Abstract
Sperm competition is thought to be a major force driving the evolution of sperm shape and function. However, previous studies investigating the relationship between the risk of sperm competition and sperm morphometry revealed inconclusive results and marked differences between taxonomic groups. In a comparative study of two families of passerines (Fringillidae and Sylviidae) and also across species belonging to different passerine families, we investigated the relative importance of the phylogenetic background on the relationship between sperm morphometry and the risk of sperm competition. The risk of sperm competition was inferred from relative testis mass as an indicator of investment in sperm production. We found: (i) a significant positive association between both midpiece length and flagellum length and relative testis mass in the Fringillidae, (ii) a significant negative association between sperm trait dimensions and relative testis mass in the Sylviidae, and (iii) no association across all species. Despite the striking difference in the patterns shown by the Sylviidae and the Fringillidae, the relationship between midpiece length and flagellum length was positive in both families and across all species with positive allometry. Reasons for the differences and similarities between passerine families are discussed.
Keywords: sperm competition, sperm size, midpiece size, Passeriformes
1. Introduction
Sperm are among the most diverse of all animal cells (Cohen 1977a). Three factors are thought to explain the diversity in sperm shape across species: (i) phylogeny, (ii) mode of fertilization and (iii) postcopulatory sexual selection, including sperm competition and cryptic female choice (Miller & Pitnick 2002; Snook 2005). Sperm competition (Parker 1970; Birkhead & Parker 1997) appears to be a particularly powerful force driving the diversity in sperm phenotype (Birkhead & Pizzari 2002; Pizzari & Birkhead 2002; Snook 2005), but the relationships between the size of sperm traits and the risk of sperm competition appear to differ markedly between taxa (e.g. Stockley et al. 1997; Balshine et al. 2001; Anderson & Dixson 2002; Gage & Freckleton 2003; Malo et al. 2006).
The role of two particular sperm traits in sperm competition is hotly debated in evolutionary biology: flagellum length (often closely correlated with total sperm length) and the size of the sperm midpiece. Theoreticians have predicted that: (i) increased flagellum length results in increased sperm velocity (Katz & Drobnis 1990) and (ii) increased midpiece size, resulting from more or larger mitochondria, results in greater power output (Cardullo & Baltz 1991). In both cases, therefore, we might expect species that experience high levels of sperm competition to have longer sperm and/or larger midpieces (see also Parker 1993). We might also expect on energetic grounds, all else being equal, a fixed relationship between midpiece size and flagellum length (Cardullo & Baltz 1991; but see Gage 1998). Empirical tests of these theoretical predictions have yielded mixed results. First, the relationship between overall sperm length and sperm competition is positive in some cases (Gomendio & Roldan 1991; Gage 1994; Breed & Taylor 2000; Morrow & Gage 2000; Balshine et al. 2001), negative in others (Stockley et al. 1997), or shows no relationship in yet other cases (Anderson & Dixson 2002; Gage & Freckleton 2003). Second, in terms of the midpiece, Anderson & Dixson (2002) found a pronounced positive association between midpiece volume and the risk of sperm competition in primates. However, contrary to theory (Cardullo & Baltz 1991), they found no association between flagellum length and sperm competition. Profound biological differences between taxonomic groups might be a potential explanation for these inconclusive results.
Passerine birds exhibit variation in both levels of sperm competition (Griffith et al. 2002) and in sperm morphometry, including sperm length and midpiece size (Retzius 1909; McFarlane 1963; Birkhead et al. 2006). However, previous studies were unable to detect a clear association between sperm length and the risk of sperm competition across passerine species belonging to different families, although there was an indirect effect mediated through the sperm storage tubules (Briskie et al. 1997). Previous studies of passerine birds have included a wide range of species from many different families. Passerine families have diverged markedly and exhibit profound biological differences which are likely to affect life history and reproductive traits (Bennett & Owens 2002). To test the possible influence of phylogeny on the relationship between sperm morphometry and the risk of sperm competition in passerine birds, we investigated how midpiece size and flagellum length covary with the risk of sperm competition within two families of passerine birds as well as across species belonging to several families.
2. Material and methods
We focused on two passerine families, namely the finches (Fringillidae) and the Old World warblers (Sylviidae). We obtained data from 18 species of Fringillidae and 22 species of Sylviidae. We chose these two passerine families owing to their well-resolved phylogenies and their accessibility. We also collected data from 33 other species belonging to a variety of passerine families.
