Abstract
As in many anurans, males of the totally aquatic species, Xenopus laevis, advertise their sexual receptivity using vocalizations. Unusually for anurans, X. laevis females also advertise producing a fertility call that results in courtship duets between partners. Although all X. laevis calls consist of repetitive click trains, male and female calls exhibit sex-specific acoustic features that might convey sexual identity. We tested the significance of the carrier frequency and the temporal pattern of calls using underwater playback experiments in which modified calls were used to evoke vocal responses in males. Since males respond differently to male and female calls, the modification of a key component of sexual identity in calls should change the male's response. We found that a female-like slow call rhythm triggers more vocal activity than a male-like fast rhythm. A call containing both a female-like temporal pattern and a female-like carrier frequency elicits higher levels of courtship display than either feature alone. In contrast, a male-like temporal pattern is sufficient to trigger typical male–male encounter vocalizations regardless of spectral cues. Thus, our evidence supports a role for temporal acoustic cues in sexual identity recognition and for spectral acoustic cues in conveying female attractiveness in X. laevis.
Keywords: inter-sexual acoustic communication, frog, Xenopus laevis, playback experiments, temporal and spectral cues
1. Introduction
For reproduction, interactions between the sexes require efficient intersexual communication. Reliable recognition of members of the same species prevents heterospecific mating and unnecessary confrontations (Dobzhansky 1940; Mayr 1942). Within a species, communication between the sexes is key for mate choice and competition for mating resources (Andersson 1994). Thus, intersexual communication is an informative model system for determining how communication systems are adapted to environmental and social constraints.
The South African clawed frog, Xenopus laevis, is a totally aquatic pipid species that lives in dark, silt-filled ponds and is active at night (Tinsley & Kobel 1996). Brief female sexual receptivity (less than 24 h when hormonally induced; Kelley 1996) and high population densities suggest that finding a mate might be an arduous task for a male. As in many other frog species (Ryan 2001), males advertise their location and sexual receptivity using vocalizations, but X. laevis belongs to the restricted group of anuran species where females produce a fertility call (Emerson & Boyd 1999) that results in courtship duets between partners (Tobias et al. 1998). Since both male and female X. laevis advertise their sexual receptivity vocally, this species offers a rare opportunity to investigate acoustic recognition of sex in males.
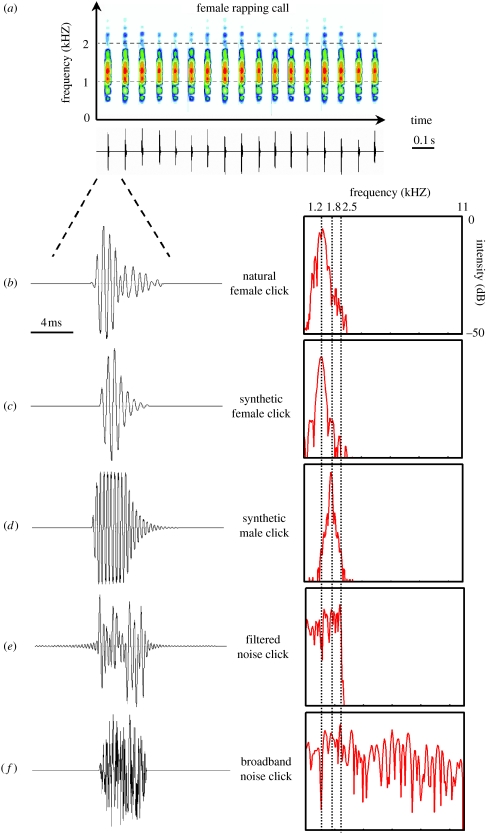
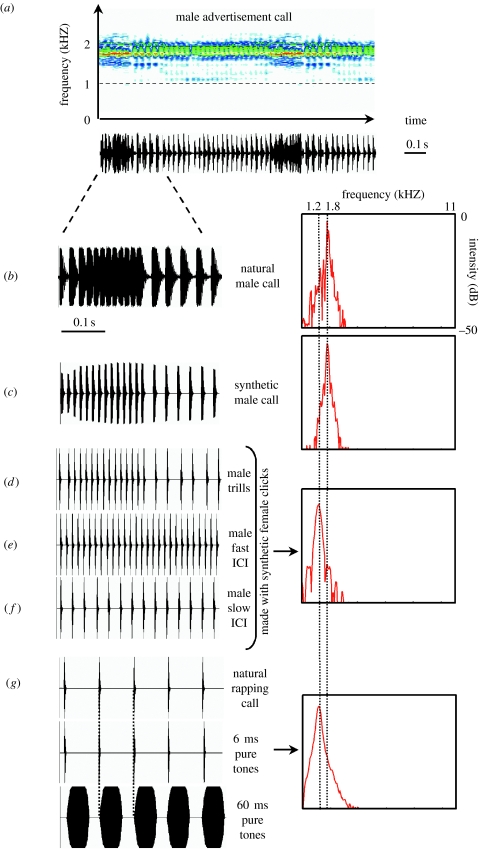
All X. laevis calls are underwater acoustic signals that consist of repetitive click trains (figures 1 and 2). Each click is a short impulse of sound (figure 1b). Click carrier frequency and click rate vary and represent sexually differentiated parameters (Wetzel & Kelley 1983; Tobias et al. 1998, 2004). Female clicks have a dominant carrier frequency ca 1.2 kHz (figure 1a) whereas most male clicks have peak frequencies between 1.7 and 2.2 kHz (figure 2a). Females produce two call types: a fast fertility call (rapping, figure 1a) that stimulates male calling and a slower unreceptive call (ticking) that suppresses male calling (Tobias et al. 1998). While these two call types differ in click rate (12 and 4 Hz, respectively), both are slower than most male calls (Tobias et al. 1998). Males exhibit socially regulated acoustic production. In the presence of females, males produce courtship-associated calls. The prevalent male advertisement call has a bimodal temporal pattern (figure 2a): the clicks are produced as alternating fast (60 Hz) and slow trills (30 Hz). The advertisement call is amplitude modulated: intensity peaks at the end of each fast trill. In the presence of a rapping female, males accelerate their advertisement call to produce an answer call with longer fast trills, shorter slow trills and increased intensity modulation (Tobias et al. 1998). During male–male encounters, the repertoire is enriched by additional call types (Kelley et al. 2001; Tobias et al. 2004): the dominant male—generally clasping the subordinate—emits a chirping call made up of short bursts of five clicks whereas the subordinate male produces a fast call, growling, with a 100 Hz click rate (Tobias et al. 2004). The male repertoire is thus adapted to the sexual identity of the audience. Since physical contacts are not required for these context-specific behaviours (Tobias et al. 1998, 2004) and social interactions in X. laevis rely mainly on the lateral line system and hearing (Elepfandt 1996a,b), the sex of the signaller could be assessed using acoustic features of the calls. These characteristics make the Xenopus vocal system a particularly good one in which to explore the significance of temporal and spectral acoustic cues in sexual recognition. Since female calls are unusual in anurans, previous studies of key acoustic features have focused on female responses (usually phonotaxis) to modified male calls (Wilczynski et al. 1995; Gerhardt & Schul 1999; Gerhardt & Hobel 2005). The prominence of female calling in X. laevis thus also presents an opportunity to open an investigation into whether the sexes use the same acoustic features for recognition and attraction.
