Abstract
The interaction structure of mutualistic relationships, in terms of relative specialization of the partners, is important to understanding their ecology and evolution. Analyses of the mutualistic interaction between anemonefish and their host sea anemones show that the relationship is highly nested in structure, generalist species interacting with one another and specialist species interacting mainly with generalists. This supports the hypothesis that the configuration of mutualistic interactions will tend towards nestedness. In this case, the structure of the interaction is at a much larger scale than previously hypothesized, across more than 180° of longitude and some 60° of latitude, probably owing to the pelagic dispersal capabilities of these species in a marine environment. Additionally, we found weak support for the hypothesis that geographically widespread species should be more generalized in their interactions than species with small ranges. This study extends understanding of the structure of mutualistic relationships into previously unexplored taxonomic and physical realms, and suggests how nestedness analysis can be applied to the conservation of obligate species interactions.
Keywords: Amphiprion, Anthozoa, coral reef, mutualism, nestedness theory, Premnas
1. Introduction
Ecological relationships between organisms are rarely, if ever, random because they are constrained by multiple interacting factors, including, in its broadest sense, the coevolutionary history of the interacting organisms. Identifying local, regional and global patterns of interaction, and their underlying causes, is therefore important for understanding the ecology and evolution of these associations. Ever-more-sophisticated statistical analytical tools are being developed to aid in the task of answering questions such as: how do species interactions affect community structure? What determines the level of specificity of an interaction? What controls the geographical occurrence of interacting species?
Recent research has extended a body of ecological theory and analysis termed ‘nestedness theory’ from its original application in the field of landscape ecology and conservation biology to consideration of patterns of mutualistic species interactions within communities (Bascompte et al. 2003; Dupont et al. 2003; Ollerton et al. 2003; Thompson 2005; Guimarães et al. 2006; Jordano et al. 2006; Lewinsohn et al. 2006). Nestedness theory, which has its origins in earlier work on island biogeographic theory, deals with the presence or the absence of species in archipelagos of islands, habitat fragments and nature reserves. Typically, within a region the species present in the smaller areas are a subset of those found in the larger areas (Patterson 1990; Atmar & Patterson 1993). The more recent work on mutualistic community structure has shown that interactions such as biotic pollination and seed dispersal are usually nested in terms of interspecific ecological specialization, i.e. how many partner species interact with any given species (Waser & Ollerton 2006). These analyses have used classical nestedness theory in a novel way to show quantitatively that at least some mutualistic associations are asymmetrical in composition. Characteristically, a core of generalist species interact with one another, while specialists interact with generalists; therefore, within networks of interacting species, reciprocal reliance of species is very rare, in relation to both the identity of the interacting partners and the strength of the interaction between them (Bascompte et al. 2006; for an alternative approach that has reached fundamentally the same conclusions see Vázquez & Aizen (2003, 2006)).
The degree of nestedness within an interaction dataset refers to the extent to which species used by specialists are subsets of species used by generalists. This can easily be tested by ranking the partner species according to the number of species with which they interact. Perfectly nested interactions fill the matrix with a triangle of positive interactions running from bottom left to top right of the matrix (table 1). In actual datasets, this triangular pattern may be strongly suggested, though not perfectly realized (Bascompte et al. 2003; Dupont et al. 2003; Ollerton et al. 2003; Jordano et al. 2006).
Table 1.
A hypothetical interaction matrix showing a perfectly nested set of species relationships. In this example, species which interact are coded 1, those which do not are designated 0. Species are ranked (top to bottom and left to right) from most ecologically generalized (large numbers of partners) to most ecologically specialized (small number of partners). Specialists interact with species which are a subset of those with which the generalists interact.
| species 1 | species 2 | species 3 | species 4 | species 5 | species 6 | species 7 | |
|---|---|---|---|---|---|---|---|
| species a | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| species b | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| species c | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| species d | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| species e | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| species f | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| species g | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
How common are nested patterns of mutualistic interactions and over what geographical scale do the patterns persist? Bascompte et al. (2003) and Thompson (2005) have hypothesized that mutualistic relationships are generally (perhaps always?) characterized by nested interactions, as opposed to random or compartmentalized patterns, because the relatively stable set of resources provided by the core of generalist–generalist associations can allow a larger number of specialized interactions to persist (henceforth the ‘nestedness hypothesis’). Support for this hypothesis to date has been provided only by local community studies of a narrow range of terrestrial mutualisms: plant–pollinator; animal–seed dispersal; and ant–plant relationships (Bascompte et al. 2003, 2006; Dupont et al. 2003; Ollerton et al. 2003; Guimarães et al. 2006; Jordano et al. 2006). These interactions are service–resource mutualisms sensu Ollerton (2006) involving plants and animals, and may therefore not be representative of the many categories of reciprocal positive interactions that exist, including resource–resource and service–service forms of ‘Biological Barter’ (Boucher 1985; Smith & Douglas 1987; Reisser 1992; Douglas 1994; Paracer & Ahmadjian 2000; Ollerton 2006).