(a) Sperm morphometry
Morphometry is defined as the measurement of shape dimensions and throughout this paper ‘sperm morphometry’ refers to the measurement of the length of sperm traits (Gage 1998). Two different methods were used to obtain sperm samples: (i) from the faeces of males in reproductive condition (Immler & Birkhead 2005) and (ii) from the seminal glomera of dissected males in reproductive condition found dead (e.g. road kills), or collected under a license. Sperm collected by different methods do not differ in their morphometry (Immler & Birkhead 2005). Samples from one to ten males per species were collected. A power analysis performed at the beginning of the study on five species of Acrocephaline Sylviidae (1–15 males per species) which show similar sperm morphometry, revealed that measuring ten males per species allowed us to detect significant differences even between closely related species with similar sperm morphometry. Five randomly chosen sperm were measured from each male since in the zebra finch, Taeniopygia guttata, five sperm per male provide a representative value for individual males (Birkhead et al. 2005; see also Morrow & Gage 2001). Sperm were fixed in a 5% formalin solution. For analysis, a sub-sample was examined using a light microscope at ×250 or ×400 magnification and digital pictures were taken. Passerine sperm are typically elongate with a short helical head and a long mitochondrial helix twisted around almost the entire length of the flagellum (McFarlane 1963). The following sperm traits were measured from digital images (using analysis software Leica IM50 Image manager): (i) head length, (ii) midpiece length (along the length of the flagellum) and (iii) flagellum length were measured to the nearest 0.5 μm, and (iv) the number of midpiece helix curves was counted to calculate straight helix length (SHL, i.e. the total length of the straightened midpiece twisted around the flagellum using the method described in Birkhead et al. 2005). Hereafter, midpiece length is used as the measurement of the straight midpiece length. Within-species repeatability (Lessels & Boag 1987) of all morphometric sperm traits was estimated.
In passerines, SHL provides a reliable measurement of the variation of midpiece size: across five species belonging to different families, midpiece volume (cylindrical volume calculated from SHL and the mitochondrial radius obtained from transmission electron microscopy pictures; coefficient of variation, CV=97.91%) varies mainly due to variation in midpiece length (CV=73.52%) whereas midpiece width shows little variation (CV=14.48%). In Anderson et al.'s (2005) dataset on mammals, variation in midpiece volume (CV=73.59%) was substantially larger than variation in midpiece length (CV=23.30%). Interestingly, an earlier study of a wider range of mammals failed to show a relationship between either midpiece volume (calculated as the volume of a cylinder subtracting the volume of the inner axoneme) or midpiece length and relative testis mass (Gage & Freckleton 2003). In Gage & Freckleton's (2003) study, the difference in variation between volume and length was smaller (midpiece length, CV=73.34%; midpiece volume, CV=90.62%) than in Anderson et al.'s (2005) study. By including the inner axoneme in their calculation of midpiece volume, Anderson et al. (2005) may have overestimated midpiece size and this may explain the discrepancy between their study and that of Gage & Freckleton (2003). Alternative hypotheses for the differences between the studies of mammals may be the variation in sample size and the different sources of data used.
(b) Testis mass and body mass
We used relative testis mass as an indicator of the risk of sperm competition (Harcourt et al. 1981; Møller & Briskie 1995; Dunn et al. 2001; Pitcher et al. 2005). Data on testis mass and body mass were obtained from the literature (Dunn et al. 2001; Calhim & Birkhead in press) and from personal observations. Testis mass was not available for all species included and accounts for varying sample sizes among analyses.
(c) Comparative methods
To account for statistical non-independence of data points due to shared ancestry of species (Felsenstein 1985; Harvey & Pagel 1991), we used a generalized least-squares (GLS) approach in a phylogenetic framework for our analyses (Pagel 1999; Freckleton et al. 2002). The GLS method allows the estimation of a phylogenetic scaling parameter λ: values of λ close to 0 correspond to traits where the similarities are likely to have evolved independently of phylogeny, whereas λ values close to 1 indicate strong phylogenetic association of the traits. A likelihood ratio test was applied to compare models including the maximum likelihood value of λ with models, including λ set to either 0 (no phylogenetic association) or 1 (complete phylogenetic association). Analyses were performed using a code for the statistical package R v. 2.1.0 (R Foundation for Statistical Computing 2005). The phylogeny including all species was obtained from the literature: the deeper nodes of the phylogenetic tree were inferred from Sibley & Ahlquist (1990) and higher nodes were obtained from different sources (see electronic supplementary material). We assumed constant branch length for our analyses.
(d) Multiple regression analysis
We performed multiple regression analyses in a phylogenetic framework as described earlier to investigate the relationship between morphometric sperm traits and relative testis mass. We conducted the following analyses: (i) across all passerine species included and (ii) within two individual passerine families as low sample size in other families did not allow statistical analyses. The Sylviidae exhibit a wide range of different mating systems which might affect testis mass (Dunn et al. 2001; Griffith et al. 2002; Leisler et al. 2002). To test for a possible influence of mating system on testis mass, we analysed socially monogamous Sylviidae species separately. We included individual sperm traits as dependent variables and both testis mass and body mass as independent variables to control for allometry between the latter (Briskie & Montgomerie 1992). The highest condition index (estimated from the matrix of the independent variables to detect collinearity between independent variables; Belsley et al. 1980) being 14.5 for body mass allowed us to exclude collinearity between independent variables. Where necessary, data were normalized using the appropriate transformation to meet parametric requirements of the GLS model.
We performed GLS analyses to establish the relationships between individual morphometric sperm traits.