Figure 1.
Modifications of female Xenopus laevis calls in the frequency domain. (a) Spectrogram (top, amplitude in colour scale; dynamic range, 35 dB; window size, 2048 points; filter bandwidth, 20 Hz) and oscillogram (bottom) of a natural rapping call. (b) Oscillogram (left) and power spectrum (right, window size, 2048 points) of a natural female click, (c) a synthetic female click, (d) a synthetic male click, (e) a filtered white noise click and (f) a broadband white noise click, used to build experimental modified calls.
Figure 2.
Test of the significance of temporal cues in Xenopus laevis calls. (a) Spectrogram (top, amplitude in colour scale; dynamic range, 35 dB; window size, 2048 points; filter bandwidth, 20 Hz) and oscillogram (bottom) of a male advertisement call. (b) Oscillogram (left) and power spectrum (right, window size, 2048 points) of a natural male call, (c) a synthetic male call, (d) a bimodal call (male-like trills) made with synthetic female clicks, (e) a male-like fast trill call (fast inter-click interval ICI) and (f) a male-like slow trill call (slow ICI) both made up with synthetic female clicks. (g) To test whether the significance of the click temporal pattern relies on the click period (the tempo) or the duty cycle (the ratio of click duration/inter-click silence), we copied the female rapping tempo (top) with 1.2 kHz pure-tone clicks of either 6 ms (middle) or 60 ms duration (bottom).
Two acoustic parameters are available to male X. laevis: click carrier frequency (pitch) and click rate (temporal pattern). These features could convey multiple pieces of information such as species identity, sex, individual identity and reproductive state or motivation. Studies in terrestrial treefrogs (Whitney & Krebs 1975; Brenowitz & Rose 1994; Gerhardt 1994) as well as in birds and mammals (Aubin 1989; Bremond & Aubin 1992; Lengagne et al. 2001; Mathevon & Aubin 2001; Charrier et al. 2002; Jouventin & Aubin 2002; Searby & Jouventin 2003) suggest two general hypotheses. One possibility is that pitch and spectral content convey information about the identity of the signaller, while temporal pattern and rhythmicity carry information about motivation or reproductive status. In another scheme, both spectral and temporal features convey identity. Here, we test these possible alternatives—joint or separate roles of spectral and temporal cues in sexual identity recognition—using playback experiments in which modified calls are used to evoke vocal responses in males. Since male X. laevis exhibit different levels of calling in response to the calls of other males than to female calls (Tobias et al. 2004), we predict that if a particular acoustic parameter is a key component of sexual identity in calls, its modification should change the male's vocal response.
2. Material and methods
(a) Subjects
Twenty-six sexually mature male X. laevis were obtained from Xenopus One (Ann Arbor, Michigan, USA) and housed in polycarbonate tanks at 20°C. Frogs were fed frog brittle (Nasco, Ft Atkinson, Wisconsin, USA) three times per week and maintained on a 12L : 12D cycle, thus all housing conditions were independent of natural seasons. Animal care procedures adhered to NIH and Columbia University's Institutional Animal Care and Use Committee guidelines (protocol number 1586). In order to ensure sexual receptivity, we injected males with gonadotropin, a treatment known to increase clasping and calling (Kelley & Pfaff 1976; Wetzel & Kelley 1983). Subjects received two injections subcutaneously of human chorionic gonadotropin (hCG; Sigma, St Louis, Missouri), usually 24 h (0.2 ml; 100 IU/0.1 ml) and 6 h (0.15 ml; 100 IU/0.1 ml) before observation.