To test the nestedness hypothesis fully, we require empirical tests of patterns of species associations from a wide range of mutualistic relationships, in aquatic as well as terrestrial habitats, and across larger geographical scales. For species with a potentially wide geographical range such as pelagic fish and invertebrates, we would hypothesize that non-random associations might hold across regions. Good data with which to test these hypotheses in the marine biome are scarce. However, many years of study by two of the authors (D.G.F. and G.R.A.), combined with records from the literature, has produced such a dataset for the entire distributional range of anemonefish and their host anemones, an obligate marine mutualism (Fautin & Allen 1997), making it a possibly unique data resource with which to explore hypotheses concerning patterns of mutualistic interaction in the ocean environment.
Anemonefish, which are members of the genera Amphiprion and Premnas (Perciformes: Pomacentridae: Amphiprioninae), comprise a monophyletic clade of some 26 currently recognized species. Two of the 28 species listed by Fautin & Allen (1997) are now recognized as natural hybrids: both Amphiprion leucokranos and Amphiprion thiellei seem to represent variants of crosses between Amphiprion chryopterus and Amphiprion sandaracinos (D. G. Fautin & G. R. Allen, unpublished data), and such hybrids can be experimentally created in captivity (Carlson 1996). All anemonefish obligately associate with one or more of 10 species of sea anemones (Anthozoa: Actiniaria) belonging to three unrelated families (Dunn 1981; Fautin & Allen 1997; Elliott et al. 1999). This mutualism extends from the east coast of Africa and the Red Sea through the Indian Ocean to the western Pacific, and from southeastern Australia to the latitude of Tokyo (figure 1). The relationship clearly benefits the fish, which are protected from predators by the tentacles of the anemone that are otherwise lethal to most fish due to their nematocysts; anemonefish eggs, which are laid beside the anemone, are likewise protected. Earlier observations and more recent experimental evidence suggest that the relationship is mutualistic for at least some species pairs (Fautin 1991; Holbrook & Schmitt 2005). Anemones of some species are protected against anemone predators, such as butterflyfishes (Godwin & Fautin 1992), and the ammonia excreted by the fish may be used by zooxanthellae, the symbiotic dinoflagellates that live within the cells of the anemones (Porat & Chadwick-Furman 2004, 2005). The biologically complex fish–anemone interaction is therefore mediated to some extent by a third mutualistic partner, the dinoflagellate (Fautin 1991). In the terminology of Ollerton (2006), the relationship is in part a service–resource (physical protection–inorganic nutrients) mutualism and in part a service–service (protection–protection) mutualism.
Figure 1.
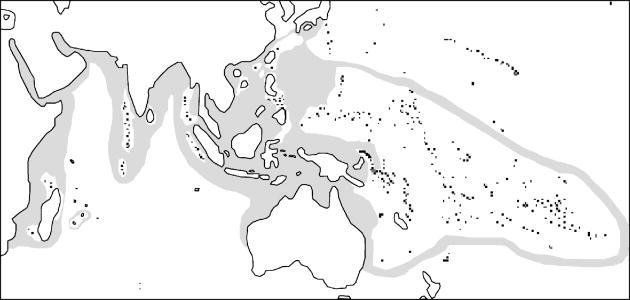
Geographical range of the anemonefish–anemone mutualism. Based on distribution maps in Fautin & Allen (1997).
The anemonefish–host mutualism has been used as a model system with which to explore several aspects of the ecology and evolution of mutualistic relationships, such as the evolution of partner specificity and host selection (Arvedlund et al. 1999; Elliott et al. 1999) and meta-population theory and species coexistence (Hattori 1995, 2002; Schmitt & Holbrook 2003). One reason for this is because, unusually for a geographically widespread mutualism, the pattern of host use by anemonefish is relatively completely known (Fautin & Allen 1997). We used this dataset to test the hypothesis that, over the entire range of the distribution of the mutualism, the pattern of anemone use by the fish follows a pattern of nested subsets: generalist fish interact with generalist and specialist anemones; generalist anemones interact with generalist and specialist fish; and specialist–specialist interactions are rare.