(e) Multiple comparisons
We performed a series of comparative analyses on different subsets of the data. However, we rejected the use of Bonferroni correction as it enhances the probability of committing type II errors, particularly in studies with small samples sizes (Nakagawa 2004). We calculated effect size to establish the strength of the relationship between sperm traits and the predicting variables (Nakagawa 2004). We calculated the effect size r from t values (Cohen 1977b) obtained from the GLS model and used Cohen's (1988) benchmarks to estimate the size of the effect. We also calculated 95% non-central confidence limits (CLs) for r which indicate statistical significance if 0 is not included in the CLs (Smithson 2003).
3. Results
(a) Association between sperm morphometry and relative testis mass
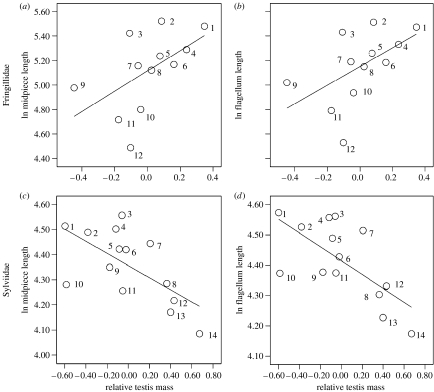
Striking differences existed for the relationship between sperm trait dimensions and relative testis mass between the Fringillidae and the Sylviidae. For the Fringillidae, a positive association existed between most sperm traits (except head length) and relative testis mass, whereas for the Sylviidae, the relationship between sperm trait dimensions and relative testis mass was negative (table 1; figure 1).
Table 1a–d.
Multiple regression analyses controlling for phylogeny (GLS) of sperm morphometry in relation to testis mass and body mass within families and across all species. (A t-test was used to compare the slopes against 0. The fitted model including the maximum likelihood value of λ was compared against the models including λ=1 and 0: superscripts after the λ value indicate significance levels of the likelihood ratio tests (first position, against λ=1; second position, λ=0; significance levels, n.s.not significant, *p<0.05). Effect size r calculated from the t value and the non-central 95% confidence intervals are presented. CLs excluding 0 indicate a significant relationship whereas CLs including 0 indicate no statistical significance. The data of the monogamous Sylviidae are a subset of the data of Sylviidae.)
| sperm trait | predictor | slope | t | p | λ | r | CL |
|---|---|---|---|---|---|---|---|
| (a) Fringillidae (n=12) | |||||||
| head | testis mass | −0.65 | −2.03 | 0.07 | <0.001*,n.s | −0.58 | −0.83 to 0.07 |
| body mass | −0.21 | −0.77 | 0.46 | −0.26 | −0.07 to 0.40 | ||
| midpiece | testis mass | 397.91 | 3.01 | 0.02 | <0.001*,n.s | 0.73 | 0.18 to 0.89 |
| body mass | −144.34 | −1.30 | 0.23 | −0.42 | −0.76 to 0.27 | ||
| flagellum | testis mass | 365.38 | 3.06 | 0.01 | <0.001*,n.s | 0.73 | 0.19 to 0.89 |
| body mass | −144.96 | −1.45 | 0.18 | −0.46 | 0.78 to 0.23 | ||
| total length | testis mass | 355.98 | 3.04 | 0.01 | <0.001*,n.s | 0.73 | 0.19 to 0.89 |
| body mass | −148.35 | −1.51 | 0.17 | −0.47 | −0.78 to 0.21 | ||
| (b) Sylviidae (n=14) | |||||||
| head | testis mass | 0.03 | 1.17 | 0.27 | <0.001n.s.,n.s | 0.35 | −0.06 to 0.79 |
| body mass | −0.24 | −2.86 | 0.02 | −0.67 | −0.85 to −0.15 | ||
| midpiece | testis mass | −0.10 | −4.43 | 0.001 | 0.14n.s.,n.s | −0.81 | −0.91 to −0.46 |
| body mass | −0.25 | −3.94 | 0.002 | −0.78 | −0.90 to −0.38 | ||
| flagellum | testis mass | −0.09 | −3.69 | 0.004 | 0.69n.s.,n.s | −0.76 | −0.89 to −0.33 |
| body mass | −0.21 | −3.55 | 0.005 | −0.75 | −0.89 to −0.33 | ||
| total length | testis mass | −0.07 | −3.40 | 0.006 | 0.80n.s.,n.s | −0.73 | −0.88 to −0.27 |
| body mass | −0.29 | −4.12 | 0.002 | −0.79 | −0.91 to −0.41 | ||
| (c) monogamous Sylviidae (n=7) | |||||||
| head | testis mass | 0.73 | 0.29 | 0.79 | <0.001n.s.,n.s | 0.17 | −0.70 to 0.79 |
| body mass | −13.60 | −3.26 | 0.03 | −0.88 | −0.97 to 0.02 | ||
| midpiece | testis mass | −0.50 | −3.69 | 0.02 | <0.001n.s.,n.s | −0.91 | −0.97 to −0.13 |
| body mass | −0.55 | −2.49 | 0.07 | −0.82 | −0.95 to 0.18 | ||
| flagellum | testis mass | −35.28 | −3.09 | 0.04 | <0.001n.s.,n.s | −0.87 | −0.96 to 0.02 |
| body mass | −25.93 | −1.40 | 0.23 | −0.63 | −0.90 to 0.47 | ||
| total length | testis mass | −33.90 | −3.31 | 0.03 | <0.001n.s.,n.s | −0.89 | −0.97 to −0.03 |
| body mass | −33.85 | −2.04 | 0.11 | −0.76 | −0.93 to 0.30 | ||
| (d) across passerine species (n=50) | |||||||
| head | testis mass | 0.04 | 2.21 | 0.03 | 0.37n.s, * | 0.31 | 0.04 to 0.61 |
| body mass | −0.08 | −3.82 | <0.001 | −0.49 | −0.82 to −0.25 | ||
| midpiece | testis mass | 3.83 | 0.52 | 0.61 | 0.84n.s.,* | 0.08 | −0.21 to 0.37 |
| body mass | −17.68 | −1.88 | 0.07 | −0.27 | −0.56 to 0.01 | ||
| flagellum | testis mass | 0.07 | 1.29 | 0.20 | 0.93n.s.,* | 0.19 | −0.09 to 0.48 |
| body mass | −0.20 | −2.59 | 0.01 | −0.26 | −0.55 to 0.02 | ||
| total length | testis mass | 7.44 | 1.15 | 0.25 | 0.80n.s.,* | 0.17 | −0.11 to 0.46 |
| body mass | −17.56 | −2.19 | 0.04 | −0.31 | −0.61 to −0.35 |
Figure 1.
