Abstract
Adenosine produces bronchoconstriction in allergic rabbits, primates, and humans by activating adenosine A1 receptors. Previously, it is reported that a high dose of L-97-1, a water-soluble, small molecule adenosine A1 receptor antagonist, blocks early and late allergic responses, and bronchial hyper-responsiveness to histamine in a hyper-responsive rabbit model of allergic asthma. Effects of a lower dose of L-97-1 are compared to montelukast, a cysteinyl leukotriene-1 receptor antagonist on early allergic response, late allergic response, bronchial hyper-responsiveness, and inflammatory cells in bronchoalveolar lavage (BAL) fluid following house dust mite administration. Rabbits received intraperitoneal injections of house dust mite extract within 24 h of birth followed by booster house dust mite injections. Hyper-responsive rabbits received aerosolized house dust mite (2500 allergen units), 1 h after intragastric administration of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) and lung dynamic compliance was measured for 6 h. Lung dynamic compliance was significantly higher following L-97-1 at all time points and with montelukast at 60-300 min following house dust mite (P < 0.05). L-97-1 blocks both early and late allergic responses. Montelukast blocks only the late allergic response. Both L-97-1 and montelukast significantly blocked bronchial hyper-responsiveness at 24 h (P < 0.05). Both L-97-1 and montelukast significantly reduced BAL eosinophils at 6 h and neutrophils at 6 and 24 h (P < 0.05). L-97-1 significantly reduced BAL lymphocytes at 6 and 24 h (P < 0.05). Montelukast significantly reduced BAL macrophages at 6 and 24 h (P < 0.05). By blocking both bronchoconstriction and airway inflammation, L-97-1 may be an effective oral anti-asthma treatment.
Keywords: Adenosine A1 receptor, Asthma, Inflammation, Bronchial hyper-responsiveness, Airway, Allergen
1. Introduction
In the U.S., asthma is one of the most common and costly diseases. The American Lung Association estimates that as many as 20 million Americans, or approximately 10% of the U.S. population, have asthma and that children account for over 6 million of these cases, that the total health care costs of asthma is $16 billion, and that over 4000 deaths in the U.S. are attributed to asthma (American Lung Association, 2001, 2002, 2004). Moreover, a high percentage of patients with asthma (20%) are not controlled with currently available therapies and a high percentage of patients (>60%) are not compliant with inhaled treatments for asthma (Bender and Rand, 2004; Jones et al., 2003; Sin et al., 2004). Thus, there is a great need for improved therapies, particularly oral therapies that enhance patient compliance.
Cysteinyl leukotrienes are key mediators of systemic allergic responses, including inflammatory responses that lead to the typical symptoms of asthma including bronchoconstriction, wheezing, increased mucus secretion, and decreased mucociliary clearance (Busse, 1998; Johnson and McNee, 1983). The effects of cysteinyl leukotrienes in asthma are mediated through the cysteinyl leukotriene-1 receptor, which is a G protein coupled receptor expressed in monocytes/macrophages, eosinophils, basophils, mast cells, neutrophils, T cells, B lymphocytes, and smooth-muscle cells (Figueroa et al., 2001; Holgate et al., 2003; Lynch et al., 1999). The leukotriene receptor antagonist montelukast sodium (montelukast) selectively blocks the cysteinyl leukotriene-1 receptor (Reiss et al., 1998).
The endogenous purine nucleoside, adenosine, is an important signaling molecule in asthma. When administered by inhalation, adenosine produces concentration dependent bronchoconstriction in patients with asthma, but not in normal subjects (Cushley et al., 1983; Polosa, 2002; Rorke and Holgate, 2002). Adenosine levels are increased in the bronchoalveolar fluid of asthmatics and also in the plasma of patients with exercise-induced asthma (Driver et al., 1993; Vizi et al., 2002). There is also an association between allergen exposure and adenosine monophosphate (AMP) responsiveness in asthmatics (Currie et al., 2003). In asthma, the airway response to AMP seems to correlate more closely with disease activity than the response to other more conventional provocative agents, such as methacholine (De Meer et al., 2002).
In asthma, adenosine-mediated airway responsiveness and airway inflammation may be mediated through its specific cell surface G protein coupled adenosine receptors (Livingston et al., 2004). Four adenosine receptor subtypes, A1,A2A,A2B and A3 are expressed in the lung, have been cloned in humans, and have been investigated as potential targets for drug development in asthma (Livingston et al., 2004; Polosa, 2002; Rorke and Holgate, 2002). By activating adenosine A1 receptors on a number of different human cell types, adenosine produces bronchoconstriction, inflammation, increased endothelial cell permeability, and mucin production, which increase airflow obstruction in asthma (Bjorck et al., 1992; Cronstein et al., 1990, 1992; Ethier and Madison, 2006; Marquardt, 1997; McNamara et al., 2004; Salmon et al., 1993; Wilson and Batra, 2002). Moreover, in steroid-naïve patients with mild, atopic asthma, adenosine A1 receptors are upregulated in the bronchial epithelium and airway smooth muscle (Brown et al., 2005).
Taken together, these studies support that in human asthmatics adenosine is an important signaling molecule, that the adenosine A1 receptor is an important target, and that activation of adenosine A1 receptors on airway smooth muscle produces bronchoconstriction. However, the role of activation of adenosine A1 receptors on airway inflammation in asthma is not known.
Previously, it was reported in studies from this laboratory, that L-97-1, a novel adenosine A1 receptor antagonist, at a high dose (10 mg/kg) blocks early (bronchoconstrictor) and late (inflammatory) allergic responses, and bronchial hyper-responsiveness to histamine following house dust mite challenge in an allergic rabbit model of asthma by blocking adenosine A1 receptors (Obiefuna et al., 2005). In the present study, in this same rabbit model of allergic asthma, the effects of a lower dose of (1 mg/kg) L-97-1, on house dust mite-induced early allergic response, late allergic response, bronchial hyper-responsiveness to histamine, and airway inflammation were compared with a cysteinyl leukotriene-1 receptor antagonist, montelukast sodium (montelukast), an FDA approved oral anti-asthma drug widely prescribed for the treatment of asthma.
