Abstract
A polychaete from the Middle Devonian Arkona Shale at Hungry Hollow, Arkona, Ontario is preserved in three dimensions in pyrite. The prostomium bears a single median antenna, a pair of lateral antennae and a pair of ventral palps. It is assumed to be fused to a reduced peristomium. The anteriormost three pairs of trunk appendages are modified as tentacular cirri, the third long and biramous. The remainder of the finely annulated trunk bears at least 21 similar biramous parapodia, some of which preserve evidence of chaetae. The postsegmental pygidium is very small and may bear up to two pairs of cirri. The polychaete, Arkonips topororum, falls within the Palpata, Aciculata, among the crown group Phyllodocida. Its remarkable preservation highlights the potential of the Arkona Shale to yield other examples of soft-tissue preservation.
Keywords: Aciculata, Palpata, pyritization, Arkona Shale, exceptional preservation
1. Introduction
The polychaete annelids are predominantly marine organisms that adopt a variety of lifestyles from swimming near the surface of the water column to burrowing in sediment. Most of the tissues of polychaetes decay readily (Briggs & Kear 1993), but their jaw elements, known as scolecodonts, are often preserved (Bergman 1989; Eriksson et al. 2004). A number of polychaetes, including serpulids, secrete calcareous dwelling tubes that are readily preserved. Whole-body fossils of polychaetes are known from a number of Palaeozoic Konservat-Lagerstätten (Briggs & Kear 1993), notably the Middle Cambrian Burgess Shale of British Columbia (Conway Morris 1979; definitive polychaetes have yet to be reported from the Cambrian Konservat-Lagerstätten at Chengjiang and Kaili in China) as well as the Carboniferous Bear Gulch biota of Central Montana and Mazon Creek biota of Illinois (Schram 1979; Thompson 1979). Whole-body fossils of polychaetes such as these, however, are normally flattened. Exceptions are very rare and include Kenostrychus from the concretions in volcanic ash that yield the Silurian Herefordshire biota of England (Briggs et al. 1996b; Sutton et al. 2001). The specimen described here is one of the very few examples of a polychaete body fossil preserved mainly in pyrite. Other examples are known from the Devonian Hunsrück Slate, Germany (Bartels & Blind 1995; Bartels et al. 1998) and the Jurassic of La Voulte-sur-Rhône, France (Alessandrello et al. 2004). Tube linings, which replicate the surface texture of their polychaete inhabitant, have been found preserved in pyrite in the Eocene La Meseta Formation in Antarctica (Schweitzer et al. 2005). A review of the fossil record of whole-body polychaetes is provided by Bracchi & Alessandrello (2005).
In general, the preservation of soft tissues in pyrite has been considered a relatively rare occurrence, confined to a few biotas such as those of the Lower Cambrian near Chengjiang, Yunnan Province, China (Gabbott et al. 2004), the Ordovician Beecher's Trilobite Bed (Briggs et al. 1991) and the Hunsrück Slate (Briggs et al. 1996a). This new study provides further evidence that it may be more common.
The phylogeny of polychaetes is not fully resolved (Fauchald & Rouse 1997). The cladistic analyses of Rouse & Fauchald (1997) provided a clearly formulated scheme, but the position of a number of living families requires further investigation. New data from the fossil record are potentially important to determining the relationships of living taxa (Donoghue et al. 1989).