Association between morphometric sperm traits and relative testis mass. Figures are not controlled for phylogeny and relative testis mass indicates the use of residual values from a linear regression of testis mass on body mass: Fringillidae (n=12), (a) association between midpiece length and relative testis mass (b=397.91, t=3.01, p=0.02, λ<0.0001), (b) association between flagellum length and relative testis mass (b=365.38, t=3.06, p=0.01, λ<0.0001); Sylviidae (n=14), (c) association between flagellum length and relative testis mass (b=−0.09, t=−3.69, p=0.004, λ=0.69), (d) association between total sperm length and relative testis mass (b=−0.07, t=−3.40, p=0.006, λ=0.80). Species list (in brackets: sample size). Fringillidae: 1, Fringilla coelebs (10); 2, Carduelis erythrinus (2); 3, Serinus serinus (1); 4, Serinus flaviventris (1); 5, Serinus canaria (10); 6, Carduelis flammea (12); 7, Carduelis tristis (6); 8, Carduelis chloris (5); 9, Carduelis cannabina (4); 10, Carpodacus mexicanus (1); 11, Carduelis carduelis (7); 12, Loxia curvirostra (3). Sylviidae: 1, Acrocephalus baeticatus (1); 2, Phylloscopus fuscatus (1); 3, Acrocephalus scirpaceus (10); 4, Anacamptis palustris (2); 5, Phylloscopus sibilatrix (4); 6, Phylloscopus collybita (5); 7, Acrocephalus melanopogon (4); 8, Sylvia curruca; 9, Phylloscopus trochilus (5); 10, Acrocephalus arundinaceus (1); 11, Acrocephalus schoenobaenus (10); 12, Acrocephalus paludicola (7); 13, Sylvia communis (4); 14, Sylvia atricapilla (10).
The values of λ for the Fringillidae were close to 0 for most traits indicating that phylogeny plays only a minor role in these relationships. Since the Fringillidae included in the analyses are all considered socially monogamous and only vary in the rate of extra-pair paternity (Dunn et al. 2001; Griffith et al. 2002), no further analyses were undertaken. For the Sylviidae, effect size was medium to large and values of λ varied considerably between sperm traits (table 1). The Sylviidae exhibit variation in mating system across species, being monogamous, polygynous and promiscuous, and males are exposed to varying copulation rate. It has been argued that the risk of sperm depletion due to frequent copulation may influence testis mass and possibly confound the relationship between sperm competition risk and testis mass. We therefore performed the analyses considering only socially monogamous Sylviidae which vary in the rate of extra-pair paternity (Dunn et al. 2001; Leisler et al. 2002); negative relationships existed between sperm traits (except head length) and relative testis mass (table 1c). Effect sizes were large. Values of λ were close to 0 indicating that factors other than phylogeny explain these patterns. The reduced major axis (RMA) regression slopes (Ricker 1973; McArdle 1988) between all Sylviidae (v1) and monogamous Sylviidae (v2) differed significantly in that in monogamous Sylviidae the slope is significantly steeper than in all Sylviidae (midpiece length, v1=−0.085±0.02 s.e., v2=−0.57±0.14 s.e., t=3.46, p<0.01; flagellum length, v1=−0.11±0.02 s.e., v2=−44.10±11.41 s.e., t=3.86, p<0.001; total sperm length, v1=−0.08±0.02 s.e., v2=−40.23±10.23 s.e., t=3.93, p<0.001). The increased effect size in monogamous Sylviidae suggests that mating system may have some influence on the relationship between sperm dimensions and relative testis mass.