We also tested a second hypothesis, suggested initially by Fautin & Allen (1997) with respect to the widespread generalist Amphiprion clarkii, that the range of widespread anemonefish is related to their ability to colonize a relatively large number of host anemones. Although A. clarkii may be widespread because it can use many host species, it is also possible that it uses many hosts because it is widespread. Distributional range data from Fautin & Allen (1997) were used to differentiate between these possibilities and to test the hypothesis more rigorously.
2. Material and methods
Data on host anemone use by amphiprionine anemonefish were taken from Fautin & Allen (1997), supplemented by information obtained during the past decade by D.G.F., including correction of an error in Fautin & Allen (1997): Macrodactyla doreensis is a host of Amphiprion chrysopterus, not Amphiprion chrysogaster. Data will be uploaded to the Interaction Web Database hosted by the National Center for Ecological Analysis and Synthesis, at the University of California, Santa Barbara, USA (http://www.nceas.ucsb.edu/interactionweb/). All data are for natural occurrences; in captivity, many anemonefish can live with host anemones that we have never seen them inhabit in nature (indeed, in captivity some anemonefish will take up residence in European or temperate Pacific anemones; Fautin & Allen 1997).
A binary matrix of naturally interacting species was subjected to nestedness analysis using the Aninhado software (http://www.guimaraes.bio.br/softwares.html; Guimarães & Guimarães 2006). Aninhado is based on the Nestedness Calculator from AICS Research Inc. (http://www.aics-research.com/research/index.html; Atmar & Patterson 1993), but extends the utility of the previous software by incorporating more realistic null models of species interactions into the analysis. These null models place restrictions on the likelihood of pairs of species interacting, based upon the aspects of their ecology which can be determined from the interaction matrix, such as how specialized a species is (see discussion by Bascompte et al. 2003). The statistic used to describe the degree of fit to the nested ideal is the matrix ‘temperature’ (defined as the ‘heat of disorder’, i.e. the degree of order or disorder in the matrix, relative to the idealized, perfectly nested pattern). A matrix temperature of 0° indicates a perfectly nested pattern and 100° a completely random pattern (Atmar & Patterson 1993). The probability that this pattern, and the resultant matrix temperature, is non-random was calculated using Monte Carlo simulations of the original dataset. Aninhado calculated 1000 randomizations for each of the four null models: ER, interactions randomly assigned to each cell (the unrestricted model of the Nestedness Calculator); CO, interactions randomly assigned within columns; LI, interactions randomly assigned within rows; and CE, generalization of each species approximately equal to that observed in the data matrix. We present the results only for the CE null model as this is the most biologically realistic (Bascompte et al. 2003). However, all the null models generated the same result.
Based on known distributional ranges, we categorized the species of fishes and host anemones as widespread, regional or local. Widespread species have distributions across ocean regions such as both the western Pacific and Indian oceans (A. clarkii), or in both the eastern and the western Indian Ocean (Amphiprion akallopisos and Amphiprion sebae); regional species are restricted to an area such as the Indo-Malaysian Archipelago (e.g. Amphiprion frenatus); local species are fish restricted to island groups (e.g. A. chrysogaster from Mauritius) or particular coastal areas (e.g. Amphiprion omanensis from the Arabian Peninsula coast). Since field data are relatively lacking for A. chagosensis, it was excluded from these analyses.
3. Results
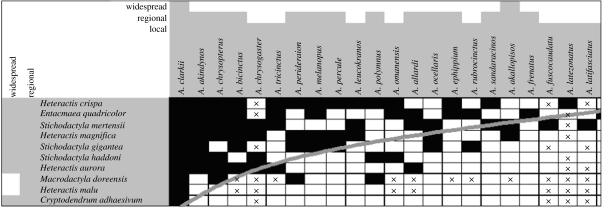
Anemonefish of the subfamily Amphiprioninae vary in host specialization by an order of magnitude, from the most generalist species, A. clarkii, which forms associations with all 10 species of host anemones, to strict specialists such as Amphiprion nigripes and Premnas biaculeatus, which associate with only one anemone species (Fautin & Allen 1997). The regional pattern of interactions between these fishes and their hosts is highly statistically significantly nested: generalist fish interact with generalist and specialist anemones; specialist fish interact with generalist anemones; and generalist anemones interact with generalist and specialist fish (table 2). The system temperature of the matrix of host–fish interactions is 16.8°; the probability of obtaining this pattern by chance is p≪0.00001.