2. Preservation
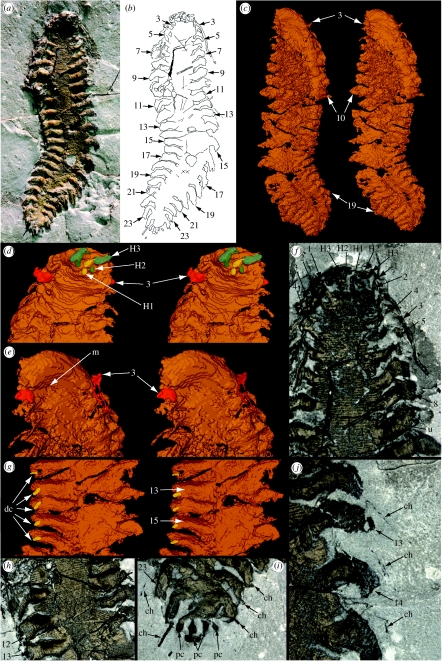
The polychaete occurs in a fine, pale grey, uniform mudstone and is preserved in pyrite in three dimensions (figure 1a). The specimen is preserved dorsal side uppermost; details of the ventral side, including a depression interpreted as the site of the mouth, were revealed by scanning (figure 1c–e; electronic supplementary material, movies S1 and S2). There is little evidence for decay apart from the flattened cross-section of the trunk which may reflect some collapse prior to mineralization. A nodular texture is evident in areas of the trunk where the annulations are not preserved, particularly near the head and in the posterior third of the body (figure 1f,h). This texture is presumably diagenetic, although it does not appear to reflect pyrite framboids. Much of the anterior right side is obscured ventrally by scattered pyrite (figure 1c; electronic supplementary material, movie S1). Where chaetae are preserved extending beyond the parapodia, they are usually preserved in a mineral other than pyrite and they are not evident in the scan. Associated with the polychaete on the same slab is a fragment of sponge (3–4 mm in dimension) as well as a number of isolated spicules preserved in pyrite. Euhedral crystals and framboids of pyrite are scattered in the mudstone. Crinoid stem fragments are present ranging from single columnals to lengths of up to 10, but these are preserved in silica which may have replaced the original calcite at a later stage.
Figure 1.
Arkonips topororum, Middle Devonian, Arkona Shale, Hungry Hollow, Ontario; UMMP 73795. (a–c) ×2: (a) dorsal view; (b) explanatory drawing; (c) stereo-pair of ventral side of reconstruction based on scanning. (d–f) Anterior end: (d) stereo-pair of dorsal side of reconstruction, ×4; (e) stereo-pair of ventral side of reconstruction, ×4; (f) dorsal side, ×5. (g) Stereo-pair of mid-trunk section, dorsal view, ×4. (h) Anterior part of trunk, dorsal view, ×5. (i) Posterior termination, dorsal view, ×5. (j) Right parapodia, dorsal view, ×9. Numbers refer to trunk appendages except where prefixed by H, when they refer to head appendages; ch, chaetae; dc, possible dorsal cirrus; pc, pygidial cirrus; u, unknown grooved structure; m, possible mouth.
Pyritization of soft tissue occurs in anaerobic sediments when bacterial sulphate reduction is focused on the organic carbon of the buried organism (Briggs et al. 1996a). Experimental results show that polychaete carcases normally collapse within 30 days (Briggs & Kear 1993). The three-dimensional nature of the fossil indicates that authigenic mineralization was relatively rapid, although pyrite tends to precipitate at a slower rate than other authigenic minerals, so some collapse is likely to have occurred. Variation in the preserved morphology of the parapodia along the length of the trunk is thought to be taphonomic, i.e. due to variation in the degree of pyritization distally or a product of preparation, or both. Bergström (1990) remarked that appendages of arthropods may be more highly pyritized where they extend beyond the carcase. He attributed this contrast to a more favourable surface area to volume ratio than in the main body of the carcase. The parapodia, which are now oval in cross-section, appear to be less flattened than the trunk. The lack of pyritization of the chaetae may reflect their chitinous composition; they did not begin to decay as soon as the soft tissues and therefore did not provide a locus for pyritization.