2. Materials and methods
2.1. Induction of allergic asthma in rabbits, lung dynamic compliance, and bronchial hyper-responsiveness measurements
A detailed description for the materials and methods for induction of allergic asthma in rabbits, lung dynamic compliance measurements, and measurement of bronchial hyper-responsiveness are described in a prior publication (Obiefuna et al., 2005). Briefly, inbred New Zealand White Pasturella-free rabbit littermates were bred and immunized intraperitoneally within 24 h of birth with house dust mite (Greer Laboratories, Lenoir, NC; 312 allergen units/animal suspended in 10% kaolin). The rabbits also received regular rabbit diet and water, ad libitum. The injections were repeated weekly for 4 weeks, biweekly for 2 months and then monthly until the end of the experiment.
Four months after birth, the rabbits were screened for histamine sensitivity by measuring lung dynamic compliance changes in response to aerosol administration of serial dilutions of histamine (0.17 mg/ml-20 mg/ml) with measurements of pulmonary function for 15 min after 2 min of aerosol for each dose. Animals were not exposed to higher doses of histamine after their PC30 (concentration in mg/ml required to produce 30% reduction in lung dynamic compliance). An initial saline aerosol was used to establish baseline bronchial hyper-responsiveness. Pulmonary function was summated every 10 breaths of even gradation. Data from spastic breathing were filtered out as artifact. The drug-induced response for each treatment was taken as the lowest consistent lung dynamic compliance value within 3 min of treatment with histamine. This point was typically achieved within the first 2 min following treatment with histamine. Allergic rabbits that do not attain a PC30 to histamine above 20 mg/ml were excluded from the study. Less than 1% of all sensitized rabbits did not attain this PC30 to histamine. All animal care and experimentation was approved and carried out in accordance with the East Carolina University Institutional Animal Care and Use Committee, and in accordance with the principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Effect of L-97-1 or montelukast sodium on allergen-induced early and late allergic responses
Sensitized rabbits exposed to allergen aerosol are susceptible to an allergic response characterized by a phasic lung dynamic compliance response. The early lung dynamic compliance response is due to bronchoconstriction (early allergic response) and the late lung dynamic compliance response is due to airway inflammation (late allergic response). The following procedure was designed to investigate the effects of L-97-1 or montelukast sodium (montelukast) on house dust mite allergen-induced early allergic response and late allergic response in the allergic rabbit model. Rabbits that had not been used for any airway provoking procedure were anesthetized and intubated as previously described (Obiefuna, et al., 2005). After a steady baseline respiration is attained, the animals were aerosolized with house dust mite (2500 allergen units) for about 10 min or until the allergen was exhausted. Pulmonary function (lung dynamic compliance) was then measured at 15-min intervals during the next 6 h to determine the effect of house dust mite on early (0-60 min) and late (120-360 min) allergic responses (early allergic response and late allergic response, respectively). The same procedure was repeated with L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) oral gavage administered 1 h before house dust mite challenge in the same animals following at least a 2-week washout period. Anesthesia was maintained with a mixture of ketamine/acepromazine (80:20) at a dose of 0.15 mg/kg given every 45 min-60 min.
Previously, it was reported that a high dose of L-97-1 (10 mg/kg) administered as an intragastric gavage 1 h prior to house dust mite, blocks early allergic response, late allergic response and bronchial hyper-responsiveness to histamine following house dust mite challenge in this allergic rabbit model of asthma by blocking adenosine A1 receptors (Obiefuna et al., 2005). Based on preclinical pharmacological and pharmacokinetic data, it is anticipated that a clinically relevant oral dose for L-97-1 in humans is approximately 1 mg/kg. In the present study, the effects of a lower dose of L-97-1 (1 mg/kg) administered as an oral gavage on house dust mite-induced early allergic response, late allergic response, bronchial hyper-responsiveness to histamine, and airway inflammation are compared to those of a clinically relevant oral dose for montelukast (0.15 mg/kg).
2.3. Effect of L-97-1 or montelukast sodium on allergen-induced bronchial hyper-responsiveness to histamine
To determine the effects of L-97-1 or montelukast on bronchial hyper-responsiveness, the following protocol was used. Allergic rabbits that had not been aerosolized with allergen or histamine for at least 2 weeks were aerosolized with histamine as described above to determine their baseline PC30 to histamine and bronchial hyper-responsiveness to histamine. Twenty-four hours later, the rabbits were given an aerosol challenge of house dust mite (2500 allergen units) and bronchial hyper-responsiveness measurement with histamine aerosolized challenge was repeated. The same protocol was used to test the effectiveness of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered as an oral gavage 1 h before house dust mite challenge on bronchial hyper-responsiveness to histamine.
2.4. Bronchoalveolar lavage (BAL)
In a separate set of experiments, allergic rabbits were challenged with house dust mite allergen (2500 allergen units) and cell counts in BAL fluid were measured at 6 and 24 h following house dust mite. Following a 2-3 week washout period, the same animals were treated with L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) orally as an oral gavage 1 h before house dust mite challenge. Cell counts in BAL fluid were measured again at 6 and 24 h following house dust mite to determine the effect of L-97-1 and montelukast on airway inflammation.
Three milliliters of normal saline was injected through the catheter into the lung via the endotracheal tube until it is gently “wedged” against an airway wall, and the return was suctioned into a sterile trap. The BAL fluid was placed into a polystyrene tube on ice. BAL fluid was centrifuged at 400 ×g for 6 min at 4 °C. After removing the supernatant, the BAL cells in the pellet were suspended in 1 ml of Phosphate Buffer Saline. The total cells were then counted manually in a hemocytometer chamber (Fisher). 1-5×103 cells were spun onto glass slides (Cytospin 3, Cytospin, Shandon, UK), air dried, fixed with methanol and stained with Diff-Quik stain set (DADE). A total of 200 cells were counted and classified as neutrophils, eosinophils, macrophages or lymphocytes based on standard morphologic criteria. The number of cells recovered per rabbit were calculated and expressed as mean ± S.E.M./ml for each group.
2.5. Plasma levels of L-97-1
An ear artery sample of blood was collected in tubes containing EDTA at 0 min, 15 min, 30 min, 1 h, 2 h, 6 h and 24 h following L-97-1 administration. The samples were centrifuged at 5000 ×g for 5 min and plasma was collected and frozen at -20 °C until used. Plasma levels of L-97-1 were measured by electrospray liquid chromatography/tandem mass spectrometry method validated at Prevalere Life Sciences, Inc. (Whitesboro NY).
2.6. Chemicals
All the reagents of analytical grade were purchased from Sigma Chemical Company (St. Louis, MO). L-97-1 (3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydropurine-2,6-dione) was custom synthesized by ChemSyn Laboratories (Lenexa KS) and provided by Constance N. Wilson, M.D., Chief Scientific Officer, Endacea, Inc. (Research Triangle Park, NC).
