicating that Stat3 is a prerequisite for EGF-induced migration (10). It has been reported that EGF receptor activation leads directly to the activation of PKC via phospholipase C-γ (23). However, PKC activation by EGF stimulation was not required for EGF-induced migration, which was resistant to GF109203X, whereas this inhibitor completely abrogated PKC-dependent migration (by stimulation with PMA plus insulin) (Fig. 4B). Seemingly, PKC activation is dispensable for migration when Stat3 signaling is intact. It is not known which isotype of PKC is involved in the PKC-dependent migration, because GF109203X at the concentration used in the present study (5 μM) attenuates the PKC activation of various subfamilies found in keratinocytes (24, 25). Activation of PKC has been reported to play an important role in keratinocyte migration through enhancement of the specific integrin-mediated motility (26), microfilament organization (27), or up-regulation of proteinases such as metalloproteinases (28) and urokinase (29). Requirement of PI3K activation for the Stat3-independent PKC-dependent signaling pathway toward migration was clearly demonstrated by in vitro studies by using wortmannin, a specific inhibitor of PI3K (Fig. 5A). Percutaneous treatment with PMA could stimulate skin components other than keratinocytes, including dermal papilla fibroblasts, vascular endothelial cells, and leukocytes. It is possible, therefore, that PMA directly activates some of these components to produce diffusible growth factors and/or cytokines, which could lead to simultaneous PI3K activation in keratinocytes. PI3K regulates a vast array of fundamental cellular responses, including proliferation, transformation, antiapoptosis, and migration (30). More recently, it has been clearly shown that PI3K-γ activation stimulated with chemotactic factors through G protein-coupled receptor was involved in leukocyte migration by using PI3K-γ knockout mice (31). Wortmannin inhibited wild-type keratinocyte migration in response to stimuli of HGF or EGF alone (Fig. 5B). These results are consistent with the recent reports that PI3K activation is required for cell migration mediated by HGF (32) or EGF (33). Taken collectively, the present study clearly shows that both Stat3-dependent and -independent pathways require PI3K activation for keratinocyte migration. In further support of these findings, Akt, a direct target of PI3K (16), was activated by HGF (Fig. 5C) and EGF (data not shown) not only in the wild-type and but also in Stat3-disrupted keratinocytes. Possible signaling pathways for keratinocyte migration are depicted in Fig. 6. Although we have shown the involvement of PI3K activation in keratinocyte migration via either pathway, it is still unclear whether PI3K activation resides at the very downstream of pathways toward migration.
Figure 6.
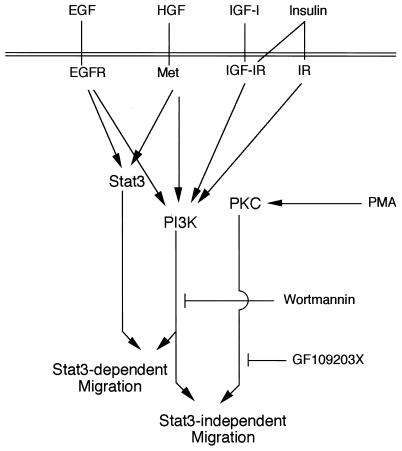
Possible migration-related signaling pathways in keratinocytes. On the basis of present findings, putative signaling pathways for migration are depicted. There are two distinct signaling pathways toward migration: Stat3-dependent and Stat3-independent (PKC-dependent) signaling pathways, both of which require PI3K activation. EGFR, EGF receptor; IGF-IR, IGF-I receptor; IR, insulin receptor.
Despite plucking-induced anagen having been used as a model for the hair cycle (13), the molecular mechanisms underlying this induction have remained largely unknown. The present study has shown that plucking-induced hair cycling is quite different from spontaneous hair cycling in its requirement for Stat3 (Fig. 2). Because basal keratinocytes of the bulge are quite resistant to physical removal and tend to remain behind after hair plucking (3), the bulge stem may respond to this “emergency microenvironment,” resulting in the regeneration of hair. It is possible that the plucking-induced milieu stimulates the bulge stem, and that PKC activation may be involved in this process. One attractive idea is that the Stat3-dependent pathway is required for spontaneous remodeling progression of anagen, whereas artificially induced or physical perturbation of the follicles induces an alternative Stat3-independent pathway for hair regrowth.