3. Material and methods
A single specimen, UMMP 73795, discovered by Mike Topor was available and donated by him and his brother John to the University of Michigan at Ann Arbor. The upper side was exposed and prepared to a limited degree on the surface of a block ca 2.5 cm thick, which was consolidated with PVA. The specimen could not be imaged in the environmental scanning electron microscope, as the chamber could not be completely evacuated due to the porosity of the block. The block was also too thick to be X-rayed. The specimen was CT scanned at intervals of 0.05295 mm at the Center for Quantitative Imaging at Penn State University, courtesy of Alan Walker and Tim Ryan. The datasets were used by M. D. Sutton (Imperial College, London) to generate a three-dimensional computerized reconstruction (electronic supplementary material, movies S1 and S2; see Sutton et al. 2001), which could be viewed using a custom on-screen visualization system with stereo capabilities.
4. Systematic palaeontology
Phylum: Annelida Lamarck, 1809
Class: Polychaeta Grube, 1850
Palpata, Aciculata, Phyllodocida sensu Rouse & Fauchald, 1997
Genus: Arkonips gen. nov.
Derivation of name: Arkona—the unit from which the fossil came + ips Gr.—a worm.
Diagnosis: the prostomium bears a single median antenna, a pair of lateral antennae and a pair of ventral palps. It appears to be fused to a reduced peristomium. The first three pairs of trunk appendages are modified as tentacular cirri, the third long and biramous. The remainder of the finely annulated trunk bears at least 21 pairs of similar biramous parapodia some of which preserve evidence of chaetae. The postsegmental pygidium is very small and may bear up to two pairs of cirri.
Species: Arkonips topororum sp. nov.
Derivation of name: for Michael and John Topor.
Diagnosis: as for genus.
Material. Holotype, University of Michigan Museum of Paleontology 73795A/B (Topor Collection), part and partial counterpart.
Type locality and horizon. Hungry Hollow, approximately 2 miles east of Arkona, West Williams Township, Middlesex County, southwestern Ontario, Canada. The fossil came from a small pit on the north side of the Ausable River, 2 miles east of Arkona, West Williams Township, Middlesex County. It was found in the Arkona Shale Formation (Hamilton Group) at a level corresponding to one yielding sponges on the southwest bank of the Ausable River (Rigby & Topor 2003, fig. 2), 6.5 m (24 ft) below the base of the overlying Hungry Hollow Formation at this locality. Apart from the polychaete and the sponges, a number of other taxa occur at the same level of the Arkona Shale, including crinoids, trilobites, cephalopods, brachiopods, starfish, brittle-stars and blastoids (M. Topor 2004, personal communication). Many of these fossils are also preserved in pyrite.
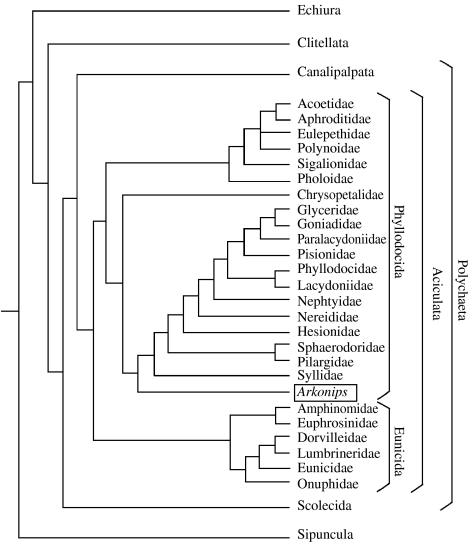
Figure 2.
Phylogeny and classification of relevant Polychaeta based on coding in table 1, electronic supplementary material (for procedure followed, see §4d). Taxonomic scheme after Rouse & Fauchald (1997, fig. 73).
(a) Size and overall form
The worm is bilaterally symmetrical and sinuously curved (figure 1a–c). It is ca 4.0 cm long outstretched and ca 1.2 cm wide at the widest point, including outstretched parapodia. Although the specimen is three dimensional, it is flattened dorso-ventrally. The body is divided into a head region, which is flexed dorsally (figure 1a,d), and a trunk with laterally projecting parapodia. It tapers slightly anteriorly, and to a more pronounced degree posteriorly. The boundary between the head and the trunk is poorly defined.
