Abstract
Tame behaviour, i.e. low wariness, in terrestrial island species is often attributed to low predation pressure. However, we know little about its physiological control and its flexibility in the face of predator introductions. Marine iguanas (Amblyrhynchus cristatus) on the Galápagos Islands are a good model to study the physiological correlates of low wariness. They have lived virtually without predation for 5–15 Myr until some populations were first confronted with feral cats and dogs some 150 years ago. We tested whether and to what extent marine iguanas can adjust their behaviour and endocrine stress response to novel predation threats. Here, we show that a corticosterone stress response to experimental chasing is absent in naive animals, but is quickly restored with experience. Initially, low wariness also increases with experience, but remains an order of magnitude too low to allow successful escape from introduced predators. Our data suggest that the ability of marine iguanas to cope with predator introductions is limited by narrow reaction norms for behavioural wariness rather than by constraints in the underlying physiological stress system. In general, we predict that island endemics show flexible physiological stress responses but are restricted by narrow behavioural plasticity.
Keywords: anti-predator behaviour, wariness, corticosterone stress response, island endemic, introduced predator, Galápagos
1. Introduction
In the absence of predators, island habitats allow for physiological and anatomical adaptations such as flightlessness (McNab 1994) which reduce the capability to escape once predators are introduced. In contrast to such hard-wired traits, behavioural patterns, e.g. the prominent tame behaviour in island endemics (Blumstein 2002; Blumstein & Daniel 2005), should be more flexible such that experience-dependent anti-predator behaviour can be quickly regained (Berger et al. 2001). This behavioural flexibility is the focus of many re-introduction programmes training naive animals to recognize and cope with introduced predators (Beck et al. 1994; Maloney & MacLean 1995; Griffin et al. 2000). Still, in most cases, it is difficult to predict the behavioural plasticity of insular animals that are in contact with introduced predators, mainly because our understanding of the physiological mechanisms controlling anti-predator behaviour such as flight is deficient. Flight is usually accompanied by a physiological stress response assumed to promote the success of flight (Hofer & East 1998). A major endocrine regulatory unit of the stress response, the hypothalamo–pituitary–adrenal (HPA) axis, can be assessed by determining corticosteroid hormones in the blood plasma, even under field conditions (Sapolsky et al. 2000). In species accustomed to the presence of local predators, the detection of a predation threat is known to activate the HPA axis and to increase circulating plasma corticosteroid concentrations within about 3 min (Scheuerlein et al. 2001; Cockrem & Silverin 2002; Romero & Reed 2005). However, little if anything is known about the interactions between wariness and the endocrine stress response in free-ranging tame animals (those not accustomed to the presence of predators).
Here, we investigate the physiological background of tame behaviour, i.e. low wariness. We experimentally test the behavioural and the physiological responses of an island endemic to a novel threat using local differences in predation risk and an unusually high incident of predation. This setting allowed the replication of our experiments at three sites with animals (i) living without predators, (ii) living with intermediate predation risk, and (iii) living with acute, heavy predation risk from introduced dogs. We chose the Galápagos marine iguana (Amblyrhynchus cristatus) as a model species owing to its exceptional low wariness and a large body of available background information on both the behaviour and the physiology of the species (Wikelski 2005).
2. Material and methods
(a) Study sites and experimental design
We studied marine iguanas at three sites in the Galápagos archipelago that differed in predation threat: (i) the small island Caamaño (0°45′31″ S 90°16′43″ W), 4 km from the town Puerto Ayora/St Cruz, never had any introduced predators, (ii) the population at Punta Nuñez (0°44′45″ S 90°15′17″ W) on the southeast coast of St Cruz is age biased towards adult animals due to predation by feral predators (Yacelgas 1995), and (iii) Our third study site on San Cristobal Island (0°55′05″ S 89°37′40″ W) is about 2.5 km west of the town of Puerto Baquerizo Moreno and 70 km from the other two sites. At the time of the study, it was the site with the highest known predation pressure, suffering from acute predation by dogs probably from the nearby town (see electronic supplementary material).
At each site, we recorded flight initiation distances as a measure of wariness. Furthermore, we caught animals to sample circulating levels of plasma corticosterone (CORT, the principal glucocorticoid ‘stress’ hormone in reptiles). We determined CORT (i) from undisturbed animals (‘naive controls’), (ii) after restraining the captured individuals in cloth bags (‘naive restrained’ group) for 30 min (a standard ‘stress’ protocol), and (iii) in a different set of animals, after experimentally chasing them for 15 min (‘chased’). We then painted small numbers on the flanks of the experimental animals and (iv) recaptured a subset of the animals previously caught for the restraint ‘stress’ protocol after three to four weeks. Before capture, these animals were experimentally chased (the ‘previously caught and chased’ group). Paint usually wears off after six to eight weeks and is not known to harm the animals.
We recorded wariness and CORT data from within a site during one season (December 2003 to January 2004 on Caamaño, March 2005 on San Cristobal), with the exception of Punta Nuñez where CORT values were taken in November to December 2003 and March 2005. Since we found no differences in the CORT values between years at Punta Nuñez (unpaired t-test with naive controls, t=0.41, d.f.=24, p=0.690), we lumped all the data for this site. A restriction to our dataset was that our study site on San Cristobal is situated in a military zone, thus no data for the previously caught and chased group could be collected due to logistic constraints.