Acknowledgments
The excellent technical assistance of Mr. K. Nishida is gratefully appreciated. We thank Drs. S. Akira and K. Takeda for providing the mice with floxed Stat3 and Stat3-null alleles. This work was supported by grants of the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations
- Stat3
signal transducer and activator of transcription 3
- EGF
epidermal growth factor
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- PMA
phorbol 12-myristate 13-acetate
- PKC
protein kinase C
- PI3K
phosphoinositide 3-kinase
- PND n
postnatal day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240303097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240303097
References
- 1.Jahoda C A, Reynolds A J. Dermatol Clin. 1996;14:573–583. doi: 10.1016/s0733-8635(05)70385-5. [DOI] [PubMed] [Google Scholar]
- 2.Peus D, Pittelkow M R. Dermatol Clin. 1996;14:559–572. doi: 10.1016/s0733-8635(05)70384-3. [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G, Sun T T, Lavker R M. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 4.Stenn K S, Combates N J, Eilertsen K J, Gordon J S, Pardinas J R, Parimoo S, Prouty S M. Dermatol Clin. 1996;14:543–558. doi: 10.1016/s0733-8635(05)70383-1. [DOI] [PubMed] [Google Scholar]
- 5.Oro A E, Scott M P. Cell. 1998;95:575–578. doi: 10.1016/s0092-8674(00)81624-4. [DOI] [PubMed] [Google Scholar]
- 6.Mann G B, Fowler K J, Gabriel A, Nice E C, Williams R L, Dunn A R. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 7.Murillas R, Larcher F, Conti C J, Santos M, Ullrich A, Jorcano J L. EMBO J. 1995;14:5216–5223. doi: 10.1002/j.1460-2075.1995.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Degenstein L, Fuchs E. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Rosenquist T A, Martin G R. Dev Dyn. 1996;205:379–386. doi: 10.1002/(SICI)1097-0177(199604)205:4<379::AID-AJA2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S. Int J Biochem Cell Biol. 1997;29:1401–1418. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- 12.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 13.Wilson C, Cotsarelis G, Wei Z G, Fryer E, Margolis-Fryer J, Ostead M, Tokarek R, Sun T T, Lavker R M. Differentiation (Berlin) 1994;55:127–136. doi: 10.1046/j.1432-0436.1994.5520127.x. [DOI] [PubMed] [Google Scholar]
- 14.Tezuka M, Ito M, Ito K, Sato Y. J Dermatol Sci. 1990;1:335–346. doi: 10.1016/0923-1811(90)90590-a. [DOI] [PubMed] [Google Scholar]
- 15.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 16.Coffer P J, Jin J, Woodgett J R. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy M H. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 18.Gat U, DasGupta R, Degenstein L, Fuchs E. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C, Swan R Z, Grachtchouk M, Bolinger M, Litingtung Y, Robertson E K, Cooper M K, Gaffield W, Westphal H, Beachy P A, Dlugosz A A. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 20.Botchkarev V A, Botchkareva N V, Roth W, Nakamura M, Chen L H, Herzog W, Lindner G, McMahon J A, Peters C, Lauster R, et al. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 21.Vasioukhin V, Degenstein L, Wise B, Fuchs E. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner G, Menrad A, Gherardi E, Merlino G, Welker P, Handjiski B, Roloff B, Paus R. FASEB J. 2000;14:319–332. doi: 10.1096/fasebj.14.2.319. [DOI] [PubMed] [Google Scholar]
- 23.Bhalla U S, Iyengar R. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 24.Dlugosz A A, Mischak H, Mushinski J F, Yuspa S H. Mol Carcinogen. 1992;5:286–292. doi: 10.1002/mc.2940050409. [DOI] [PubMed] [Google Scholar]
- 25.Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke H H. J Cell Biol. 1999;144:413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Wu J, Spong S, Sheppard D. J Cell Sci. 1998;111:2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 27.Masson-Gadais B, Salers P, Bongrand P, Lissitzky J C. Exp Cell Res. 1997;236:238–247. doi: 10.1006/excr.1997.3721. [DOI] [PubMed] [Google Scholar]
- 28.Dunsmore S E, Rubin J S, Kovacs S O, Chedid M, Parks W C, Welgus H G. J Biol Chem. 1996;271:24576–24582. doi: 10.1074/jbc.271.40.24576. [DOI] [PubMed] [Google Scholar]
- 29.Ando Y, Jensen P J. J Cell Physiol. 1996;167:500–511. doi: 10.1002/(SICI)1097-4652(199606)167:3<500::AID-JCP14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki T, Irie-Sasaki J, Jones R G, Oliveira-dos-Santos A J, Stanford W L, Bolon B, Wakeham A, Itie A, Bouchard D, et al. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 32.Lai J F, Kao S C, Jiang S T, Tang M J, Chan P C, Chen H C. J Biol Chem. 2000;275:7474–7480. doi: 10.1074/jbc.275.11.7474. [DOI] [PubMed] [Google Scholar]
- 33.Price J T, Tiganis T, Agarwal A, Djakiew D, Thompson E W. Cancer Res. 1999;59:5475–5478. [PubMed] [Google Scholar]