(b) Head
The head tapers anteriorly to a blunt termination, which is not much narrower than the rest of the body (approx. 4 mm; figure 1d,e). The scanned reconstruction reveals an arcuate structure, convex anteriorly, on the ventral surface of the anterior extremity of the head (figure 1e). This structure is bounded posteriorly by a pronounced transverse depression. The head of polychaetes is highly variable (Rouse & Fauchald 1997), and it is difficult to identify homologies between structures in a fossil and those in the living taxa.
The head appendages are difficult to enumerate, but one median and two paired lateral appendages apparently can be discerned by combining the evidence of the specimen viewed under the microscope and the data from the scan (figure 1d,f). These appendages are short and project anteriorly. The first paired appendage is medial and attached posterior of the second pair that flanks it. An additional appendage lies between the first pair. These appendages are interpreted as a median and lateral antennae and sensory palps (H1, H2 and H3, respectively, in figure 1d,f); the distal terminations of the palps may not be preserved. The antennae and palps are attached to a small raised area at the anterior of the head, which presumably represents the prostomium. There is no evidence of jaws, but they could be concealed within the pyrite.
(c) Trunk
The first three appendages of the trunk are cephalized, i.e. differentiated from those lying further posteriorly (figure 1d–f). They are attached laterally and increase in size posteriorly; they are better preserved on the left side of the animal. The first and second trunk appendages are directed postero-laterally and then flex sharply and project ventrally. The attitude of the third is similar, but it extends posteriorly into a tentacle-like structure. The one on the right is evident where it extends about 9 mm overlapping the eighth trunk appendage (figure 1a,b,f), but it is not evident in the scan as it is not pyritized distally. The one on the left is concealed beneath the specimen and is evident only in the scan (figure 1e). It bifurcates into two slender rami, but appears shorter than its counterpart on the right side. These first three pairs of trunk appendages are interpreted as tentacular cirri, the first two pairs uniramous, the third biramous.
The postero-lateral margin of the arcuate structure on the ventral margin of the head corresponds to the position of attachment of the third trunk appendage (figure 1e). Posterior of this, the body bears a series of appendages that are clearly parapodia. The first trunk annulations (which are evident on the specimen but not in the computer reconstruction) are evident between the first and the second parapodia (the fourth and fifth trunk appendages), but the preservation anterior of this may not be adequate to reveal annulations. The scan also reveals a linear trace down the centre of the posterior part of the trunk, which forms a groove on the ventral side, and may reflect the position of the gut (figure 1c).
The trunk is finely annulated (figure 1f,h) and bears at least 21 pairs of parapodia (trunk appendages 4–24), probably 22 (figure 1a–c). The posteriormost pair of parapodia is considerably smaller than the others, but there is no evidence that they differ in morphology. The trunk is ca 6 mm wide, excluding the appendages, and shows lateral flexibility just posterior of its mid-length. Apart from the distribution of appendage pairs, there is no evidence of segmentation. There are six to seven annulations per millimetre, the length occupied by each appendage pair corresponding to approximately eight annulations (figure 1h). The annulations are not preserved in the posterior part of the trunk. The first nine pairs of parapodia (trunk appendages 4–12) are preserved projecting near normal to the trunk, where it shows no lateral flexure. Those on the left side of the body, in particular, are inclined just above the dorsal surface of the trunk in their proximal part, before curving ventrally distally (figure 1a,h). Just posterior of the mid-length, the trunk is curved laterally and the attitude of the parapodia reflects the flexure of the body: they are more closely spaced and converge on the concave (left) side whereas they diverge on the convex (right) side (figure 1a–c). The parapodia in the posteriormost part of the trunk bend posteriorly almost parallel to the trunk axis.