Across all passerines, we found no association between any sperm trait dimensions (except head length) and relative testis mass (table 1d). Accordingly, effect size was small and CLs were large, indicating a weak effect. However, there was a significant negative association between sperm dimensions and body mass (table 1d). This negative association exists mainly due to the inclusion of the two Corvus species which have extremely small sperm compared with their body mass and disappears when the two Corvus species are excluded. Values of λ were close to 1 for all sperm traits except for sperm head, indicating a very strong phylogenetic component.
For all morphometric sperm traits, within-species repeatability was very high (see electronic supplementary material).
(b) Relationships between sperm traits
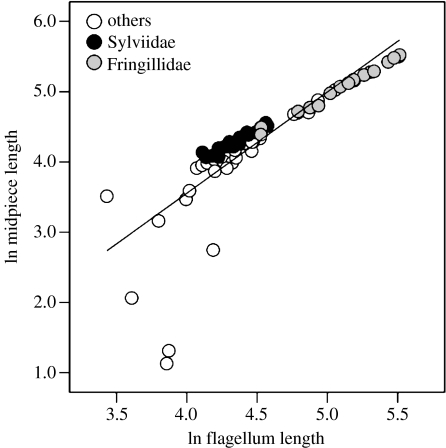
Across all the species, midpiece length was significantly positively associated with both flagellum length (r=0.84, p<0.0001, n=73; figure 2) and total sperm length (r=0.69, p<0.0001, n=73) after controlling for phylogeny. To assess whether an allometric relationship exists between midpiece length and flagellum length (as a possible indicator of the metabolic regulation of sperm) as predicted by Cardullo & Baltz (1991; see also Gage 1998), we calculated the slope v of a RMA regression (Ricker 1973; McArdle 1988), using the standard errors (s.e.) obtained from the GLS regression as an approximation (Sokal & Rohlf 1995) and performed a t-test of v against 1. We found a significant positive allometric relationship between midpiece length and flagellum length across all species included, and also within the Fringillidae. Similarly, across all the Sylviidae (excluding Locustella spp.) and across only the monogamous Sylviidae, the RMA regression slopes were significantly larger than 1 (table 2).
Figure 2.
Association between midpiece length and flagellum length across 73 passerine species belonging to different families. The results of RMA regression revealed a positive allometry between midpiece length and flagellum length with the slope v=1.76±0.11 s.e. (t-test against 1: t=6.91, p<0.005). Similarly, a positive allometry existed within the Fringillidae (v=1.14±0.03 s.e., t=4.67, p<0.005) and the Sylviidae (v=1.11±0.06 s.e., t=1.83, p<0.05).
Table 2.
Relationship between midpiece length and flagellum length: correlation coefficient r and slope b obtained from the GLS regression was used to calculate the RMA regression slope v. (Standard errors (s.e.) for b were used to compare v against 1 and t and p are given from a one sample t-test of the slope against 1. The data of the monogamous Sylviidae are a subset of the data of Sylviidae.)
| family | b | v | s.e. | r | n | t | p |
|---|---|---|---|---|---|---|---|
| all species | 1.48 | 1.76 | 0.11 | 0.99 | 73 | 6.91 | <0.005 |
| Fringillidae | 1.13 | 1.14 | 0.03 | 0.99 | 16 | 4.67 | <0.005 |
| Sylviidae | 1.03 | 1.11 | 0.06 | 0.93 | 22 | 1.83 | <0.05 |
| monogamous Sylviidae | 1.23 | 1.26 | 0.11 | 0.97 | 7 | 2.36 | <0.05 |
| Alaudidae | 1.18 | 1.19 | 0.02 | 0.99 | 10 | 9.50 | <0.005 |
| Turdidae | 1.29 | 1.30 | 0.05 | 0.99 | 8 | 6.00 | <0.005 |
Head length was positively correlated with all other sperm traits across all species: midpiece length (r=0.13, p=0.001, n=73), flagellum length (r=0.16, p=0.0003, n=73) and total sperm length (r=0.18, p=0.0001, n=73).
4. Discussion
Our results revealed striking differences for the relationship between sperm trait dimensions and the risk of sperm competition inferred from relative testis mass between passerine families, being positive in the Fringillidae and negative in the Sylviidae. Across the passerine species belonging to different families, including Fringillidae and Sylviidae, we found no association between sperm trait dimensions and the risk of sperm competition. This is consistent with previous studies of passerine birds (Briskie & Montgomerie 1992; Briskie et al. 1997). Our study highlights the variation across different taxonomic groups which may explain the results obtained in earlier studies. Despite the marked differences in sperm dimensions between passerine families, the relationship between midpiece length and flagellum length was positive with a positive allometry. This suggests that essential biological functions (e.g. energetic principles) determine gross passerine sperm morphology.
(a) The importance of phylogeny
Our results highlight the importance of phylogeny for the investigation of trait coevolution and emphasize that statistical analyses correcting for phylogeny sometimes deal insufficiently with differences between taxonomic groups. The size and composition of taxonomic groups used in comparative studies differ markedly. Despite the rigorous control for phylogeny as applied in most comparative studies (Felsenstein 1985; Harvey & Pagel 1991), it is possible that inter-taxonomic variation in other life-history traits, such as breeding cycle, number of eggs per clutch or number of copulations prior and during ovulation may confound the results (see also Arnold & Owens 2002; Bennett & Owens 2002). This variation is likely to be reduced within smaller taxonomic groups such as orders and families. It is therefore important for future comparative studies to investigate trait coevolution at different taxonomic levels.