2.7. Statistical analysis
To assess the effect of L-97-1 or montelukast on the changes in pulmonary function after histamine and house dust mite challenge, the area under the curve in cm2 is digitized by computer assisted plenometry for each rabbit during a 6 h period following house dust mite challenge and at 24 h following histamine and house dust mite challenge. The early and late phase responses are determined at 0-60 min and 120-360 min, respectively, as previously established in this allergic rabbit model. The percentage change in lung dynamic compliance is calculated for each time point following house dust mite challenge (every 15 min for 6 h). Statistical significance in time series between the control and drug-treated groups were determined by two-way multiple ANOVA (MANOVA). Comparisons between control and test values at the same time point were determined by post-hoc comparison of two means. Bronchial hyper-responsiveness for histamine is calculated by determining the concentration of histamine (mg/ml) required to reduce the lung dynamic compliance by 30% from baseline (PC30). Histamine responses in house dust mite-challenged animals before and after L-97-1 or montelukast were compared using analysis of variance (ANOVA) with appropriate post-hoc analysis. Airway inflammation was assessed using BAL cell counts (Mean ± S.E.M./ml). Cell counts of house dust mite-challenged animals before and after L-97-1 or montelukast were compared utilizing the paired t-test. Results are expressed as Mean ± S.E.M. A value of P < 0.05 is considered significant.
3. Results
3.1. Effect of L-97-1 or montelukast on early allergic response and late allergic response
Fig. 1 shows the effect of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) oral administration on house dust mite-induced early allergic response and late allergic response. L-97-1-treated rabbits showed significantly higher lung dynamic compliance, up to 6 h compared to the untreated control group. The curve is significant at all time points after treatment with L-97-1 (P < 0.05; MANOVA). Montelukast treated animals showed a significant difference for lung dynamic compliance from control between 60-300 min time points (P < 0.05; MANOVA).
Fig. 1.
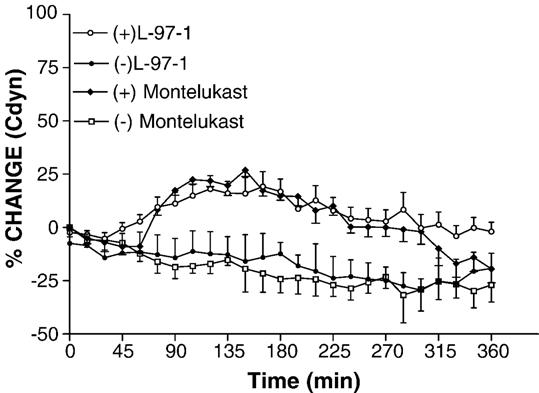
Lung dynamic compliance (Cdyn) in house dust mite (HDM) challenged rabbits with (+) and without (-) L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) treatment. House dust mite was administered to sensitized rabbits by aerosolization (2500 allergen units). L-97-1 or montelukast was administered by intragastric tube 1 h before house dust mite administration. Ventilation was monitored in the anesthetized rabbits up to 6 h after house dust mite challenge. The curve is significant at all time points after treatment with L-97-1 (n =4; P < 0.05; MANOVA). The montelukast curve showed significant difference from control between 60-300 min time points (n =5; P < 0.05; MANOVA). Data is expressed as Mean ± S.E.M.
3.2. Effect of L-97-1 or montelukast on bronchial hyper-responsiveness to histamine
Fig. 2 shows the effect of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) oral administration on house dust mite-induced bronchial hyper-responsiveness to histamine 24 h following house dust mite challenge in allergic rabbits. House dust mite challenge significantly reduced PC30 histamine to 1.88 ± 0.36 mg/ml (74.6% decrease from baseline) and 2.67 ± 0.89 mg/ml (63.9% decrease from baseline) 24 h following house dust mite challenge from baseline measurements for PC30 histamine of 7.38 ± 1.02 mg/ml and 7.33 ± 1.45 mg/ml (no house dust mite, no drug), for L-97-1 and montelukast treatment groups, respectively (P < 0.05; ANOVA). PC30 histamine significantly increased from 1.88 ± 0.36 mg/ml and 2.67 ± 0.89 mg/ml (24 h following house dust mite challenge with no drug, L-97-1 and montelukast, respectively), to 5.0 ± 1.22 mg/ml (167% increase 24 h following house dust mite challenge plus L-97-1; P < 0.05; ANOVA) and 4.5 ± 0.5 mg/ml (70 % increase 24 h following house dust mite challenge plus montelukast; P < 0.05; ANOVA).
Fig. 2.
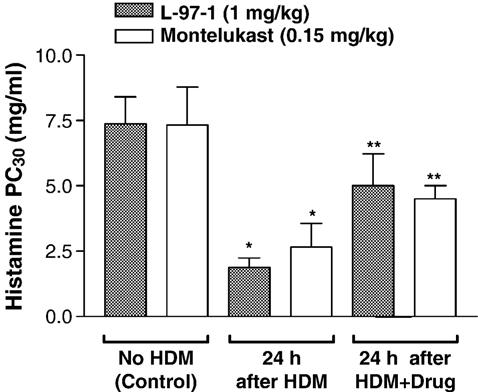
Histamine responses (Histamine PC30, mg/ml) in allergic rabbits aerosolized with house dust mite (HDM) allergen with or without L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered by intragastric tube 1 h before house dust mite challenge. Control measurement PC30 histamine was taken without house dust mite and without prior drug treatment. PC30 histamine was measured again 24 h after HDM (no drug treatment), after HDM + L-97-1 (n =4) or after HDM + montelukast treatment (n = 5). Data is expressed as Mean± S.E.M.*P<0.05 as compared to respective Control;**P<0.05 as compared to respective 24 h after HDM treatment (ANOVA).
3.3. Effect of L-97-1 or montelukast on airway inflammation
Both L-97-1 and montelukast significantly reduced bronchoalveolar lavage (BAL) eosinophils at 6 h and neutrophils at 6 and 24 h (P < 0.05; paired t-test) (Figs. 3 and 4, respectively). As opposed to montelukast, L-97-1 significantly reduced BAL lymphocytes at both 6 and 24 h (Fig. 5; paired t-test), while montelukast significantly reduced BAL macrophages at both 6 and 24 h (Fig. 6)(P < 0.05; paired t-test). In a separate group of animals without house dust mite challenge, repeat BAL did not significantly affect total leukocyte counts. BAL total leukocyte counts were not significantly different at 0, 6, or 24 h on day 1 or day 14 or at day 1 versus day 14 at 0, 6, and 24 h (n =4; ANOVA; data not shown).