The morphology of the parapodia varies due to differences in preservation. They are annulated (figure 1h), the annulations showing a similar spacing but more variability than the trunk, perhaps reflecting flexure. The most completely preserved parapodia are those near the posterior right of the specimen (appendages 17–24; figure 1a,b). These parapodia taper distally. Some appear to be divided axially by a groove. Additional evidence from the scan suggests that two rami may lie one above the other corresponding to the notopodium and the neuropodium. Trunk appendages 12–17 on the left side show small postero-lateral projections on the dorsal side that may represent dorsal cirri (figure 1g). Similarly, obvious structures are not evident on the ventral side, but may have been present. Elytra (scales formed by modification of dorsal cirri) are unlikely to have been present and subsequently lost since cirriform dorsal cirri are found on successive segments.
Evidence for chaetae is limited. On the right side, there are traces of very slender linear structures projecting beyond trunk appendages 12–15 and 21–23 which are interpreted as chaetae (figure 1i,j). They do not appear to be pyritized and are not evident on the computer reconstruction. Traces of up to two per parapodium are preserved and they reach lengths of at least 4 mm, but their number and length are uncertain; there may be many more concealed in the sediment. Spine-like structures also project from some of the appendages at the posterior end of the animal (22–24 on the right and 23–25 on the left), which may also be chaetae (figure 1i). The most obvious lies posterior (but not directly connected) to 24 on the left (figure 1i); its preserved length is at least 2 mm. The projections on the adjacent appendages are less obvious and less than 1 mm long. They are oriented parallel to the appendage axes. The blunt extremities of these spine-like structures suggest that their full extent may not be preserved, probably reflecting preparation loss. The detailed morphology of the chaetae cannot be determined, but they appear to be simple linear structures.
There is no obvious postsegmental pygidium. It was clearly a very small structure. The nature of the projections at the posterior extremity of the animal is unknown. There are four, which may represent two pairs of very short pygidial cirri (figure 1i).
A number of randomly oriented near-straight linear structures are present on the surface of the trunk and on some of the appendages (figure 1a,b). The majority are roughly equal in diameter (less than 0.1 mm) and 4–5 mm long. It is difficult to determine whether these structures represent sponge spicules (which are also present in the matrix) or chaetae that have separated from the appendages. The latter interpretation is less likely, however, as the specimen does not appear to have undergone much decay prior to pyritization. One larger example of a linear structure traverses the trunk from the proximal part of appendage 5 on the right (figure 1f). It is grooved along its length and may have a different origin to the others.
(d) Classification and affinities
Arkonips falls into Annelida, Polychaeta based on the annulated body, the parapodia-like appendages with chaetae and the presence of head appendages that represent antennae and palps. The classification of living polychaetes remains an area of controversy, even the monophyly of Annelida and Polychaeta (Westheide et al. 1999; Rouse & Pleijel 2003; Merz & Woodin 2006), although the work of Fauchald and Rouse is a landmark (Fauchald & Rouse 1997; Rouse & Fauchald 1997). Depending on the details of the analysis, Polychaeta is supported by the presence of palps and mixonephridia, and nuchal organs and parapodia (Westheide et al. 1999, p. 294). Rouse & Fauchald's (1997) system is used as a basis for discussing the position of Arkonips, as theirs is the most comprehensive morphological cladogram, even though they were reluctant to propose a new classification due to the range of uncertainties in the analysis. But attaching diagnostic characters to the clades that can be used to assign the fossil is difficult due to homoplasy and reversals (Rouse & Fauchald 1997). More recent cladograms contain additional characters (Rouse 1999, 2000), but as these are all embryological, they are of limited relevance to the coding of Arkonips.