(b) Sperm morphometry and risk of sperm competition in passerine birds
Our finding of an inconsistent pattern of the relationship between midpiece length and relative testis mass in passerines contrasts with Anderson & Dixson's (2002) finding of a positive relationship between midpiece volume and relative testis size in primates (Anderson & Dixson 2002) and across mammals in general (Anderson et al. 2005). We consider four possible non-exclusive reasons for the existence of different relationships between sperm trait dimensions and relative testis mass in the Fringillidae and in the Sylviidae:
Mating systems. Relative testis size is known to be a reasonable index of the risk of sperm competition as relative testis size is positively correlated with the rate of sperm production (Harcourt et al. 1981; Møller 1988a,b, 1991; Møller & Briskie 1995; Hosken & Ward 2001; Pitnick et al. 2001). But testis size may also provide an index of increased sperm production due to polygynous or promiscuous mating systems which may entail the risk of sperm depletion (Cartar 1985; Wedell et al. 2002). All Fringillidae included in this study are socially monogamous with varying rates of extra-pair paternity (Dunn et al. 2001; Griffith et al. 2002), whereas the Sylviidae exhibit a range of mating systems, including monogamy, polygyny and promiscuity (Dunn et al. 2001; Leisler et al. 2002) and testis mass in the two families might be subject to differential selection. However, the results including all Sylviidae did not differ from those that consider only monogamous Sylviidae and, therefore, variation in mating systems can be excluded as a possible explanation for the difference between the two families.
Trade-off between sperm size and number. Parker (1993) assumed that a trade-off might exist between sperm size and sperm number. If so, the different results in the Fringillidae and the Sylviidae could be explained by a possible advantage of few larger sperm in the Fringillidae, whereas in the Sylviidae, increased sperm numbers might be favoured at the expense of sperm size. In general, the Fringillidae produce sperm that are twice the size of Sylviidae sperm (see electronic supplementary material). In addition, the lack of a significant difference in testis size between the two families (see electronic supplementary material) indicates that the overall expenditure on sperm production is constant and might therefore indicate a trade-off between sperm size and number. Future studies will have to take sperm numbers produced into account to specifically investigate this issue.
Sperm–female coevolution. The differences in sperm morphometry between closely related taxonomic groups such as the Fringillidae and the Sylviidae may be the result of coevolution between sperm and the female reproductive tract rather than sperm competition (Briskie & Montgomerie 1992; Briskie et al. 1997). The anatomy of the female reproductive tract may have an equal (or stronger) impact on sperm morphometry than sperm competition (Briskie & Montgomerie 1992; Briskie et al. 1997; Miller & Pitnick 2002; Minder et al. 2005) and may interfere with and even reverse the relative impact of sperm competition, as suggested by the opposite associations between sperm morphometry and the risk of sperm competition in the Fringillidae and the Sylviidae. To test this, we would need information on female reproductive anatomy.
Sperm survival. A trade-off between sperm size and sperm longevity (as proposed in mammals (Cardullo & Baltz 1991; Gomendio & Roldan 1991, 1993) and fish (Stockley et al. 1997) might explain the divergent results in the Fringillidae and Sylviidae: the smaller sperm of the Sylviidae might have to survive for longer after ejaculation than the larger sperm of the Fringillidae. The biological bases for any trade-off between sperm size and sperm longevity are still poorly understood. In mammals, it has been suggested that the trade-off results from the negative allometry between midpiece size and flagellum length (i.e. longer sperm have a relatively shorter, but absolutely longer midpiece; Cardullo & Baltz 1991; but see Gage 1998). In other words, the relatively small midpiece of longer sperm generates less power per unit length of flagellum, resulting in rapid energy consumption and early death.
In contrast to the situation in mammals, we found a positive allometry between midpiece length and flagellum length in passerines (i.e. longer sperm have a relatively and absolutely longer midpiece; figure 2). Using the same logic as applied to mammalian sperm, all else being equal we might expect longer passerine sperm to survive longer than short sperm. However, in a preliminary in vitro study, we found exactly the opposite pattern: shorter sperm with a smaller midpiece survived longer than longer sperm (S. Immler & T. R. Birkhead, unpublished data). This suggests that in passerines the increased metabolic rate of longer sperm is generated by an absolutely longer midpiece.
5. Conclusions
The results of this study emphasize how little we still understand about the evolution of sperm design and function. We can almost certainly exclude mating system as a possible explanation for the opposite relationship between sperm morphometry and testis size in Fringillidae and Sylviidae. The difference between Fringillidae and Sylviidae might indicate some crucial biological limitations to sperm production and a possible trade-off between sperm size against sperm number at the extremes, but no firm conclusions can be drawn at this stage. Future studies should concentrate on both broad evolutionary patterns within and across a variety of taxonomic groups and on the detailed investigation of the functional significance of specific sperm traits and their role in postcopulatory sexual selection including both sperm competition and female reproductive biology.