Table 2.
Interaction matrix between amphiprionine anemonefish (columns) and their sea anemone hosts (rows). Filled squares indicate a known interaction. The grey line is the isocline for this dataset, above which all interactions would lie in a fully nested matrix. The extent of the grey shading around the species names indicates whether the species is local, regional or widespread in distribution. An X in a cell indicates that the fish and the anemone do not overlap in their distributional ranges (n=43 out of 137 zero-interaction cells). Note that M. doreensis is a host of A. chrysopterus and not A. chrysogaster as incorrectly recorded in Fautin & Allen (1997).
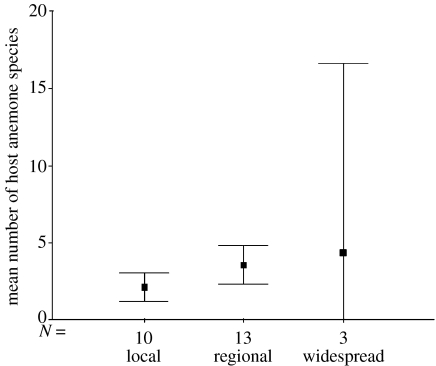
Two of the anemone hosts are regionally distributed and the rest are widespread; however, the fish range from extremely widespread to very local (Fautin & Allen 1997). There is no clear pattern to the distributional range of the fish in relation to the level of host specialization, except perhaps a tendency for specialist fish to be locally distributed, and for them to be more frequently found outside of the distributional range of potential hosts (table 2). Widespread fish include extreme generalists (A. clarkii) and moderate to strict specialists (A. akallopisos and A. sebae), while fish with local distributions can be moderate generalists (e.g. A. chrysogaster and Amphiprion tricinctus) or strict specialists (e.g. Amphiprion fuscocaudatus and A. nigripes). There is no statistically significant difference between the mean number of anemone hosts used by the local, regional and widespread species (figure 2; univariate general linear model: F2,26=1.74, p=0.198). However, when we remove the small sample of widespread species from the analysis, there is a marginally statistically significant difference between local and regional fish species, with locally restricted species having fewer hosts than regional species (figure 2; univariate general linear model: F1,23=3.8, p=0.064).
Figure 2.
The relationship between fish specialization on anemone hosts and distributional range (local, regional or widespread). N=number of fish species in each range category. Bars show 95% confidence limits.
4. Discussion
Ecological theory relating to the community structure of interacting mutualists is more advanced for plant–animal interactions than for animal–animal interactions. Lewinsohn et al. (2006) developed an analytical framework within which to search for pattern and process in plant–animal interaction assemblages, and stressed the importance of assessing for a range of non-random interaction structures (e.g. compartmentalization or gradients) rather than focussing on a single ‘expected’ pattern. Our results show clearly that the anemonefish and their anemone hosts interact in a non-random, highly structured and predictable manner across a very wide geographical region—more than 180° of longitude and some 60° of latitude. There is no suggestion of structure within table 2 other than nested subsets of interactions, with specialist fish and anemones using generalist partners, and generalist fish and anemones associating with both specialist and generalist partners. Further analyses and ordination of the data do not support other patterns of interaction (results not shown). The highly statistically significantly nested arrangement of an animal–animal tropical marine interaction in table 2 supports the hypothesis that nestedness is a feature of most, or possibly all, mutualistic relationships (Bascompte et al. 2003; Thompson 2005).
The maintenance of this pattern over a large geographical area shows that nestedness is not confined to local sets of interactions. At least in this case, the pattern is consistent with the evolutionary history of the animals that we infer involves the fishes tending to become genetically isolated (on island groups, for example) while retaining the host specificity of the ancestral species. This follows from the observation that closely related fish species tend to share host specificities (Dunn 1981), and from recent genetic evidence that a large proportion of larvae of Amphiprion polymnus, which occurs from the East China Sea in the north to the Gulf of Carpentaria in the south (Fautin & Allen 1997), disperse only a few hundred metres from their hatching sites (Jones et al. 2005). The host anemones, being far longer-lived than the fishes, are more likely to maintain genetic continuity through space and time, as evidenced by all of them being broadly distributed. Despite genetic evidence of limited dispersal by some sea anemones (e.g. Monteiro et al. 1997; Perrin et al. 1999; note that these are temperate animals that are much smaller and more abundant than those that host anemonefish), populations of anemones with apparently poor larval dispersal can be genetically uniform over scales of thousands of kilometres (Solecava et al. 1994).