The ‘A/Pwr’ analysis preferred by Rouse & Fauchald (1997) was run with the addition of Arkonips (figure 2). It was possible to code 59 characters from the fossil out of the 124 used by Rouse & Fauchald (1997) (table 1, electronic supplementary material). The remaining undetermined characters were coded as ‘?’. Characters 0–6, 9 and 31–35 (table 1, electronic supplementary material), which are based on some of the finer details of the prostomium, the peristomium and the first segment, are the most difficult to determine with certainty. The absence of ventral cirri (character 55, table 1, electronic supplementary material) is potentially due to preservational loss, and therefore a sequence of analyses was run to test what effect different coding of this character would have on the resulting cladogram. Euarthropoda and Onychophora were removed from the matrix (following Sutton et al. 2001). The analysis was not subjected to successive approximations character weighting (SW) contrary to Rouse & Fauchald (1997, p. 146). Rouse & Fauchald (1997) recovered essentially the same solution with or without weighting, except that the clade, including the Arenicolidae, Capitellidae and Maldanidae, shifted from the Canalipalpata to a more basal position, within the Scolecida.
Arkonips fell within the crown-group Phyllodocida (figure 2), the largest group within the Aciculata. Changing the coding of character 55 did not alter the position of Arkonips in the cladogram. It is potentially therefore the oldest soft-bodied crown-group Phyllodocid known, with the possible exception of Canadia from the Burgess Shale. Canadia was placed among the Phyllodocida by Butterfield (1990), but its position there is contested (Eibye-Jacobsen 2004). Although we cannot determine whether or not Arkonips had aciculae, characters such as the prostomial palps, tentacular cirri and median antenna are consistent with its placement among the Aciculata and ultimately the Phyllodocida. The apparent absence of compound chaetae argues against an affinity with Phyllodocida, but their structure across the range of living polychaetes is poorly known (Fauchald & Rouse 1997).
5. Comparison with other faunas
Brett (1999) highlighted the status of the Arkona Shale as a crinoid Lagerstätte (see also McIntosh 2001, p. 786) and compared it with the near age-equivalent Silica Shale in Ohio. The crinoid assemblage is of relatively low diversity and dominated by the camerate Arthroacantha. Brett (1999) interpreted these assemblages as representative of offshore, deeper water, muddy bottoms with low-oxygen conditions at least below the sediment–water interface. He envisaged the crinoid assemblages as buried during storms. The crinoids were capable of anchoring in soft mud as well as attaching to skeletal debris in the mud.
Although there are varying amounts of pyrite found in the crinoid fossils (M. Topor & J. Topor 2006, personal communication), the polychaete is the first reported incidence of soft-bodied preservation from these localities. This occurrence indicates that conditions occasionally favoured the pyritization of soft tissues in a manner similar to that in the Hunsrück Slate (Briggs et al. 1996a; Bartels et al. 1998).
Acknowledgments
We are grateful to Mike and John Topor for generously donating the specimen to the University of Michigan Museum of Paleontology, where it was drawn to our attention by Daniel Miller who arranged a loan. Carlton Brett and Cam Tsujita provided advice on the setting of the Arkona Shale. Alan Walker and Tim Ryan at Pennsylvania State University kindly provided facilities for scanning the specimen. Mark Sutton of Imperial College, London rendered the scanned images into the computer reconstruction. Danny Eibye-Jacobsen of the Zoological Museum, Copenhagen, provided insightful comments on an earlier draft. Krister Smith helped us use PAUP, and Heather Wilson assisted with the photography.
Supplementary Material
Arkonips toporon, Middle Devonian, Arkona Shale, Hungry Hollow, Ontario; UMMP 73795. Reconstruction of entire specimen based on CT scanning at intervals of 0.05295 mm at the Center for Quantitative Imaging at Penn State University.
Movie S2: Arkonips toporon, Middle Devonian, Arkona Shale, Hungry Hollow, Ontario; UMMP 73795. Reconstruction of head based on CT scanning at intervals of 0.05295 mm at the Center for Quantitative Imaging at Penn State University.