(b) Playback signals
All natural calls used in this study were obtained from recordings during the breeding season in a clear-water concrete pond (Tobias et al. 1998) in the vicinity of Cape Town (South Africa) using a Cornell Bioacoustics hydrophone (output sensitivity, −163 dB at 1 V μ−1 Pa−1; frequency sensitivity, 0.015–10 kHz; sampling frequency, 44.1 kHz). These natural calls (female rapping and male advertisement calling) originated from different recordings (avoiding pseudoreplication); none of the frogs tested had experience with any of the individuals from which the calls were recorded. Calls to be used as stimuli in playback studies were filtered (0.5–2.5 kHz bandpass) to remove background noise occurring outside the natural frequency range of X. laevis clicks. To control every acoustic feature in the signal and to avoid potential information on individual identity, we built synthetic copies of these natural calls: we replaced natural female clicks (figure 1b) with synthetic female clicks (figure 1c; 1.2 kHz carrier frequency, 1.95 ms linear rise time, 1 ms plateau, 3.53 ms exponential fall time) and natural male clicks (figure 2b) with synthetic male clicks (figure 2c; 1.8 kHz carrier frequency, 1 ms linear rise time, 4 ms plateau, 7 ms exponential fall time). Natural amplitude modulation (AM) of the male advertisement call was added to the synthetic male calls using PRAAT software package (v. 4.0.48, www.praat.org; figure 2c). Synthetic female rapping calls (mean inter-click interval (ICI) ± S.D. measured between the starts of two successive clicks: 87±2 ms, 1.2 kHz carrier frequency) were modified in the frequency, temporal and intensity domains.
(i) Modifications in the frequency domain
To test the significance of the carrier frequency of clicks in female rapping, we built a call with the temporal features of rapping, but composed of synthetic male clicks (figure 1d; 1.8 kHz carrier frequency) using Goldwave (v. 5.12, www.goldwave.com). White noise clicks were built using computer-generated white noise (0–11.025 kHz) and then low-pass filtered at 2.5 kHz using Syntana software (Aubin 1994) according to the natural spectral range of X. laevis clicks (Tobias et al. 2004). Phase distortions introduced by signal filtering are likely to be very small in comparison with the reverberations within the experimental tank. These reverberations create, for all experimental signals, unpredictable phase distortions at all frequency ranges. However, reverberations did not appear to affect call recognition by the frogs, as revealed by behavioural responses to playbacks of natural female and male control calls (see §3). Filtered clicks (figure 1e) and broadband clicks (figure 1f) were used to build two experimental rapping calls.
(ii) Modifications in the temporal domain
To test the significance of the click temporal pattern in female rapping calls, female clicks were used to build a male-like bimodal call (male trills, figure 2d) (fast trill duration: 200±5 ms, ICI, 15 ±2 ms; slow trill duration: 800±5 ms, ICI, 30±2 ms) using Goldwave. To test the effect of each male trill type separately, a fast trill (male fast ICI, figure 2e; ICI, 15±2 ms) and a slow trill (male slow ICI, figure 2f; ICI, 30±2 ms) were also built using female clicks.
The significance of the click temporal pattern could be due to either the click period (the tempo) or the click duty cycle (the ratio of click duration/inter-click silence). We built 1.2 kHz pure-tone clicks of either 6 ms (2 ms linear rise time, 1 ms plateau, 3 ms exponential fall time, figure 2g) or 60 ms duration (12 ms linear rise time, 36 ms plateau, 12 ms linear fall time, figure 2g) using PRAAT, and these clicks were used to create a female rapping tempo (ICI: 87±2 ms). Thus, the pulse period remained the same but the duration of silence was 81±2 ms for the first stimulus and 27±2 ms for the second.
(iii) Modifications in the intensity domain
To test the significance of AM, the natural AM of a male advertisement call was applied to a female rapping call using PRAAT.
(c) Playback procedure
All the observations were conducted between 15.00 and 20.00 h during the first hours of the dark phase of the light: dark cycle, from October 2005 to March 2006. For playback tests, each frog was isolated from other individuals, moved from the colony room and placed in an experimental fibreglass tank (100 (width)×100 (length)×80 (height) cm). The tank stood on foam and plywood to isolate it from ground vibrations was filled two-thirds with dechlorinated water (water temperature, 18°C) and placed in a dark room (room temperature, 22°C). All experimental stimuli were broadcast through an underwater loudspeaker (University Sound, UW30, frequency response 0.1–10 kHz) placed on the bottom corner of the tank and connected to an amplifier (Realistic MPA30). Broadcast of the stimuli was controlled by a PC laptop (Sony VAIO). Signals were played at a natural sound pressure level mimicking a real frog (165 dB re μPa measured 2 cm in front of the snout of a frog; Elepfandt 1996b). The amplitude of all stimuli was adjusted to match the amplitude of the control (RMS levels adjusted using Goldwave). Two hydrophones (Cornell Bioacoustics, output sensitivity, −163 dB at 1 V μ−1 Pa−1; frequency sensitivity, 0.015–10 kHz) were placed in the tank; the one near the loudspeaker monitored the broadcast stimuli, while the other, in the middle of the tank, recorded the vocalizations emitted by the test frog. Both hydrophones were connected to a digital recorder (Marantz PMD670) and sound files were saved in stereo as MP3 files (48 kHz, 320 kpb). The acoustic characteristics of the tank had been checked prior to the experiment by broadcasting X. laevis calls and white noise using the same playback setup. While sound propagation in the tank resulted in a slight enhancement of the frequencies between 1.85 and 2.45 kHz, this enhancement did not appear to affect call recognition by the frogs, as revealed by the behavioural response to the playback of natural female and male control calls (see §3). Click duration was not significantly modified by propagation in the tank (see §2b(i) for a discussion of signal phase distortion).