Fig. 3.
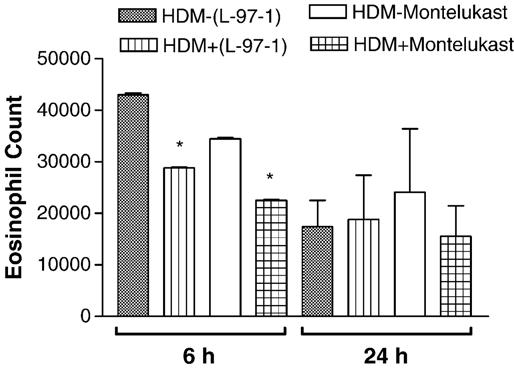
Effects of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered by intragastric tube 1 h before house dust mite (HDM) challenge on eosinophils in BAL fluid measured at 6 h and 24 h in allergic rabbits. Comparison is made between respective HDM-challenged animals without drug treatment (control) versus HDM plus L-97-1 (n = 5) or HDM plus montelukast (n = 5).*P<0.05 as compared to respective HDM-challenged group without drug (paired t-test). Data is expressed as Mean± S.E.M./ml.
Fig. 4.
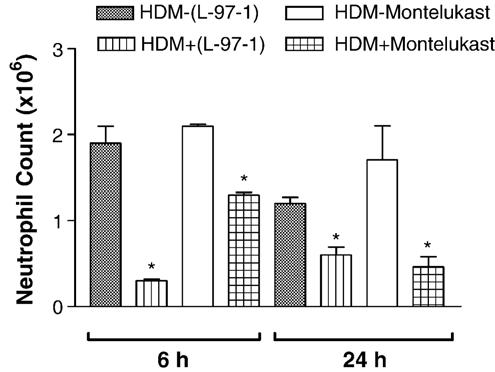
Effects of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered by intragastric tube 1 h before house dust mite (HDM) challenge on neutrophils in BAL fluid measured at 6 h and 24 h in allergic rabbits. Comparison is made between respective HDM-challenged animals without drug treatment (control) versus HDM plus L-97-1 (n = 5) or HDM plus montelukast (n = 5).*P<0.05 as compared to respective HDM-challenged group without drug (paired t-test). Data is expressed as Mean ± S.E.M./ml.
Fig. 5.
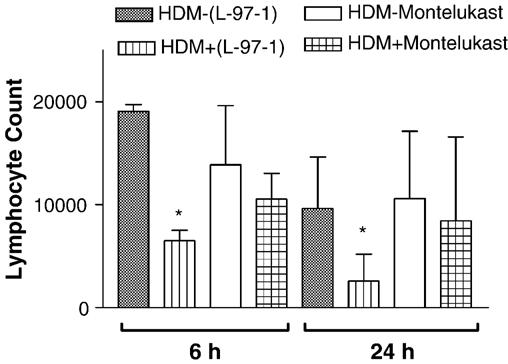
Effects of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered by intragastric tube 1 h before house dust mite (HDM) challenge on lymphocytes in BAL fluid measured at 6 h and 24 h in allergic rabbits. Comparison is made between respective HDM-challenged animals without drug treatment (control) versus HDM plus L-97-1 (n = 5) or HDM plus montelukast (n = 5).*P<0.05 as compared to respective HDM-challenged group without drug (paired t-test). Data is expressed as Mean ± S.E.M./ml.
Fig. 6.
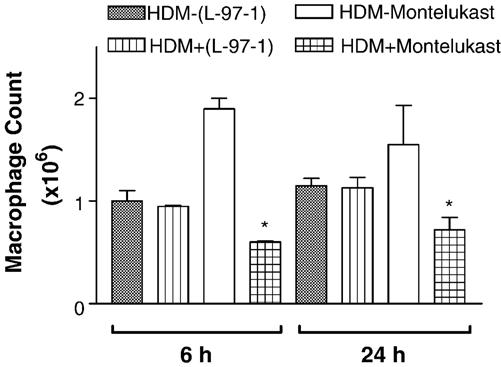
Effects of L-97-1 (1 mg/kg) or montelukast (0.15 mg/kg) administered by intragastric tube 1 h before house dust mite (HDM) challenge on macrophages in BAL fluid measured at 6 h and 24 h in allergic rabbits. Comparison is made between respective HDM-challenged animals without drug treatment (control) versus HDM plus L-97-1 (n = 5) or HDM plus montelukast (n = 5).*P<0.05 as compared to respective HDM-challenged group without drug (paired t-test). Data is expressed as Mean± S.E.M./ml.
3.4. Plasma levels of L-97-1
Table 1 shows the levels of L-97-1 in a 24 h period following a single oral administration of L-97-1 (1 mg/kg) in allergic rabbits. A plasma level of L-97-1 of (47.5 ± 20.5 ng/ml) blocks early allergic response at 30 min, a plasma level of (2.7 ng/ml) blocks late allergic response at 6 h, and a plasma level of L-97-1 (1.84 ± 0.83 ng/ml) blocks bronchial hyper-responsiveness at 24 h following house dust mite.
Table 1.
Plasma levels (ng/ml) of L-97-1 in a 24-h period following oral administration of L-97-1 (1 mg/kg) in allergic rabbits
| Sample | 15 min | 30 min | 1 h | 2 h | 6 h | 24 h |
|---|---|---|---|---|---|---|
| Mean | 48 | 47.5 | 32.1 | 8.02 | 2.7 | 1.84 |
| S.E.M. | 13.7 | 20.5 | 12.4 | 2.5 | 0 | 0.83 |
Data is presented as Mean+S.E.M., n=4.