References
- Alessandrello A, Bracchi G, Riou B. Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. Vol. 32. 2004. Polychaete, sipunculan and enteropneust worms from the Lower Callovian (Middle Jurassic) of La Voulte-sur-Rhône (Ardèche, France) pp. 16. [Google Scholar]
- Bartels C, Blind W. Röntgenuntersuchung pyritisch vererzter Fossilien aus dem Hunsrückschiefer (Unter-Devon, Rheinisches Schiefergebirge) Metalla. 1995;2:79–100. [Google Scholar]
- Bartels C, Briggs D.E.G, Brassel G. Cambridge University Press; Cambridge, UK: 1998. Fossils of the Hunsrück Slate—marine life in the Devonian. pp. xiv +309. [Google Scholar]
- Bergman C.F. Silurian paulinitid polychaetes from Gotland. Fossils Strata. 1989;25:1–128. [Google Scholar]
- Bergström J. Hunsrück Slate. In: Briggs D.E.G, Crowther P.R, editors. Palaeobiology—a synthesis. Blackwell Scientific; Oxford, UK: 1990. pp. 277–279. [Google Scholar]
- Bracchi G, Alessandrello A. Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano. Vol. 32. 2005. Paleodiversity of the free-living polychaetes (Annelida, Polychaeta) and description of new taxa from the Upper Cretaceous Lagerstätten of Haqel, Hadjula and Al-Namoura (Lebanon) pp. 64. [Google Scholar]
- Brett C.E. Middle Devonian Arkona Shale of Ontario, Canada, and Silica Shale of Ohio, USA. In: Hess H, Ausich W.I, Brett C.E, Simms M.J, editors. Fossil Crinoids. Cambridge University Press; Cambridge, UK: 1999. pp. 137–141. [Google Scholar]
- Briggs D.E.G, Kear A. Decay and preservation of polychaetes: taphonomic thresholds in soft-bodied organisms. Paleobiology. 1993;19:107–135. [Google Scholar]
- Briggs D.E.G, Bottrell S.H, Raiswell R. Pyritization of soft-bodied fossils: Beecher's Trilobite Bed, Upper Ordovician, New York State. Geology. 1991;19:1221–1224. doi:10.1130/0091-7613(1991)019<1221:POSBFB>2.3.CO;2 [Google Scholar]
- Briggs D.E.G, Raiswell R, Bottrell S.H, Hatfield D, Bartels C. Controls on the pyritization of exceptionally preserved fossils: an analysis of the Lower Devonian Hunsrück slate of Germany. Am. J. Sci. 1996a;296:633–663. [Google Scholar]
- Briggs D.E.G, Siveter D.J, Siveter D.J. Soft-bodied fossils from a Silurian volcaniclastic deposit. Nature. 1996b;382:248–250. doi:10.1038/382248a0 [Google Scholar]
- Butterfield N.J. A reassessment of the enigmatic Burgess Shale fossil Wiwaxia corrugata (Matthew) and its relationship to the polychaete Canadia spinosa (Walcott) Paleobiology. 1990;16:287–303. [Google Scholar]
- Conway Morris S. Middle Cambrian polychaetes from the Burgess Shale of British Columbia. Phil. Trans. R. Soc. B. 1979;285:227–274. [Google Scholar]
- Donoghue M.J, Doyle J.A, Gauthier J, Kluge A.G, Rowe T. The importance of fossils in phylogeny reconstruction. Annu. Rev. Ecol. Syst. 1989;20:431–460. doi:10.1146/annurev.es.20.110189.002243 [Google Scholar]
- Eibye-Jacobsen D. A reevaluation of Wiwaxia and the polychaetes of the Burgess Shale. Lethaia. 2004;37:317–335. doi:10.1080/00241160410002027 [Google Scholar]
- Eriksson M.E, Bergman C.F, Jeppsson L. Silurian scolecodonts. Rev. Palaeobot. Palynol. 2004;131:269–300. doi:10.1016/j.revpalbo.2004.04.001 [Google Scholar]
- Fauchald K, Rouse G. Polychaete systematics: past and present. Zool. Scr. 1997;26:71–138. doi:10.1111/j.1463-6409.1997.tb00411.x [Google Scholar]