The playback procedure was as follows: after 15 min of acclimatization, the spontaneous calling of the test frog was recorded for 6 min. Then, a control stimulus (6 min of a natural female rapping call) was broadcast. If no vocal activity was recorded during this control stimulus, the test was stopped and the frog was eliminated from the study. Thus, this first control stimulus served to control for the general responsiveness of the frog as well as to avoid a surprise effect of the first playback that could modify the arousal of the animal. The frog next heard playbacks of seven stimuli (6 min each): four experimental stimuli (modified calls), one natural male advertisement call, one synthetic male advertisement call and one synthetic female rapping call. Consequently, not all nine modified calls described above (frequency, temporal and intensity domains) were tested on each individual frog: only a subset of four modified calls out of nine was randomly chosen for each individual frog. Moreover, the order of these stimuli was randomized for each tested frog. No frog was tested more than once with any one experimental stimulus. In order to control for any habituation to the playback stimuli, the test ended with playback of a natural female rapping call. If no vocal activity was recorded during this final control stimulus, the test was eliminated from the study. Twenty-six out of 50 frogs (52%) passed the two control checkpoints (first and final stimuli of the test). Since these controls selected only males that were highly receptive to female calls, results reflect responses of males with high sexual motivation. The entire playback experiment lasted for 1 h. Each stimulus presentation of 6 min consisted of three repetitions of a 30 s set followed by 1.5 min of silence. Each set presented four playback blocks of 5 s each separated by 3 s of silence. This design was chosen to ensure discontinuous stimulation. Since the natural mean durations of call production differ by sex but have overlapping ranges, this design mimicked a mean calling duration that could have been produced by either sex.
(d) Response criteria and statistical analysis
The frog's response to the playback was determined by how much time it spent producing the advertisement call (in seconds), measured during each stimulus (6 min). Since the time spent advertisement calling during the entire test was highly variable across individuals (mean±s.d.=1632.23±836.45 s, min=326 s, max=2855 s), the time spent advertisement calling during each stimulus was divided by the total time spent advertisement calling by the individual during the entire test. This procedure produced a parameter called an advertisement call allocation factor that reflects the proportion of time spent advertisement calling to each stimulus. Allocation factor values obtained for each experimental stimulus were normally distributed (Shapiro–Wilk test, p>0.05) and respected homoscedasticity (Levene test, F=1.593, p=0.088), allowing us to use a parametric analysis of variance (ANOVA). Moreover, the individual identity of the tested frog had no effect on the allocation factor values (one-way ANOVA F(25,208)=0.08, p=1). Thus, we could pool all individual measures. The effects of the playback of experimental stimuli were assessed using a one-way ANOVA. The amount of spontaneous advertisement calling was compared with the amount of advertisement calling in response to natural male and female calls using a t-test with the Bonferroni correction for multiple comparisons. The advertisement calling response to each experimental stimulus was compared with the response to the control natural female rapping call using a Dunnett post hoc test.
The call that a sexually mature male X. laevis produces depends on social context. In the presence of a rapping female, males accelerate their advertisement call to produce an answer call with shorter slow trills (mean duration, 265 ms) and longer fast trills (mean duration, 281 ms) and with increased intensity modulation (Tobias et al. 1998). The answer call is often given during duets with a rapping female: each sex responds to the other which produces overlapping calls. During male–male encounters, the dominant male (generally clasping the subordinate) emits a chirping call made up of very short trills (five clicks) with an inter-trill interval ca 200 ms (Tobias et al. 2004). Although the advertisement call was the main call type produced during the playback tests, these two other call types (answer calls and chirping) were also recorded. Since these call types were relatively rare, the time spent producing these calls was not normally distributed. Thus, non-parametric statistical analyses were performed (Kruskal–Wallis test followed by Wald–Wolfowitz runs test with Bonferroni-corrected p-values). All statistical tests were carried out using Statistica software v. 6.
3. Results
(a) Effect of female call modifications on male advertisement calling
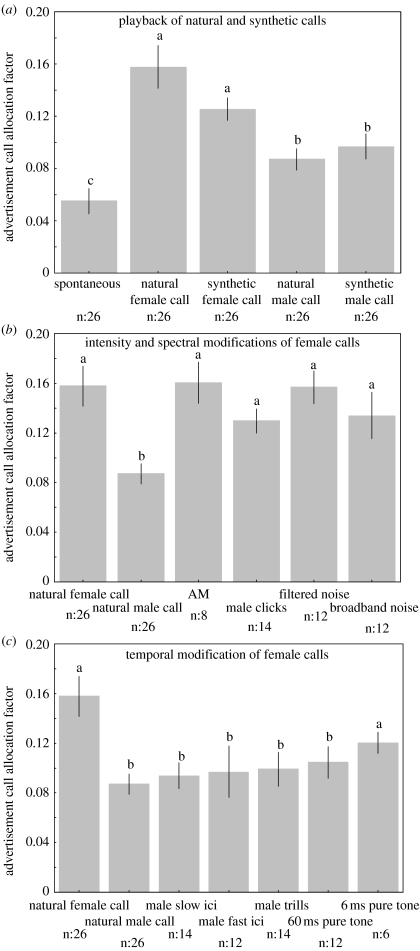
Male X. laevis reacted differentially to different playback stimuli, and therefore effects of playbacks on the advertisement call allocation factor value were significant (F(13,220)=6.129, p<10−5). Male and female natural calls effectively evoked males to call, increasing the level of advertisement calling (spontaneous versus natural female call p=2×10−6, spontaneous versus natural male call p=0.031; figure 3a). Thus, playbacks were effective stimuli for advertisement calling activity. Synthetic calls were also effective stimuli. The advertisement calling response to female rapping calls built with synthetic female clicks is not significantly different from the response to natural female calls (p=0.261; figure 3a). However, the advertisement calling response to natural male advertisement calls and to synthetic male advertisement calls made up of synthetic male clicks was significantly lower than to natural female rapping (p=6×10−5 and 7×10−4, respectively; figure 3a).
Figure 3.