The limited diversity of anemonefish and the relatively few widespread species make it difficult to test our supplementary hypothesis that more widely distributed anemonefish should interact with more host anemone species because they are likely to encounter more species. Although it is only partially and weakly supported (table 2, figure 2), these results make clear that the non-random pattern of host use by anemonefish is not simply a result of the different distributional ranges of the fish, but is a fundamental aspect of their biology and ecology.
Host choice seems largely due to innate preference of the fishes, an attribute that is difficult to alter experimentally (e.g. Elliott et al. 1995; Arvedlund et al. 1999); among the preferred hosts, which fish occupies a particular anemone is at least partly a function of competition for hosts, as demonstrated in two localities by Fautin (1986, 1992). This pattern is suggestive also at large scale: in the Comoros, where it occurs alone, the anemonefish A. akallopisos occupies the anemones Heteractis magnifica and Stichodactyla mertensii. In the Seychelles and the Maldives, where it occurs with A. fuscocaudatus and A. clarkia, respectively, A. akallopisos is restricted to H. magnifica, while S. mertensii is used by the other anemonefish (Dunn 1981).
In the case of extreme anemone specialists such as P. biaculeatus, there is probably strong natural selection for individuals to out-compete those of other species for the single species with which it lives. The phenomenon of nursery anemones, which contain only immature anemonefish (Moyer 1976; Dunn 1981; Chadwick & Arvedlund 2005), and from which a fish must move if it is to reproduce, is further evidence that choice of anemone can influence fish fitness.
Whatever proximate and evolutionary factors govern host distribution, across the range of this association, specialized anemonefish tend to use generalist host anemones, which are used by many other amphiprionines. Even the most specialist–specialist interaction involves A. sebae with Stichodactyla haddoni, an anemone which hosts six species of anemonefish (table 2).
In summary, we suggest a model for the structuring of the mutualistic interaction between amphiprionine fishes and their host anemones at both geographically small and large scales. Local selection of hosts by a fish is determined primarily by a combination of competition with other species of fish (Dunn 1981) for preferred anemones (Fautin & Allen 1997; Richardson 1999; Srinivasan et al. 1999; Elliott & Mariscal 2001), and perhaps secondarily by habitat preferences (e.g. water depth and position on the reef; Dunn 1981). The potential for interaction is determined by organism distributions (table 2, figure 2). Although evidence is limited and more field studies are required, local assemblages of anemonefish and their hosts do not exhibit a nested structure of interactions: a reanalysis by us of the assemblage of nine anemonefish and nine host anemones from Madang, Papua New Guinea presented by Elliott & Mariscal (2001) showed that these local interactions were not statistically significantly nested (system temperature of confirmed interactions=30.0°; p=0.29). This is in contrast to previous work on other mutualisms at a local scale which consistently finds nested interaction structure (see §1). However, at larger geographical scales, the tendency of host-specialist fish to associate with generalist anemones produced a highly non-random, nested pattern (table 2).
Why do specialist fish interact with generalist hosts? There seems to be a slight influence of the limited distributional ranges of some species, though certainly not all, and this effect is not strong (figure 2); more important may be local population size of the fish or anemones, with abundant species interacting more frequently with one another. The effect of relative abundance has been rarely examined in mutualistic networks, but in a nested matrix of flower visitors to an assemblage of plants in a South African grassland, Ollerton et al. (2003) found that the insect species which visited a wide range of plant species were also the most abundant. Similar results have been noted by Dupont et al. (2003) and Vázquez & Aizen (2004). At the local level, anemones of one species from which anemonefish of two species had been removed were repopulated by the two species in proportion to their local abundance (Fautin 1992). Clearly, more research is needed if we are to test the hypothesis that the relative abundance of interacting species is a factor in generating a nested pattern of interactions.