- Gabbott S.E, Hou X.-G, Norry M.J, Siveter D.J. Preservation of Early Cambrian animals of the Chengjiang biota. Geology. 2004;32:901–904. doi:10.1130/G20640.1 [Google Scholar]
- Grube A.E. Archiv für Naturgeschichte. vol. 16. Springer; Berlin, Germany: 1850. Die Familien der Anneliden. pp. 249–364. [Google Scholar]
- Lamarck, J. B. 1809 Philosophie Zoologique, ou exposition des considerations relatives a l'histoire naturelle des Animaux. Dentu, Paris
- McIntosh G.C. Devonian cladid crinoids: families Glossocrinidae Goldring, 1923, and Rutkowskicrinidae, new family. J. Paleontol. 2001;75:783–807. doi:10.1666/0022-3360(2001)075<0783:DCCFGG>2.0.CO;2 [Google Scholar]
- Merz R.A, Woodin S.A. Polychaete chaetae: function, fossils and phylogeny. Integr. Comp. Biol. 2006;46:481–496. doi: 10.1093/icb/icj057. doi:10.1093/icb/icj057 [DOI] [PubMed] [Google Scholar]
- Rigby J.K, Topor M. Brigham Young University Geology Studies. Vol. 47. 2003. Juvenile hexactinellid sponges from the Middle Devonian Arkona Shale at Hungry Hollow, southwestern Ontario. pp. 133–137. [Google Scholar]
- Rouse G.W. Trochophore concepts: ciliary bands and the evolution of larvae in spiralian Metazoa. Biol. J. Linn. Soc. 1999;66:411–464. doi:10.1006/bijl.1998.0278 [Google Scholar]
- Rouse G.W. The epitome of hand waving? Larval feeding and hypotheses of metazoan phylogeny. Evol. Dev. 2000;2:222–233. doi: 10.1046/j.1525-142x.2000.00063.x. doi:10.1046/j.1525-142x.2000.00063.x [DOI] [PubMed] [Google Scholar]
- Rouse G, Fauchald K. Cladistics and polychaetes. Zool. Scr. 1997;26:139–204. doi:10.1111/j.1463-6409.1997.tb00412.x [Google Scholar]
- Rouse G.W, Pleijel F. Problems in polychaete systematics. Hydrobiologia. 2003;496:175–189. doi:10.1023/A:1026188630116 [Google Scholar]
- Schram F.R. Worms of the Mississippian Bear Gulch Limestone of central Montana, USA. Trans. San Diego Soc. Nat. Hist. 1979;19:107–120. [Google Scholar]
- Schweitzer C.E, Feldmann R.M, Marenssi S, Waugh D.A. Remarkably preserved annelid worms from the La Meseta Formation (Eocene), Seymour Island, Antarctica. Palaeontology. 2005;48:1–13. doi:10.1111/j.1475-4983.2004.00440.x [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. A three-dimensionally preserved fossil polychaete worm from the Silurian of Herefordshire, England. Proc. R. Soc. B. 2001;268:2355–2363. doi: 10.1098/rspb.2001.1788. doi:10.1098/rspb.2001.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I. Errant polychaetes (Annelida) from the Pennsylvanian Essex Fauna of Northern Illinois. Palaeontographica A. 1979;163:169–199. [Google Scholar]
- Westheide W, McHugh D, Purschke G, Rouse G. Systematization of the Annelida: different approaches. Hydrobiologia. 1999;402:291–307. doi:10.1023/A:1003713230485 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arkonips toporon, Middle Devonian, Arkona Shale, Hungry Hollow, Ontario; UMMP 73795. Reconstruction of entire specimen based on CT scanning at intervals of 0.05295 mm at the Center for Quantitative Imaging at Penn State University.
Movie S2: Arkonips toporon, Middle Devonian, Arkona Shale, Hungry Hollow, Ontario; UMMP 73795. Reconstruction of head based on CT scanning at intervals of 0.05295 mm at the Center for Quantitative Imaging at Penn State University.