Results of playbacks of non-modified calls and modified female calls on male Xenopus laevis advertisement call allocation factors (mean±s.e.m). (a) Spontaneous calling compared with the response to natural female rapping call, synthetic female call, natural male advertisement call and synthetic male call. (b) Response to intensity modification (AM) and frequency modifications (male clicks, filtered noise, broadband noise) of female calls compared with natural controls. (c) Response to temporal modifications (male slow ICI, male fast ICI, male trills, 60 ms pure tone, 6 ms pure tone) of female calls compared with natural controls. Comparisons between responses to different stimuli were performed using a one-way ANOVA (F(13,220)=6.129, p<10−5), followed by t-test and Dunnett post hoc test with Bonferroni correction. Means represented with different letters are significantly different (p<0.05). Sample sizes are indicated for each stimulus.
Males showed similar advertisement calling responses to the natural female call and to the female call with spectral modifications (male clicks, p=0.683; filtered noise clicks, p=1; broadband noise clicks, p=0.89) or with intensity modification (AM, p=1; figure 3b). Thus, these modified stimuli (spectral and intensity modifications of female calls) can be operationally classified as ‘female-like’.
In contrast, modifications of the temporal pattern of the female calls significantly decreased males' advertisement calling responses (male slow ICI, p=4×10−3; male fast ICI, p=0.015; male trills, p=0.013; figure 3c). Since these temporal modifications of female calls reduced advertisement calling as compared with natural female rapping (as male calls do), they can be operationally classified as ‘male-like’. Thus, while spectral and amplitude modifications of female rapping calls did not affect the male's advertisement calling response, temporal modifications did.
Which aspects of the temporal pattern do males use to recognize female rhythms? The significance of the temporal pattern could rely either on the click period (the tempo) or the duty cycle (the ratio of click duration/inter-click silence). Whereas a 60 ms pure-tone stimulus produced a nearly significantly depressed advertisement calling response (p=0.053), a 6 ms pure-tone stimulus clearly did not (p=0.715; figure 3c). Therefore, we tentatively conclude that while click period or tempo is insufficient for recognition, drastic modification of click duration does impair male advertisement calling responses. This preliminary experiment gives supporting evidence for a role of duty cycle (ratio of click duration/inter-click silence) as discriminative cue.
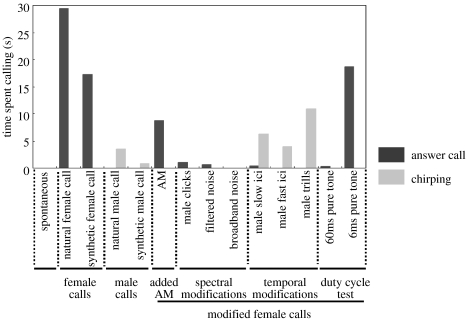
(b) Effect of female call modifications on male answer calling
Although the advertisement call was the main call type produced during the playback tests, 69% (18 out of 26) of the frogs tested also produced answer calls, which is characteristic of a male responding to a female rapping call. The answer call was generally produced during positive phonotaxis (movement towards the speaker). The proportion of time spent producing answer calls was linearly correlated with the total time spent calling by the frog (r=0.507, p=0.008): the more the frog called during the test, the more it used answer calls in response to the playback. The ‘male-like’ stimuli (i.e. modifications of female temporal pattern: male trills, male fast ICI and male slow ICI) never or very rarely evoked answer calling (figure 4). On the contrary, all ‘female-like’ stimuli (AM, male clicks, filtered noise clicks and broadband noise clicks) evoked answer calling though with great variability (figure 4). Among stimuli that evoked answer calling, the extent of the vocal response was significantly affected by stimulus type (H(7, 38)=16.34, p=0.022). The response to female rapping calls built with synthetic female clicks was not significantly different from the response to natural rapping calls (Z=0.60, p=1). Both natural and synthetic female rapping triggered more answer calls than female calls modified in the spectral domain (male clicks: Z=2.516, p=0.05; filtered noise clicks: Z=2.516, p=0.05; broadband noise clicks). Added intensity modulation failed to abolish answer calling (Z=1.225, p=1). Differences in answer calling to 6 ms (Z=0.557, p=1) and 60 ms pure-tone stimuli confirmed that the former is recognized as ‘female-like’ and the latter as ‘male-like’. Thus, both a female-like temporal pattern and a female-like spectral content are necessary to trigger the full variety of male courtship vocal responses.
Figure 4.
Time spent answer calling (courtship call, dark bars) and chirping (aggressive call, light bars) by male Xenopus laevis during playbacks of non-modified calls and modified female calls (time in seconds normalized by the number of stimulus presentations).
(c) Effect of female call modifications on male chirping
Only 23% (6 out of 26) of the tested frogs chirped during the playback. All frogs that used this typical male–male encounter call also answer called during the experiment. Thus, individuals with high level of vocal activity are also those that express a larger vocal repertoire. Nevertheless, chirping and answer calls were produced differentially to different stimuli (χ2; p<0.001; figure 4). Males chirped in response to all ‘male-like’ modifications of the female call (i.e. modifications of female temporal pattern: male trills, male fast ICI and male slow ICI) as they do in response to natural and synthetic male calls (H(4, 23)=4.55; p=0.336). Thus, a male-like temporal pattern is sufficient to enhance typical male–male encounter vocalizations.
4. Discussion
Several studies dealing with acoustic communication in frogs have investigated species recognition or discrimination among potential mates by females (Gerhardt 1994; Ryan & Rand 2001). Since both male and female X. laevis advertise their sexual receptivity vocally, this species offers a rare opportunity to explore sexual acoustic discrimination by males. In this study, we used playbacks of synthetic calls in which sex-specific acoustic features were modified to show that male X. laevis use the temporal pattern of calls to identify the sex of an individual. A male-like temporal pattern is sufficient to trigger typical male–male encounter vocalizations (low level of advertisement calling and aggressive chirping) regardless of spectral cues, but both female-like temporal pattern and female-like spectral content are required to drive the complete panoply of male courtship vocal responses to a receptive female (high level of advertisement calling and duetting answer calls). Consequently, the attractiveness of female calls also relies on spectral properties. Our evidence suggests that temporal acoustic cues support sexual identity recognition and spectral acoustic cues contribute to female attractiveness in X. laevis.