4. Discussion
Previously, it was reported in studies from this laboratory, that L-97-1, a novel adenosine A1 receptor antagonist, at a high dose (10 mg/kg) blocks early allergic response, late allergic response and bronchial hyper-responsiveness to histamine following house dust mite in an allergic rabbit model of asthma by blocking adenosine A1 receptors (Obiefuna et al., 2005). In the present study, in this same rabbit model of allergic asthma, the effects of a lower (1 mg/kg), clinically relevant dose of L-97-1, on house dust mite-induced early allergic response, late allergic response, bronchial hyper-responsiveness to histamine, and airway inflammation following house dust mite were compared with a cysteinyl leukotriene-1 receptor antagonist, montelukast sodium (montelukast). Pretreatment with L-97-1 orally to allergic rabbits significantly improved lung dynamic compliance following house dust mite challenge at all time points up to 6 h following house dust mite. Thus, in this animal model of allergic asthma, this lower dose of L-97-1 blocks both the early allergic response and the late allergic response following house dust mite. Pretreatment with montelukast orally to allergic rabbits significantly improved lung dynamic compliance following house dust mite allergen challenge at time points between 60 to 300 min following house dust mite. Thus, in this allergic rabbit model of asthma, montelukast has no effect on early allergic response (less than 60 min) and blocks late allergic response following house dust mite.
Pretreatment with either L-97-1 or montelukast before house dust mite challenge also significantly reduced bronchial hyper-responsiveness to histamine 24 h following house dust mite and airway inflammation at 6 and 24 h following house dust mite in allergic rabbits. At 6 and 24 h following house dust mite, both L-97-1 and montelukast inhibited the neutrophil influx into the airways of allergic rabbits, although L-97-1 had a greater effect on neutrophil bronchoalveolar lavage (BAL) cell counts at 6 h than montelukast. Moreover, at 6 h, L-97-1 significantly reduced BAL lymphocyte and eosinophil cell counts and montelukast significantly reduced macrophage and eosinophil cell counts. Furthermore, at 24 h following house dust mite, L-97-1 significantly reduced lymphocyte and montelukast significantly reduced macrophage cell counts in BAL fluid of allergic rabbits. Pharmacokinetic profile of L-97-1 in rabbit plasma shows that L-97-1 produced these anti-inflammatory effects in the airways of allergic rabbits following house dust mite at plasma levels of approximately 2 ng/ml.
Airway hyper-responsiveness and airway inflammation following an inhalational challenge with an allergen are considered to be hallmarks of allergic asthma. Both allergic rabbits and allergic humans share features of asthma, such as airway hyperreactivity to adenosine, histamine, acetylcholine, platelet activating factor, development of inflammation and permeability changes in the airways, release of mediators including neutrophil and eosinophil chemotactic factors, production of antigen-specific IgE antibodies, and mast cell dependency (Gascoigne et al., 2003; Gozzard et al., 1997; Herd and Page, 1996; Larsen et al., 1984; Metzger et al., 1989). Moreover, adenosine levels are increased in the BAL fluid of both humans and rabbits with allergic asthma (Ali et al., 1992b; Driver et al., 1993). Because of these shared features of asthma in allergic rabbits and humans with asthma, the rabbit model of allergic asthma has been previously used to test anti-asthma drugs (Ali et al., 1992a,c, 1994a). Drugs, which currently are in use to treat human asthma, theophylline and beclomethasone, inhibit early allergic response and late allergic response, respectively in this rabbit model of allergic asthma (Ali et al., 1992c, 1994a). Furthermore, the results from the present study, wherein montelukast, a widely prescribed oral anti-asthma drug for humans, blocks late allergic response and airway inflammation in allergic rabbits, further supports that the allergic rabbit model simulates that of human asthma and is acceptable for preclinical testing of anti-asthma drugs.
The endogenous nucleoside, adenosine, produces acute bronchoconstriction through indirect effects by inducing the release of preformed and newly formed mediators from mast cells and possibly direct effects on airway smooth muscle and adrenergic nerve endings (Livingston et al., 2004; Polosa, 2002). Previously, it is reported that adenosine A1 receptors on airway smooth muscle are a direct target for adenosine in humans and allergic rabbits (Ali et al., 1994b; Brown et al., 2005; Mundell et al., 2001; Nyce and Metzger, 1997). In the present study, L-97-1 administered 1 h before house dust mite challenge significantly increased lung dynamic compliance at all time points up to 6 h, thus preventing the decline in lung dynamic compliance and blocking both early allergic response and late allergic response following house dust mite challenge in rabbits with allergic asthma. The increase in lung dynamic compliance following L-97-1 during the early allergic response can be explained by the direct blocking of adenosine A1 receptors on airway smooth muscle as some of the previous studies have suggested (Ali et al., 1994b; Nyce and Metzger, 1997). In small airways from allergic rabbits, the expression of the adenosine A1 receptor is increased compared to that in small airways from normal rabbits (Ali et al., 1994b). Moreover, EPI 2010, a respiratory antisense oligonucleotide to the human adenosine A1 receptor, reduces early allergic response and decreases the expression of adenosine A1 receptors in airway smooth muscle in allergic rabbits (Nyce and Metzger, 1997). In human bronchial smooth muscle cells, adenosine A1 receptors are coupled to pertussis-toxin sensitive G proteins and calcium signaling, which is an important intracellular signaling pathway that controls bronchomotor tone (Ethier and Madison, 2006). Furthermore, in adenosine A1 receptor knock-out mice, following inhalational challenge, 5’-N-ethylcarboxamidoadenosine produced a significantly lower enhanced pause indicative of airway resistance, compared to their wild type counterparts. In wild type animals, this inhibition of enhanced pause was significantly attenuated by the selective adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine before 5’-N-ethylcarboxamidoadenosine challenge suggesting a role for adenosine A1 receptor in acute bronchoconstriction in this adenosine A1 receptor knockout mouse model of allergic asthma (Oldenburg and Mustafa, 2004).
In addition to blocking adenosine A1 receptors on airway smooth muscle, L-97-1 may block early allergic response following house dust mite by blocking adenosine A1 receptors on mast cells and the release of preformed mediators, i.e., histamine (Forsythe et al., 1999; Peachell et al., 1988). Airway responses in allergic rabbits are mast cell dependent (Gascoigne et al., 2003; Gozzard et al., 1997; Larsen et al., 1984). Moreover, in mast cells obtained from human lung parenchyma, adenosine alone did not induce histamine release (Peachell et al., 1988). However, in immunologically activated human lung mast cells, adenosine and adenosine A1 receptor agonists, (R)-N6 -phenylisopropyladenosine and 5’-N-ethylcarboxamidoadenosine enhanced histamine release. Thus, in the present study, L-97-1 may block allergen-induced early allergic responses in allergic rabbits by directly blocking activation of adenosine A1 receptors on airway smooth muscle cells, and perhaps by blocking adenosine A1 receptors on mast cells and the release of preformed and newly formed mediators, i.e. histamine and cysteinyl leukotrienes. These effects of L-97-1 on immunologically sensitized mast cells, as well as, the effects of L-97-1 on blocking adenosine A1 receptors on adrenergic nerve terminals in allergic rabbits to determine the relative contribution of these important mechanisms to early allergic response following house dust mite are areas for future investigations.