Jordano et al. (2006) and Medan et al. (2006) used the term ‘forbidden interactions’ to describe interspecific relationships within a network which cannot occur owing to physical, biochemical or phenological mismatch between species. Fewer than one-third (31%) of the zero interactions between anemonefish and host anemones are due to spatially forbidden interactions, in which the distributional ranges of fish and anemone do not overlap (table 2). Given the wide geographical range of the host anemones, however, some of the remaining 69% of the zero interactions are possibly caused by local factors which ‘forbid’ these interactions even when there is overlap in the range of potential host–fish combinations. For example, the highly generalist Entacmaea quadricolor is found within the ranges of most anemonefish (Fautin & Allen 1997), yet it interacts with only about half the fishes. Some of the species with which it does not interact are widespread (A. sebae and A. akallopisos), and so these may be examples of forbidden interactions, mediated by factors such as competition (Fautin 1992), host–fish biochemical signalling or habitat preferences.
The title of this paper is, of course, slightly tongue-in-cheek. However, our analyses support previous assertions that Nestedness is Engendered by Mutualistic Organization (NEMO): nested patterns of mutualistic interactions seem to be widely distributed within nature, across many kinds of mutualism, involving a wide array of organisms, in marine as well as terrestrial environments (although this does not preclude other kinds of interaction pattern; Lewinsohn et al. 2006). Further analyses are required of mutualisms of all kinds, but NEMO appears to be an emerging ecological rule, and one which should be considered in relation to the conservation of species and their interactions. Specialist–specialist mutualistic interactions are likely to be most vulnerable to extinction because the loss of one species inevitably leads to the loss of the other (Bond 1994; Waser & Ollerton 2006). The ecological redundancy provided by using multiple partners may buffer even the more locally distributed species from the likelihood of natural (and possibly anthropogenic) extinction.
Although this analysis might suggest that anemonefish–host interactions should be robust to the local extirpation of either partner, as through over-collecting for the aquarium trade (Shuman et al. 2005), the obligate nature of the relationship means that both the fish and the anemone are locally vulnerable. Collection of an anemone will perforce reduce the anemonefish population owing to habitat loss. This is exacerbated by the great longevity and apparent low recruitment and slow average growth of the anemones (Fautin 1991), which means that anemones are not readily replaced. Reciprocally, removal of all fish from an anemone can lead, within a matter of minutes or hours, to removal of the anemone by predators (Godwin & Fautin 1992).
Since the fish is the active partner in establishing the interaction (Dunn 1981), perhaps anemone hosts that associate with a small number of anemonefish species should not be considered specialists in the same way that fish associating with anemones of one species are. The anemones Heteractis malu and Cryptodendrum adhaesivum host in nature only A. clarkii, the extreme generalist anemonefish, although most specimens of C. adhaesivum in most places lack anemonefish symbionts and are perfectly able to survive without fish. Thus, rather than a specialist, this anemone can be viewed as a marginal host tolerated only by the least selective fish and only when no other host is available. The existence of such anemones may contribute to local anemonefish diversity by allowing differential use of reef habitats and/or host anemones (Elliott & Mariscal 2001).
Mutualistic networks may in general be relatively resilient to disruption via species extinctions (Memmott et al. 2004; Jordano et al. 2006), perhaps because specialist–specialist interactions have largely been removed from the network by past natural ecological filtering (Ollerton et al. 2003). By identifying reciprocally specialist relationships, nestedness analysis can be a useful tool for making an initial assessment of the vulnerability of mutualistic (and other) interactions to extinction via anthropogenic disturbance. This should be backed up, however, by in-depth understanding of the biology of the interaction, including the local and regional processes which determine the pattern.
Acknowledgments
We thank Jordi Bascompte for valuable comments on an earlier version of the manuscript, John Thompson for his encouragement, Paulo Guimarães for advice on using the Aninhado software and two anonymous referees for their support and suggestions.