The results of the present study can be seen in the light of the two general alternatives for acoustic communication systems: joint or separate roles for spectral and temporal cues. In the neotropical túngara frog, Physalaemus pustulosus, females use different components of male advertisement calls for species recognition and attractiveness. While the downward frequency sweep of the ‘whine’ is required for species recognition, a call is more likely to attract females when it is accompanied by one or several multiharmonic ‘chuck’ notes (Rand & Ryan 1981; Ryan 1983; Ryan & Rand 1990, 1998; Wilczynski et al. 1995). The chuck is an example of an ‘acoustic adornment’ (Ryan & Rand 1990), a feature that increases attractiveness but is not required for recognition. In X. laevis, while the temporal pattern of clicks conveys sexual identity and thus determines the intensity of the male's vocal response, click carrier frequency enhances attractiveness. Thus, spectral cues serve as acoustic adornments for the Xenopus male's response to female vocal signals much as chucks serve as acoustic adornments for the túngara female frog's response to male vocal signals.
In the green treefrog, Hyla cinerea, addition of an artificial component of intermediate frequency to a male advertisement call that naturally includes two spectral peaks reduced the call's attractiveness to females (Gerhardt 1974; Gerhardt & Hobel 2005). In our experiment, addition of spectral components to the female rapping call (broadband noise clicks or filtered noise clicks) reduced attractiveness to males, revealed by the intensity of the male courtship display. Thus, our results suggest the existence of a comparable phenomenon of frequency suppression in X. laevis. Further investigations would be necessary to define the existence, if any, of a precise suppression range.
An influential hypothesis in the field of animal communication is ‘sensory exploitation’, the idea that pre-existing female sensory biases—rather than receiver–sender coevolution—can explain the phylogenetic appearance of male traits (Ryan & Rand 1990). Whether the bias is confined to females or whether it is also present in males has not been determined primarily because female calling is rare in anurans. Our study in X. laevis reveals key acoustic features used by males in determining the sexual identity and the attractiveness of the caller. Whether the same acoustic features are used for the same purposes by females is an intriguing question. In the Puerto Rican treefrog, Eleutherodacylus coqui, males have a two-note call; the first note—a constant frequency ‘Co’—is used in male–male territorial encounters, whereas the second note—an upsweeping ‘Qui’—is attractive to females (Narins & Capranica 1976, 1978). Whether male and female X. laevis also process calls differently remains to be determined.
An alternative hypothesis is that a female-like spectral content secures information about sexual identity initially conveyed by temporal cues. Females cannot produce rapid click trains: the rate of click production in females is constrained by the physiology of the vocal organ. Female larynges contain slow twitch muscle fibres which cannot contract and relax as rapidly as male laryngeal muscles (Tobias & Kelley 1987; Tobias et al. 1991). A very fast click rate thus easily discriminates male from female callers, whereas a slow click rate could be produced by either sex (the male amplectant call, e.g. a call made by males while clasping females is slow). Thus, a male may perform a double identity check: it may need to rely on other information in addition to click rate, such as spectral content, to unambiguously identify a female.
Our results show that the vocal activity level of male X. laevis is modulated by the temporal pattern of the calls. A male-like fast call rhythm triggers less vocal activity than a female-like slow rhythm. This weaker response is evoked by both monotonous calls presenting a constant click rate (stimuli ‘male fast ICI’ or ‘male slow ICI’) and by bimodal calls with alternating slow and fast click rates (stimulus ‘male trills’). Thus, the categorization of sexual identity may depend on identifying temporal pattern: fast click rhythms are classified as male. Repetitive trains of pulse-like stereotyped clicks are a reliable way to encode information during underwater acoustic communication (Elepfandt 1996b; Bass & Clark 2003). Our results are consistent with previous studies in some fishes (Bass & McKibben 2003) showing that recognition relies on the temporal pattern of the strong intensity variations represented by the clicks. In male X. laevis calls, click intensity is also modulated over time. However, we do not observe any effect of intensity modulation on recognition of sexual identity in calls. Although it is a highly male-specific acoustic feature, this slow intensity modulation may be an unreliable cue since it could be modified during sound propagation. For example, this feature could be added to female calls due to sound modification during propagation. Indeed, reflection phenomena of water-borne sounds at the surface of shallow waters like X. laevis natural ponds result in phase inversion of the reflected waves (Elepfandt 1996b) and thus variation in sound amplitude.
Our preliminary test of the significance of the duty cycle suggests that an increase in click duration without perturbation of click period suppresses males' preferences for female-like rhythms. While we used only two acoustic stimuli with vastly different duty cycles and our data must be considered as tentative, our results suggest that perception of temporal pattern in X. laevis might rely on duty cycle rather than tempo. Further playback experiments will be necessary to validate this hypothesis and to determine threshold duty cycle values for temporal pattern perception. Indeed, the absolute durations of intervals and clicks could be significant cues for temporal pattern identification, as is true in other frog species such as the grey treefrog, Hyla versicolor (Schul & Bush 2002), and in other taxa that use acoustic signals made up of repetitive sound burst trains such as Cicada (Fonseca & Revez 2002; Sueur & Aubin 2003) or Tettigonia (Schul 1998). Previous studies in terrestrial frog species (Gerhardt & Schul 1999; Edwards et al. 2005) have revealed an important role for pulse rise time in temporal perception. To completely understand how male X. laevis use the temporal pattern of clicks for sex recognition, further playback experiments using varied click shapes are needed. Recent studies in the northern leopard frog (Rana pipiens) and the Pacific treefrog (Hyla regilla; Alder & Rose 1998; Brenowitz & Rose 1999; Edwards et al. 2002; Edwards & Rose 2003) have investigated the basis of selective temporal filters and have identified interval-counting midbrain neurons. Our results indicate that X. laevis is also a candidate for the study of the neural substrates that function as detectors of temporal patterns in calls and could provide new support for interval-counting processes as a general mechanism of temporal perception in frogs.