Acknowledgments
We are extremely grateful to ornithologists in Europe, Africa and the USA for their assistance with sperm sample collection. We would like to thank Shinichi Nakagawa and Ally Phillimore for their statistical advice and Dave Hosken, Bob Montgomerie and three anonymous referees for their useful comments on an earlier version of the manuscript. This project was funded by the Roche Research Foundation.
Supplementary Material
Phylogeny of passerine birds as used for statistical analyses with source references Within-species repeatability of morphometric sperm traits Statistics to test for differences in total sperm length and testis mass between the Fringillidae and Sylviidae.
References
- Anderson M.J, Dixson A.F. Motility and the midpiece in primates. Nature. 2002;416:496. doi: 10.1038/416496a. doi:10.1038/416496a [DOI] [PubMed] [Google Scholar]
- Anderson M.J, Nyholt J, Dixson A.F. Sperm competition and the evolution of sperm midpiece volume in mammals. J. Zool. Lond. 2005;267:135–142. [Google Scholar]
- Arnold K.E, Owens I.P.F. Extra-pair paternity and egg dumping in birds: life history, parental care and the risk of retaliation. Proc. R. Soc. B. 2002;269:1263–1269. doi: 10.1098/rspb.2002.2013. doi:10.1098/rspb.2002.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshine S, Leach B.J, Neat F, Werner N.Y, Montgomerie R. Sperm size of African cichlids in relation to sperm competition. Behav. Ecol. 2001;12:726–731. doi:10.1093/beheco/12.6.726 [Google Scholar]
- Belsley D.A, Kuh E, Welsch R.E. New York, NY; Wiley: 1980. Regression diagnostics: identifying influential data and sources of collinearity. [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life history, mating system and extinction. [Google Scholar]
- Birkhead T.R, Parker G.A. Sperm competition and mating systems. In: Krebs J.R, Davies N.B, editors. Behavioral ecology: an evolutionary approach. Blackwell Science; Oxford, UK: 1997. pp. 121–145. [Google Scholar]
- Birkhead T.R, Pizzari T. Postcopulatory sexual selection. Nat. Rev. 2002;3:262–273. doi: 10.1038/nrg774. doi:10.1038/nrg774 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Pellatt E.J, Brekke P, Yeates R, Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. doi:10.1038/nature03374 [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Immler S, Pellatt A.J, Freckleton R.P. Unusual sperm morphology in the Northern bullfinch (Pyrrhula pyrrhula) Auk. 2006;123:383–392. doi:10.1642/0004-8038(2006)123[383:USMITE]2.0.CO;2 [Google Scholar]
- Breed W.G, Taylor J. Body mass, testes mass, and sperm size in murine rodents. J. Mammal. 2000;81:758–768. doi:10.1644/1545-1542(2000)081<0758:BMTMAS>2.3.CO;2 [Google Scholar]
- Briskie J.V, Montgomerie R. Sperm size and sperm competition in birds. Proc. R. Soc. B. 1992;247:89–95. doi: 10.1098/rspb.1992.0013. [DOI] [PubMed] [Google Scholar]
- Briskie J.V, Montgomerie R, Birkhead T.R. The evolution of sperm size in birds. Evolution. 1997;51:937–945. doi: 10.1111/j.1558-5646.1997.tb03674.x. doi:10.2307/2411167 [DOI] [PubMed] [Google Scholar]
- Calhim, S. & Birkhead, T. R. In press. Testes size in birds: quality versus quantity—assumptions, errors and estimates. Behav. Ecol
- Cardullo R.A, Baltz J.M. Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil. Cytoskel. 1991;19:180–188. doi: 10.1002/cm.970190306. doi:10.1002/cm.970190306 [DOI] [PubMed] [Google Scholar]
- Cartar R.V. Testis size in sandpipers. Naturwissenschaften. 1985;72:157–158. doi:10.1007/BF00490407 [Google Scholar]
- Cohen J. London, UK; Butterworths: 1977. Reproduction. [Google Scholar]
- Cohen J. Erlbaum, Hillsdale; New Jersey, NJ: 1977. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Cohen J. 2nd edn. Erlbaum, Hillsdale; New Jersey, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Dunn P.O, Whittingham L.A, Pitcher T.E. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. doi:10.1554/0014-3820(2001)055[0161:MSSCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–725. doi: 10.1086/343873. doi:10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G. Associations between body size, mating pattern, testis size and sperm length across butterflies. Proc. R. Soc. B. 1994;258:247–254. [Google Scholar]
- Gage M.J.G. Mammalian sperm morphometry. Proc. R. Soc. B. 1998;265:97–103. doi: 10.1098/rspb.1998.0269. doi:10.1098/rspb.1998.0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage M.J.G, Freckleton R.P. Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc. R. Soc. B. 2003;270:625–632. doi: 10.1098/rspb.2002.2258. doi:10.1098/rspb.2002.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M, Roldan E.R.S. Sperm competition influences sperm size in mammals. Proc. R. Soc. B. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Gomendio M, Roldan E.R.S. Coevolution between male ejaculates and female reproductive biology in eutherian mammals. Proc. R. Soc. B. 1993;252:7–12. doi: 10.1098/rspb.1993.0039. [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Harcourt A.H, Harvey P.H, Larson S.G, Short R.V. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. doi:10.1038/293055a0 [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hosken D.J, Ward P.I. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 2001;4:10–13. doi:10.1046/j.1461-0248.2001.00198.x [Google Scholar]