References
- Arvedlund M, McCormick M.I, Fautin D.G, Bildsoe M. Host recognition and possible imprinting in the anemonefish Amphiprion melanopus (Pisces: Pomacentridae) Mar. Ecol. Prog. Ser. 1999;188:207–218. [Google Scholar]
- Atmar W, Patterson B.D. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia. 1993;96:373–382. doi: 10.1007/BF00317508. doi:10.1007/BF00317508 [DOI] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Melián C.J, Olesen J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. doi:10.1073/pnas.1633576100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Olesen J.M. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. doi:10.1126/science.1123412 [DOI] [PubMed] [Google Scholar]
- Bond W.J. Do mutualisms matter—assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. B. 1994;344:83–90. [Google Scholar]
- Boucher D.H, editor. The biology of mutualism: ecology and evolution. Croom Helm; London, UK: 1985. [Google Scholar]
- Carlson B. The Amphiprion leucokranos mystery. Aquarium Frontiers. 1996;3:34–37. [Google Scholar]
- Chadwick N.E, Arvedlund M. Abundance of giant sea anemones and patterns of association with anemonefish in the northern Red Sea. J. Mar. Biol. Assoc. UK. 2005;85:1287–1292. doi:10.1017/S0025315405012440 [Google Scholar]
- Douglas A.E. Oxford University Press; Oxford, UK: 1994. Symbiotic interactions. [Google Scholar]
- Dunn D.F. The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Trans. Am. Phil. Soc. 1981;71:1–115. [Google Scholar]
- Dupont Y.L, Hansen D.M, Olesen J.M. Structure of a plant–pollinator network in the high altitude sub-alpine desert of Tenerife, Canary Islands. Ecography. 2003;26:301–310. doi:10.1034/j.1600-0587.2003.03443.x [Google Scholar]
- Elliott J.K, Mariscal R.N. Coexistence of nine anemonefish species: differential host and habitat utilization, size and recruitment. Mar. Biol. 2001;138:23–36. doi:10.1007/s002270000441 [Google Scholar]
- Elliott J.K, Elliott J.M, Mariscal R.N. Host selection, location, and association behaviors of anemonefishes in-field settlement experiments. Mar. Biol. 1995;122:377–389. doi:10.1007/BF00350870 [Google Scholar]
- Elliott J.K, Lougheed S.C, Bateman B, McPhee L.K, Boag P.T. Molecular phylogenetic evidence for the evolution of specialization in anemonefishes. Proc. R. Soc. B. 1999;266:677–685. doi: 10.1098/rspb.1999.0689. doi:10.1098/rspb.1999.0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautin D.G. Why do anemonefishes inhabit only some host actinians? Environ. Biol. Fishes. 1986;15:171–180. doi:10.1007/BF00002992 [Google Scholar]
- Fautin D.G. The anemonefish symbiosis—what is known and what is not. Symbiosis. 1991;10:23–46. [Google Scholar]
- Fautin D.G. Anemonefish recruitment: the roles of order and chance. Symbiosis. 1992;14:143–160. [Google Scholar]
- Fautin D.G, Allen G.R. Western Australian Museum; Perth, Australia: 1997. Anemonefishes and their host sea anemones. Revised edition. [Google Scholar]
- Godwin J, Fautin D.G. Defense of host actinians by anemonefishes. Copeia. 1992;3:903–908. [Google Scholar]
- Guimarães P.R, Guimarães P. Improving the analyses of nestedness for large sets of matrices. Environ. Model. Software. 2006;21:1512–1513. doi:10.1016/j.envsoft.2006.04.002 [Google Scholar]
- Guimarães P.R, Rico-Gray V, Furtado do Reis S, Thompson J.N. Asymmetries in specialization in ant–plant mutualistic networks. Proc. R. Soc. B. 2006;273:2041–2047. doi: 10.1098/rspb.2006.3548. doi:10.1098/rspb.2006.3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A. Coexistence of 2 anemonefishes, Amphiprion clarkii and A. perideraion, which utilize the same host sea-anemone. Environ. Biol. Fishes. 1995;42:345–353. doi:10.1007/BF00001464 [Google Scholar]
- Hattori A. Small and large anemonefishes can coexist using the same patchy resources on a coral reef, before habitat destruction. J. Anim. Ecol. 2002;71:824–831. doi:10.1046/j.1365-2656.2002.00649.x [Google Scholar]
- Holbrook S.J, Schmitt R.J. Growth, reproduction and survival of a tropical sea anemone (Actiniaria): benefits of hosting anemonefish. Coral Reefs. 2005;24:67–73. doi:10.1007/s00338-004-0432-8 [Google Scholar]
- Jones G.P, Planes S, Thorrold S.R. Coral reef fish larvae settle close to home. Curr. Biol. 2005;15:1314–1318. doi: 10.1016/j.cub.2005.06.061. doi:10.1016/j.cub.2005.06.061 [DOI] [PubMed] [Google Scholar]
- Jordano P, Bascompte J, Olesen J.M. The ecological consequences of complex topology and nested structure in pollination webs. In: Waser N.M, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago, IL: 2006. pp. 173–199. [Google Scholar]