Acknowledgments
The authors are grateful to Taffeta Elliott for help with the playback setup and Jakob Christensen-Dalsgaard for providing the synthetic clicks. Thanks to Taffeta Elliott, Elizabeth Leininger, Brian Nasipak, Hédi Soula, Martha Tobias, Eun-Jin Yang, Erik Zornik and four anonymous referees for their constructive comments on the manuscript. This work was supported by a fellowship from the French Région Rhône-Alpes. Research in the authors' laboratory is supported by a grant (NS23864) from the National Institutes of Health.
References
- Alder T.B, Rose G.J. Long-term temporal integration in the anuran auditory system. Nat. Neurosci. 1998;1:519–523. doi: 10.1038/2237. doi:10.1038/2237 [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Aubin T. The role of frequency modulation in the process of distress calls recognition by the starling (Sturnus vulgaris) Behaviour. 1989;108:57–72. [Google Scholar]
- Aubin T. Syntana: a software for the synthesis and analysis of animal sounds. Bioacoustics. 1994;6:80–81. [Google Scholar]
- Bass A.H, Clark C.W. The physical acoustics of underwater sound communication. In: Simmons A.M, Popper A.N, Fay R.R, editors. Acoustic communication. Springer; New York, NY: 2003. pp. 15–63. [Google Scholar]
- Bass A.H, McKibben J.R. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. doi:10.1016/S0301-0082(03)00004-2 [DOI] [PubMed] [Google Scholar]
- Bremond J.C, Aubin T. The role of amplitude modulation in distress call recognition by the black-headed gull (Larus ridibundus) Ethol. Ecol. Evol. 1992;4:187–191. [Google Scholar]
- Brenowitz E.A, Rose G.J. Behavioral plasticity mediates aggression in choruses of the Pacific treefrog. Anim. Behav. 1994;47:633–641. doi:10.1006/anbe.1994.1086 [Google Scholar]
- Brenowitz E.A, Rose G.J. Female choice and plasticity of male calling behaviour in the Pacific treefrog. Anim. Behav. 1999;57:1337–1342. doi: 10.1006/anbe.1999.1111. doi:10.1006/anbe.1999.1111 [DOI] [PubMed] [Google Scholar]
- Charrier I, Mathevon N, Jouventin P. How does a fur seal mother recognize the voice of her pup? An experimental study of Arctocephalus tropicalis. J. Exp. Biol. 2002;205:603–612. doi: 10.1242/jeb.205.5.603. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. Am. Nat. 1940;74:312–321. doi:10.1086/280899 [Google Scholar]
- Edwards C.J, Rose G.J. Interval-integration underlies amplitude modulation band-suppression selectivity in the anuran midbrain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189:907–914. doi: 10.1007/s00359-003-0467-2. doi:10.1007/s00359-003-0467-2 [DOI] [PubMed] [Google Scholar]
- Edwards C.J, Alder T.B, Rose G.J. Auditory midbrain neurons that count. Nat. Neurosci. 2002;5:934–936. doi: 10.1038/nn916. doi:10.1038/nn916 [DOI] [PubMed] [Google Scholar]
- Edwards C.J, Alder T.B, Rose G.J. Pulse rise time but not duty cycle affects the temporal selectivity of neurons in the anuran midbrain that prefer slow AM rates. J. Neurophysiol. 2005;93:1336–1341. doi: 10.1152/jn.00797.2004. doi:10.1152/jn.00797.2004 [DOI] [PubMed] [Google Scholar]
- Elepfandt A. Sensory perception and the lateral line system in the clawed frog, Xenopus laevis. In: Tinsley R, Kobel H, editors. The biology of Xenopus. Clarendon Press; Oxford, UK: 1996a. pp. 97–120. [Google Scholar]
- Elepfandt A. Underwater acoustics and hearing in the clawed frog, Xenopus. In: Tinsley R, Kobel H, editors. The biology of Xenopus. Oxford University Press; Oxford, UK: 1996b. pp. 177–193. [Google Scholar]
- Emerson S.B, Boyd S.K. Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav. Evol. 1999;53:187–197. doi: 10.1159/000006594. doi:10.1159/000006594 [DOI] [PubMed] [Google Scholar]
- Fonseca P.J, Revez M.A. Song discrimination by male cicadas Cicada barbara lusitanica (Homoptera, Cicadidae) J. Exp. Biol. 2002;205:1285–1292. doi: 10.1242/jeb.205.9.1285. [DOI] [PubMed] [Google Scholar]
- Gerhardt H.C. The significance of some spectral features in mating call recognition in the green treefrog (Hyla cinerea) J. Exp. Biol. 1974;61:229–241. doi: 10.1242/jeb.61.1.229. [DOI] [PubMed] [Google Scholar]
- Gerhardt H.C. The evolution of vocalization in frogs and toads. Annu. Rev. Ecol. Syst. 1994;25:293–324. doi:10.1146/annurev.es.25.110194.001453 [Google Scholar]
- Gerhardt H.C, Hobel G. Mid-frequency suppression in the green treefrog (Hyla cinerea): mechanisms and implications for the evolution of acoustic communication. J. Comp. Physiol. A. 2005;191:707–714. doi: 10.1007/s00359-005-0626-8. doi:10.1007/s00359-005-0626-8 [DOI] [PubMed] [Google Scholar]
- Gerhardt H.C, Schul J. A quantitative analysis of behavioral selectivity for pulse rise-time in the gray treefrog, Hyla versicolor. J. Comp. Physiol. A. 1999;185:33–40. doi: 10.1007/s003590050363. doi:10.1007/s003590050363 [DOI] [PubMed] [Google Scholar]
- Jouventin P, Aubin T. Acoustic systems are adapted to breeding ecologies: individual recognition in nesting penguins. Anim. Behav. 2002;64:747–757. doi:10.1006/anbe.2002.4002 [Google Scholar]