Allergen causes the release of preformed mediators such as histamine and initiates metabolism of arachidonic acid leading to newly generated mediators such as cysteinyl leukotrienes (Lane and Lee, 1996). Moreover, activation of T cells, mast cells, and macrophages, to produce inflammatory cytokines after allergen challenge results in the recruitment of inflammatory cells into the airways, including eosinophils, monocytes and neutrophils that may contribute to both acute and chronic inflammation (Busse and Lemanske, 2001). In the present study, increased migration of inflammatory cells into the airways induced by house dust mite seems to involve the action of adenosine on adenosine A1 receptors, because L-97-1, a specific adenosine A1 antagonist, significantly decreased BAL inflammatory cell counts at 6 and 24 h following house dust mite. Activation of adenosine A1 receptors on human neutrophils and macrophages has been shown to produce pro-inflammatory effects (Cronstein et al., 1990, 1992; Salmon et al., 1993). The improvement in lung dynamic compliance at 6 h following house dust mite, i.e. attenuation of late allergic response by L-97-1 may be due to the inhibition of influx of inflammatory cells into the airways of allergic rabbits, since this improvement in lung dynamic compliance correlates with a reduction in BAL lymphocytes, eosinophils, and neutrophils at 6 h following house dust mite.
In the present study, montelukast also attenuated house dust mite-induced airway inflammation, significantly reducing BAL eosinophils at 6 h and neutrophils and macrophages at 6 and 24 h. This effect of montelukast on airway inflammation following house dust mite in allergic rabbits may be due to the blockade of leukotriene cysteinyl-1 receptors on inflammatory cells, since cysteinyl leukotrienes are known to act as chemoattractants for leukocyte trafficking into the airways (Fregonese et al., 2002; Spada et al., 1997) and also act in a paracrine and autocrine fashion on eosinophils and macrophages (Figueroa et al., 2001), thereby amplifying lung airway inflammation. The attenuation of late allergic response by montelukast in the present study may be due to inhibition of influx of inflammatory cells into the airways of allergic rabbits, since the improvement in lung dynamic compliance by montelukast from 60 min to 300 min following house dust mite correlates with a reduction in BAL eosinophils, neutrophils, and macrophages.
Combined inhibition of both early allergic response and late allergic response following house dust mite in allergic rabbits by L-97-1 could be due to a diverse antagonism of adenosine A1 receptors found on a number of cell types, including antigen presenting cells, mast cells, airway smooth muscle cells, lymphocytes, neutrophils, and macrophages which actively participate in these responses after allergen challenge (Bjorck et al., 1992; Cronstein et al., 1990, 1992; Forsythe et al., 1999; Marquardt, 1997; Panther et al., 2001; Salmon et al., 1993). The effect of L-97-1 on early allergic response suggests that activation of adenosine A1 receptors is an important early event in the cascade of mediator release and inflammatory cell infiltration of airways compared to the effect of montelukast on the late allergic response, which suggests that activation of cysteinyl leukotriene-1 receptor appears to be a later event in this cascade following house dust mite challenge. Moreover, in addition to their direct effects on inflammatory cells described above, L-97-1 and montelukast may block late allergic response and airway inflammation following house dust mite by preventing the release of chemoattractants and further amplification of the initial signal following house dust mite. Previously, it was reported that adenosine A1 receptor antagonists, 1,3-dipropyl-8-p-sulfophenylxanthine and 1,3-dipropyl-8-cyclopentylxanthine significantly reduced the chemoattractant response of neutrophils to formyl-Met-Leu-Phe, which induced the release of adenosine sufficient to activate adenosine A1 receptors (Forman et al., 2000).
Airway inflammation is a characteristic feature of asthma and likely contributes to bronchial hyper-responsiveness following allergen challenge. Influx of inflammatory cells into the airways may be responsible for the activation and liberation of inflammatory mediators such as reactive oxygen species and cysteinyl leukotrienes that can cause increased bronchial hyper-responsiveness to spasmogens (Barnes et al., 1998). In this study, L-97-1 attenuates bronchial hyper-responsiveness to histamine following house dust mite, possibly through blocking the activation of adenosine A1 receptors by adenosine on mast cells, lymphocytes, neutrophils, and macrophages which are coupled to the release of both preformed mediators such as histamine and newly formed mediators, i.e. cysteinyl leukotrienes, as well as, release of cytokines from lymphocytes and reactive oxygen species from neutrophils which contribute either directly or indirectly via their chemoattractant effects on inflammation and increase bronchial hyper-responsiveness. Montelukast significantly attenuated bronchial hyper-responsiveness to histamine following house dust mite although to a lesser extent than that seen with L-97-1 (70%, montelukast versus 167%, L-97-1). These effects of montelukast on bronchial hyper-responsiveness are consistent with the downstream effects of cysteinyl leukotrienes on cysteinyl leukotriene-1 receptor activation in the cascade of mediator release and inflammation in asthma.
In this study, the effects of L-97-1 on early allergic response, late allergic response, bronchial hyper-responsiveness to histamine, and airway inflammation following house dust mite challenge are not due to the inhibition of phosphodiesterase class of enzymes, since the plasma levels of L-97-1 after oral administration are too low to cause inhibition of phosphodiesterase enzymes (Obiefuna et al., 2005). Compared to montelukast, following house dust mite, the effect of L-97-1 was greater on early allergic response, bronchial hyper-responsiveness to histamine and lymphocyte BAL cell counts at 6 and 24 h. The effects of L-97-1 on BAL lymphocytes in these studies suggest that as opposed to montelukast, L-97-1 may block the Th2 lymphocyte response and cytokine release, i.e. IL-4, IL-5, and IL-13, associated with asthmatic airway responses following allergen challenge.