- Immler S, Birkhead T.R. A non-invasive method for obtaining spermatozoa from birds. Ibis. 2005;147:827–830. doi:10.1111/j.1474-919x.2005.00456.x [Google Scholar]
- Katz D.F, Drobnis E.Z. Analysis and interpretation of the forces generated by spermatozoa. In: Bavister B.D, Cummins J.M, Roldan E.R.S, editors. Fertilization in mammals. Serono Symposia; Norwell, MA: 1990. pp. 125–137. [Google Scholar]
- Leisler B, Winkler H, Wink M. Evolution of breeding systems in acrocephaline warblers. Auk. 2002;119:379–390. doi:10.1642/0004-8038(2002)119[0379:EOBSIA]2.0.CO;2 [Google Scholar]
- Lessels C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Malo A, Gomendio M, Garde J, Lang-Lenton B, Soler A.J, Roldan E.R.S. Sperm design and sperm function. Biol. Lett. 2006;2:246–249. doi: 10.1098/rsbl.2006.0449. doi:10.1098/rsbl.2006.0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle B.H. The structural relationship—regression in biology. Can. J. Zool. 1988;66:2329–2339. [Google Scholar]
- McFarlane R.W. The taxonomic significance of avian sperm. Proc. 13th Int. Ornith. Congr. 1963;1:91–102. [Google Scholar]
- Miller G.T, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. doi:10.1126/science.1076968 [DOI] [PubMed] [Google Scholar]
- Minder A.M, Hosken D.J, Ward P.I. Co-evolution of male and female reproductive characters across the Scatophagidae (Diptera) J. Evol. Biol. 2005;18:60–69. doi: 10.1111/j.1420-9101.2004.00799.x. doi:10.1111/j.1420-9101.2004.00799.x [DOI] [PubMed] [Google Scholar]
- Møller A.P. Ejaculate quality, testes size and sperm competition in primates. J. Hum. Evol. 1988;17:479–488. doi:10.1016/0047-2484(88)90037-1 [Google Scholar]
- Møller A.P. Testes size, ejaculate quality and sperm competition in birds. Biol. J. Linn. Soc. 1988;33:273–283. [Google Scholar]
- Møller A.P. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 1991;137:882–906. doi:10.1086/285199 [Google Scholar]
- Møller A.P, Briskie J.V. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 1995;36:357–365. [Google Scholar]
- Morrow E.H, Gage M.J.G. The evolution of sperm length in moths. Proc. R. Soc. B. 2000;267:307–313. doi: 10.1098/rspb.2000.1001. doi:10.1098/rspb.2000.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H, Gage M.J.G. Consistent significant variation between individual males in spermatozoal morphometry. J. Zool. Lond. 2001;254:147–153. [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 2004;15:1044–1045. doi:10.1093/beheco/arh107 [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. doi:10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Sperm competition games: sperm size and sperm number under adult control. Proc. R. Soc. B. 1993;253:245–254. doi: 10.1098/rspb.1993.0110. [DOI] [PubMed] [Google Scholar]
- Pitcher T.E, Dunn P.O, Wittingham L.A. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005;18:557–567. doi: 10.1111/j.1420-9101.2004.00874.x. doi:10.1111/j.1420-9101.2004.00874.x [DOI] [PubMed] [Google Scholar]
- Pitnick S, Miller G.T, Reagan J, Holland B. Males' evolutionary responses to experimental removal of sexual selection. Proc. R. Soc. B. 2001;268:1071–1080. doi: 10.1098/rspb.2001.1621. doi:10.1098/rspb.2001.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Birkhead T.R. The sexually-selected sperm hypothesis: sex-biased inheritance and sexual antagonism. Biol. Rev. 2002;77:183–209. doi: 10.1017/s1464793101005863. doi:10.1017/S1464793101005863 [DOI] [PubMed] [Google Scholar]
- Retzius G. Die Spermien der Vögel. Biol. Unters. New Ser. 1909;14:89–122. [Google Scholar]
- Ricker W.E. Linear regression in fishery research. J. Fish Res. Board Can. 1973;30:409–434. [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; Yale, NY: 1990. The phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Smithson M. Sage Publications; London, UK: 2003. Confidence intervals. [Google Scholar]
- Snook R.R. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. doi:10.1016/j.tree.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman and Co; New York, NY: 1995. Biometry. [Google Scholar]
- Stockley P, Gage M.J.G, Parker G.A, Møller A.P. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 1997;149:933–954. doi: 10.1086/286031. doi:10.1086/286031 [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogeny of passerine birds as used for statistical analyses with source references Within-species repeatability of morphometric sperm traits Statistics to test for differences in total sperm length and testis mass between the Fringillidae and Sylviidae.