- Kelley D.B.K. Sexual differenciation in Xenopus laevis. In: Tinsley R, Kobel H, editors. The biology of Xenopus. The Zoological Society of London; Oxford, UK: 1996. pp. 143–176. [Google Scholar]
- Kelley D.B, Pfaff D.W. Hormone effects on male sex behavior in adult South African clawed frogs, Xenopus laevis. Horm. Behav. 1976;7:159–182. doi: 10.1016/0018-506x(76)90045-3. doi:10.1016/0018-506X(76)90045-3 [DOI] [PubMed] [Google Scholar]
- Kelley D.B.K, Tobias M.L, Horng S. Producing and perceiving frog songs. Dissecting the neural bases for vocal behaviors in Xenopus laevis. In: Ryan M.J, editor. Anuran communication. Smithsonian Institution Press; Washington, DC: 2001. p. 252. [Google Scholar]
- Lengagne T, Lauga J, Aubin T. Intra-syllabic acoustic signatures used by the king penguin in parent–chick recognition: an experimental approach. J. Exp. Biol. 2001;204:663–672. doi: 10.1242/jeb.204.4.663. [DOI] [PubMed] [Google Scholar]
- Mathevon N, Aubin T. Sound-based species-specific recognition in the blackcap Sylvia atricapilla shows high tolerance to signal modifications. Behaviour. 2001;138:511–524. doi:10.1163/156853901750382133 [Google Scholar]
- Mayr E. Columbia University Press; New York, NY: 1942. Systematics and the origin of species. [Google Scholar]
- Narins P.M, Capranica R.R. Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science. 1976;192:378–380. doi: 10.1126/science.1257772. [DOI] [PubMed] [Google Scholar]
- Narins P.M, Capranica R.R. Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J. Comp. Physiol. A. 1978;127:1–9. doi:10.1007/BF00611921 [Google Scholar]
- Rand A.S, Ryan M.J. The adaptative significance of a complex vocal repertoire in a neotropical frog. Z. Tierpsychol. 1981;57:209–214. [Google Scholar]
- Ryan M.J. Frequency modulated calls and species recognition in a neotropical frog. J. Comp. Physiol. A. 1983;150:217–221. doi:10.1007/BF00606371 [Google Scholar]
- Ryan M.J. Smithsonian Institution Press; Washington, DC: 2001. Anuran communication. [Google Scholar]
- Ryan M.J, Rand A.S. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation) Evolution. 1990;44:305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x. doi:10.2307/2409409 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Rand A.S. Evoked vocal response in male túngara frogs: pre-existing biases in male responses? Anim. Behav. 1998;56:1509–1516. doi: 10.1006/anbe.1998.0928. doi:10.1006/anbe.1998.0928 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Rand A.S. Feature weighting in signal recognition and discrimination by túngara frogs. In: Ryan M.J, editor. Anuran communication. Smithsonian Institution Press; Washington, DC: 2001. pp. 86–101. [Google Scholar]
- Schul J. Song recognition by temporal cues in a group of closely related bushcricket species (genus Tettigonia) J. Comp. Physiol. A. 1998;183:401–410. doi:10.1007/s003590050266 [Google Scholar]
- Schul J, Bush S.L. Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc. R. Soc. B. 2002;269:1847–1852. doi: 10.1098/rspb.2002.2092. doi:10.1098/rspb.2002.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searby A, Jouventin P. Mother–lamb acoustic recognition in sheep: a frequency coding. Proc. R. Soc. B. 2003;270:1765–1771. doi: 10.1098/rspb.2003.2442. doi:10.1098/rspb.2003.2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueur J, Aubin T. Specificity of cicada calling songs in the genus Tibicina (Hemiptera: Cicadidae) Syst. Entomol. 2003;28:481–492. doi:10.1046/j.1365-3113.2003.00222.x [Google Scholar]
- Tinsley R, Kobel H. The Zoological Society of London; Oxford, UK: 1996. The biology of Xenopus. [Google Scholar]
- Tobias M.L, Kelley D.B. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J. Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M.L, Marin M.L, Kelley D.B. Development of functional sex differences in the larynx of Xenopus laevis. Dev. Biol. 1991;147:251–259. doi: 10.1016/s0012-1606(05)80022-3. doi:10.1016/S0012-1606(05)80022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M.L, Viswanathan S.S, Kelley D.B. Rapping, a female receptive call, initiates male–female duets in the South African clawed frog. Proc. Natl Acad. Sci. USA. 1998;95:1870–1875. doi: 10.1073/pnas.95.4.1870. doi:10.1073/pnas.95.4.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias M.L, Barnard C, O'Hagan R, Horng S.H, Rand M, Kelley D.B.K. Vocal communication between male Xenopus laevis. Anim. Behav. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. doi:10.1016/j.anbehav.2003.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel D.M, Kelley D.B. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm. Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. doi:10.1016/0018-506X(83)90048-X [DOI] [PubMed] [Google Scholar]
- Whitney C.L, Krebs J.R. Mate selection in Pacific tree frogs. Nature. 1975;255:325–326. doi:10.1038/255325a0 [Google Scholar]
- Wilczynski W, Rand A.S, Ryan M.J. The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Anim. Behav. 1995;49:911–929. doi:10.1006/anbe.1995.0123 [Google Scholar]