L-97-1 is a novel adenosine A1 receptor antagonist which blocks house dust mite-induced acute airway bronchoconstrictor responses and airway inflammation in allergic rabbits by blocking adenosine A1 receptors and may be beneficial as an oral drug in the treatment of human asthma. In humans, activation of adenosine A1 receptors in human airway epithelial cells increased expression of MUC 2 mucin gene and MUC 2 is upregulated in asthmatic airways, suggesting that activation of adenosine A1 receptors in human asthmatic airways produces increased mucin production. (McNamara et al., 2004). Moreover, activation of adenosine A1 receptors induces angiogenesis, a hallmark of airway remodeling of human asthma (Clark et al., 2004). In addition to blocking acute bronchoconstriction and airway inflammation following allergen challenge, adenosine A1 receptor antagonists may provide additional beneficial effects in the treatment of asthma including blocking of adenosine A1 receptor-induced increase in MUC 2 in human asthmatic airway epithelium and mucin production in asthmatics, blocking the release of Th2 lymphocyte cytokines, i.e. IL-13, and angiogenesis which play important roles in airway remodeling of asthma (Clark et al., 2004; Cohn et al., 2004; McNamara et al., 2004). The effects of L-97-1 on MUC 2 gene expression and mucin production, Th2 lymphocyte cytokine release, and airway remodeling are areas for future investigations.
Acknowledgements
Supported by North Carolina Biotechnology Center Kenan Award CFA #2000 CFG 8002 and STTR Phase I Grant # HL070458.
References
- Ali S, Mustafa SJ, Metzger WJ. Adenosine-induced bronchoconstriction in an allergic rabbit model: antagonism by theophylline aerosol. Agents Actions. 1992a;37:165–167. doi: 10.1007/BF02028098. [DOI] [PubMed] [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Allergen-induced release of adenosine in bronchoalveolar lavage (BAL) fluid in allergic rabbits: effect of anti-asthmatic drugs. J. Allergy Clin. Immunol. 1992b;89(Suppl 2):163. [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Modification of allergen-induced airway obstruction and bronchial hyper-responsiveness in the allergic rabbit by theophylline aerosol. Agents Actions. 1992c;37:68–170. doi: 10.1007/BF02028099. [DOI] [PubMed] [Google Scholar]
- Ali S, Mustafa SJ, Knox C, Metzger WJ. Effects of beclomethasone on allergen-induced airway obstruction, bronchial hyper-responsiveness, and cell infiltration in allergic rabbits. J. Allergy Clin. Immunol. 1994a;93:186. [Google Scholar]
- Ali S, Mustafa SJ, Metzger WJ. Adenosine-induced bronchoconstriction and contraction of airway smooth muscle from allergic rabbits with late-phase airway obstruction: evidence for an inducible adenosine A1 receptor. J. Pharmacol. Exp. Ther. 1994b;268:1328–1334. [PubMed] [Google Scholar]
- American Lung Association . National Center for Health Statistics. Report of Final Mortality Statistics; 2001. [Google Scholar]
- American Lung Association . National Center for Health Statistics. Raw Data from the National Health Interview Survey; 2002. [Google Scholar]
- American Lung Association . National Heart, Lung, and Blood Institute Chartbook, U.S. Department of Health and Human Services; 2004. [Google Scholar]
- Barnes PJ, Fan CK, Page CP. Inflammatory mediators of asthma: an update. Pharmacol. Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr. Opin. Allergy Clin. Immunol. 2004;4:191–195. doi: 10.1097/00130832-200406000-00009. [DOI] [PubMed] [Google Scholar]
- Bjorck T, Gustafsson LE, Dahlen SE. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am. Rev. Respir. Dis. 1992;145:1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- Brown RA, Clarke GW, Hurle MJ, Denyer JC, Savage TJ, Ratoff JC, Ledbetter CL, Murdoch RD, O'Connor BJ, Page CP, Spina D. Immunolocalization of adenosine A1 receptors in asthmatic bronchial biopsy sections. Am. J. Respir. Crit. Care Med. 2005;171:A805. [Google Scholar]
- Busse WW. Leukotrienes and inflammation. Am. J. Respir. Crit. Care Med. 1998;157:S210–S213. [PubMed] [Google Scholar]
- Busse WW, Lemanske RF. Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Clark A, Youkey R, Jia L, Sullivan G, Linden J, Tucker A. Stimulation of angiogenesis through adenosine A1 receptor activation: possible role of vascular endothelial growth factor release from mononuclear cells; 4th International Symposium of Nucleosides and Nucleotides; Purines. Chapel Hill, NC. 2004. [Google Scholar]
- Cohn L, Elis JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J.Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Currie GP, Jackson CM, Lee DK, Lipworth BJ. Allergen sensitization and bronchial hyper-responsiveness to adenosine monophosphate in asthmatic patients. Clin. Exp. Allergy. 2003;33:1405–1408. doi: 10.1046/j.1365-2222.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br. J. Clin. Pharmacol. 1983;15:161–165. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meer G, Heederik D, Postma DS. Bronchial responsiveness to adenosine 5-monophosphate (AMP) and methacholine differ in their relationship with airway allergy and baseline FEV1. Am. J. Respir. Crit. Care Med. 2002;165:327–331. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am. J. Resp Cell Mol. Biol. 2006 doi: 10.1165/rcmb.2005-0290OC. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa DJ, Breyer RM, Defoe SK, Kargman S, Daugherty BL, Waldburger K, Liu Q, Clements M, Zheng Z, O'Neill GP, Jones TR, Lynch KR, Austin CP, Evans JF. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am. J. Respir. Crit. Care Med. 2001;163:226–233. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- Forman MB, Vitola JV, Velasco CE, Murray JJ, Raghvendra KD, Jackson EK. Sustained reduction in myocardial reperfusion injuy with an adenosine receptor antagonist: possible role of the neutrophil chemoattractant response. J. Pharmacol. Exp. Ther. 2000;292:929–938. [PubMed] [Google Scholar]
- Forsythe P, McGarvey LPA, Heaney LG, MacMahon J, Ennis M. Adenosine induces histamine release from human bronchoalveolar lavage mast cells. Clin. Sci. 1999;96:349–355. [PubMed] [Google Scholar]