- Lewinsohn T.M, Prado P.I, Jordano P, Bascompte J, Olesen J.M. Structure in plant–animal interaction assemblages. Oikos. 2006;113:174–184. doi:10.1111/j.0030-1299.2006.14583.x [Google Scholar]
- Medan D, Basilio A.M, Devoto M, Bartoloni N.J, Torretta J.P, Petanidou T. Measuring generalization and connectance in temperate, year-long active systems. In: Waser N.M, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago, IL: 2006. pp. 245–259. [Google Scholar]
- Memmott J, Waser N.M, Price M.V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. B. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. doi:10.1098/rspb.2004.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro F.A, Sole-Cava A.M, Thorpe J.P. Extensive genetic divergence between populations of the common intertidal sea anemone Actinia equina from Britain, the Mediterranean and the Cape Verde Islands. Mar. Biol. 1997;129:425–433. doi:10.1007/s002270050183 [Google Scholar]
- Moyer J.T. Geographical variation and social dominance in Japanese populations of the anemonefish Amphiprion clarkii. Jpn J. Ichth. 1976;23:12–22. [Google Scholar]
- Ollerton J. “Biological Barter”: patterns of specialization compared across different mutualisms. In: Waser N.M, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago, IL: 2006. pp. 411–435. [Google Scholar]
- Ollerton J, Johnson S.D, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Ann. Bot. 2003;92:807–834. doi: 10.1093/aob/mcg206. doi:10.1093/aob/mcg206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracer S, Ahmadjian V. Oxford University Press; Oxford, UK: 2000. Symbiosis: an introduction to biological associations. [Google Scholar]
- Patterson B.D. On the temporal development of nested subset patterns of species composition. Oikos. 1990;59:330–342. [Google Scholar]
- Perrin M.C, Thorpe J.P, Sole-Cava A.M. Population structuring, gene dispersal and reproduction in the Actinia equina species group. Oceanog. Mar. Biol. 1999;37:129–152. [Google Scholar]
- Porat D, Chadwick-Furman N.E. Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia. 2004;530:513–520. doi:10.1007/s10750-004-2688-y [Google Scholar]
- Porat D, Chadwick-Furman N.E. Effects of anemonefish on giant sea anemones: ammonium uptake, zooxanthella content and tissue regeneration. Mar. Freshw. Behav. Phys. 2005;38:43–51. doi:10.1080/10236240500057929 [Google Scholar]
- Reisser W, editor. Algae and symbioses: plants, animals, fungi, viruses, interactions explored. Biopress Ltd; Bristol, UK: 1992. [Google Scholar]
- Richardson D.L. Correlates of environmental variables with patterns in the distribution and abundance of two anemonefishes (Pomacentridae: Amphiprion) on an eastern Australian sub-tropical reef system. Environ. Biol. Fishes. 1999;55:255–263. doi:10.1023/A:1007596330476 [Google Scholar]
- Schmitt R.J, Holbrook S.J. Mutualism can mediate competition and promote coexistence. Ecol. Lett. 2003;6:898–902. doi:10.1046/j.1461-0248.2003.00514.x [Google Scholar]
- Shuman C.S, Hodgson G, Ambrose R.F. Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs. 2005;24:564–573. doi:10.1007/s00338-005-0027-z [Google Scholar]
- Smith D.C, Douglas A.E. Edward Arnold; London, UK: 1987. The biology of symbiosis. [Google Scholar]
- Solecava A.M, Thorpe J.P, Todd C.D. High genetic similarity between geographically distant populations in a sea-anemone with low dispersal capabilities. J. Mar. Biol. Assoc. UK. 1994;74:895–902. [Google Scholar]
- Srinivasan M, Jones G.P, Caley M.J. Experimental evaluation of the roles of habitat selection and interspecific competition in determining patterns of host use by two anemonefishes. Mar. Ecol. Prog. Ser. 1999;186:283–292. [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 2005. The geographic mosaic of coevolution. [Google Scholar]
- Vázquez D.P, Aizen M.A. Null model analyses of specialization in plant–pollinator interactions. Ecology. 2003;84:2493–2501. [Google Scholar]
- Vázquez D.P, Aizen M.A. Asymmetric specialization: a pervasive feature of plant–pollinator interactions. Ecology. 2004;85:1251–1257. [Google Scholar]
- Vázquez D.P, Aizen M.A. Community-wide patterns of specialization in plant–pollinator interactions revealed by null models. In: Waser N.M, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago, IL: 2006. pp. 200–219. [Google Scholar]
- Waser N.M, Ollerton J, editors. Plant–pollinator interactions: from specialization to generalization. University of Chicago Press; Chicago, IL: 2006. [Google Scholar]