- Fregonese L, Silvestri M, Sabatini F, Rossi GA. Cysteinyl leukotrienes induce human eosinophil locomotion and adhesion molecule expression via a CysLT1 receptor-mediated mechanism. Clin. Exp. Allergy. 2002;32:745–750. doi: 10.1046/j.1365-2222.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- Gascoigne MH, Holland K, Page CP, Shock A, Robinson M, Foulkes R, Gozzard N. The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm. Pharmacol. Ther. 2003;16:279–285. doi: 10.1016/S1094-5539(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Gozzard N, Kingaby R, Higgs G, Hughes B. Characterization of antigen-induced airway responses in the neonatally immunized rabbit. Am. J. Resp. Crit. Care Med. 1997;155:A163. [Google Scholar]
- Herd CM, Page CP. The rabbit model of asthma and late asthmatic response. In: Raeburn D, Giembycz MA, editors. Airways Smooth Muscle: Modeling the Asthmatic Response in Vivo. Birkhauser Verlag Basel; Switzerland: 1996. pp. 147–169. [Google Scholar]
- Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J. Allergy Clin. Immunol. 2003;111:S18–S34. doi: 10.1067/mai.2003.25. [DOI] [PubMed] [Google Scholar]
- Johnson HG, McNee ML. Secretogogue responses of leukotriene C4,D4: comparison of potency in canine trachea in vivo. Prostaglandins. 1983;25:237–243. doi: 10.1016/0090-6980(83)90107-7. [DOI] [PubMed] [Google Scholar]
- Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J. Asthma. 2003;40:93–101. doi: 10.1081/jas-120017212. [DOI] [PubMed] [Google Scholar]
- Lane SJ, Lee TH. Mast cell effector mechanisms. J. Allergy Clin. Immunol. 1996;98:S67–S72. doi: 10.1016/s0091-6749(96)80131-x. [DOI] [PubMed] [Google Scholar]
- Larsen GL, Shampain MP, Marsh WR, Behrens BL. An animal model of the late asthmatic response to antigen challenge. In: Kay AB, Austen KF, Lichtenstein LM, editors. Asthma: Physiology, Immunopharmacology, and Treatment. Academic Press; London UK: 1984. pp. 245–259. [Google Scholar]
- Livingston M, Heaney LG, Ennis M. Adenosine, inflammation, and asthma — a review. Inflamm. Res. 2004;53:171–178. doi: 10.1007/s00011-004-1248-2. [DOI] [PubMed] [Google Scholar]
- Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfiled E, Williams DL, Jr., Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Marquardt DL. Adenosine. In: Barnes PJ, Grunstein MM, Leff AR, Wool Cock AJ, editors. Asthma. Lipincott-Raven Publishers; Philadelphia, PA: 1997. pp. 585–591. [Google Scholar]
- McNamara N, Gallup M, Khong A, Sucher A, Maltseva I, Fahy JV, Basbaum C. Adenosine up-regulation of the mucin gene, MUC2, in asthma. FASEB J. 2004;18:1770–1772. doi: 10.1096/fj.04-1964fje. [DOI] [PubMed] [Google Scholar]
- Metzger WJ, Brown L, Sjoerdsma K, Mustafa SJ. Bronchial hyperreactivity in a rabbit model by allergic asthma: responses to histamine, acetylcholine and platelet activating factor. In: Holme G, Morley J, editors. PAF in Asthma. Academic Press; New York: 1989. pp. 347–354. [Google Scholar]
- Mundell SJ, Olah ME, Panettieri RA, Jr., Benovic JL, Penn RB. Regulation of G protein-coupled receptor-adenylyl cyclase responsiveness in human airway smooth muscle by exogenous and autocrine adenosine. Am. J. Respir. Cell Mol. Biol. 2001;24:155–163. doi: 10.1165/ajrcmb.24.2.4243. [DOI] [PubMed] [Google Scholar]
- Nyce JW, Metzger WJ. DNA antisense therapy for asthma in an animal model. Nature. 1997;385:721–725. doi: 10.1038/385721a0. [DOI] [PubMed] [Google Scholar]
- Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, Mustafa SJ. A Novel A1 adenosine receptor antagonist (L-97-1) reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J. Pharmacol. Exp. Ther. 2005;315:329–336. doi: 10.1124/jpet.105.088179. [DOI] [PubMed] [Google Scholar]
- Oldenburg PJ, Mustafa SJ. Involvement of A1 adenosine receptors in adenosine induced bronchoconstriction in an allergic mouse model. Am. J. Respir. Crit. Care Med. 2004;169:A197. [Google Scholar]
- Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- Peachell PT, Columbo M, Kagey-Sobotka A, Lichtenstein LM, Marone G. Adenosine potentiates mediator release from human lung mast cells. Am. Rev. Respir. Dis. 1988;138:1143–1151. doi: 10.1164/ajrccm/138.5.1143. [DOI] [PubMed] [Google Scholar]
- Polosa R. Adenosine-receptor subtypes: their relevance to adenosinemediated responses in asthma and chronic obstructed pulmonary disease. Eur. Respir. J. 2002;20:488–496. doi: 10.1183/09031936.02.01132002. [DOI] [PubMed] [Google Scholar]
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast clinical research study group. Arch. Intern. Med. 1998;158:1213–1220. doi: 10.1001/archinte.158.11.1213. [DOI] [PubMed] [Google Scholar]
- Rorke S, Holgate ST. Targeting adenosine receptors: novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Med. 2002;1:99–105. doi: 10.1007/BF03256599. [DOI] [PubMed] [Google Scholar]
- Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen BX, Erlanger BF. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J. Immunol. 1993;151:2775–2785. [PubMed] [Google Scholar]
- Sin DD, Man J, Sharpe H, Gan WQ, Man SFP. Pharmacological management to reduce exacerbations in adults with asthma. JAMA. 2004;292:367–376. doi: 10.1001/jama.292.3.367. [DOI] [PubMed] [Google Scholar]
- Spada CS, Krauss AH, Nieves AL, Woodward DF. Effects of leukotrienes B4 (LTB4) and D4 (LTD4) on motility of isolated normodense human eosinophils and neutrophils. Adv. Exp. Med. Biol. 1997;400:699–706. [PubMed] [Google Scholar]
- Vizi E, Huszar E, Csoma Z, Boszormenyi-Nagy G, Barat E, Horvath I, Herjaveez I, Kollai M. Plasma adenosine concentration increases during exercise: a possible contributing factor in exercised-induced bronchoconstriction in asthma. J. Allergy Clin. Immunol. 2002;109:446–448. doi: 10.1067/mai.2002.121955. [DOI] [PubMed] [Google Scholar]
- Wilson CN, Batra VK. Lipopolysaccharide binds to and activates A1 adenosine receptors on human pulmonary artery endothelial cells. J. Endotoxin Res. 2002;8:263–271. doi: 10.1179/096805102125000470. [DOI] [PubMed] [Google Scholar]