We excluded females, bachelor males and young animals from our main analysis to reduce variability due to sex- and status-specific characteristics (Berger et al. in preparation). However, we used additional young animals (between 2 and 3 years of age) to test for differences between naive controls and chased groups. We expected young animals to behave differently because they are more prone to predation by natural predators (Galápagos hawk and herons) than adults.
(b) Flight initiation distances and experimental chasing
We randomly chose our study animals (territorial male iguanas) from a distance where we did not obviously influence their behaviour (no change in body posture due to our presence). One observer approached the animal in a standardized way along a straight line, with a constant speed of about 0.5 m s−1, avoiding rapid movements and not wearing sunglasses as this could influence responses (Burger & Gochfeld 1990). The location of the observer at which the animal started to move was marked, and the shortest distance to the animal's original location defined the flight initiation distance (FID; Cooper 2003; Stankowich & Blumstein 2005). During the experimental chasing protocol, we essentially repeated the above-described flight excitation for a period of 15 min. During 15 min, the observer briefly halted each time the focal animal moved, but continued to slowly walk towards the animal after it had stopped moving, which usually lasted a few seconds. We then caught the experimental animals immediately at the end of the 15 min of chasing and took CORT samples.
(c) Blood sampling and processing
Within 3 min of our first capture attempt for an individual, we drew up to 0.5 ml of blood using heparinized vacutainers (Becton Dickinson, Franklin Lakes, New Jersey) from the caudal vein (Romero & Wikelski 2001). We stored the blood in an insulated container with cool gels for no more than a few hours and then centrifuged it at about 200 g for 5 min. The plasma was then transferred into a gas powered camping refrigerator in the field (3–7°C), for one to three weeks and subsequently frozen at −20°C and transported to Princeton University on dry ice. Laboratory analysis involved a dichloromethane extraction for steroids and a competitive binding radioimmuno assay (RIA) as described elsewhere (Wingfield et al. 1992). Average sample volume of the RIA was 50 μl, average intra- and inter-assay variation was 3.0 and 17.23%, respectively, and the detection limit averaged at 0.55 ng ml−1.
(d) Testing potential confounds
Many studies on FIDs in wild animals assume that the animals' responses towards humans reflect similar responses towards natural predators (Stone et al. 1994; Sapolsky et al. 2000; Frid & Dill 2002). We tested this assumption for both parameters used in this study (FID and CORT response to chasing) by comparing results from (i) a person accompanied by a trained dog on a leash approaching and chasing (see electronic supplementary material), with (ii) a person approaching and chasing without a dog. FIDs of animals close to Puerto Ayora, St Cruz Island, which were approached with a dog (mean±s.e.m.=2.52±1.52, n=13) were not significantly different from animals approached without a dog (mean±s.e.m.=2.04±1.41, n=30, t=1.329, d.f.=41, p=0.191). Similarly, CORT concentrations from animals at Punta Nuñez were not significantly different when chased by the experimenter with (mean±s.e.m.=4.81±1.71, n=10) or without a dog (mean±s.e.m.=7.32±2.48, n=8, t=0.666, d.f.=16, p=0.515).
(e) Statistical analyses
Unless otherwise noted, all data from within a site were tested with unpaired t-tests. We used a one-way repeated measure ANOVA to compare naive controls with the restrained group from all three sites. We compared FIDs among treatments and sites using a one-way ANOVA and Tukey post hoc comparisons. Statistical adjustments for multiple testing (Wright 1992) were taken into account for FIDs but not for the comparisons of CORT data because the only group repeatedly used, the naive control, serves as CORT baseline reference. To meet normality assumptions, confirmed by Kolmogorov–Smirnov one-sample test, all CORT data were log transformed before testing; wariness data were additionally square-root transformed. All tests were two tailed, alpha was set to 0.05. From the previously caught and chased group on Caamaño, one obvious CORT outlier (94.55 ng ml−1) that was 13 times the standard deviation above the mean was removed prior to the analysis. The outlier could be due to a sick animal, problems with the plasma sample, or, alternatively, it could represent a small fraction of hyper-responding individuals (according to the concept of responders and non-responders, see Blanchard et al. 1995). Inclusion of this data point would not change the statistical interpretation of the graph.
3. Results
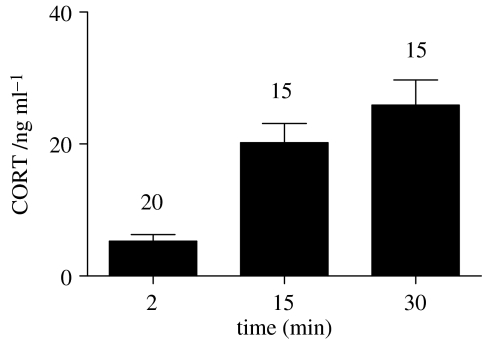
As in continental vertebrates, a serious threat such as capture and subsequent restraint of marine iguanas in an opaque bag causes a significant increase of CORT (figure 1). After 15 min of restraint, CORT concentrations were significantly elevated from pre-stressed, or baseline, levels (paired t-test, t=7.710, d.f.=12, p<0.001). There was no significant further increase between 15 and 30 min. CORT responses of similar strength in marine iguanas have previously been shown to result from metabolic disruptions during an El Nino episode or an oil spill (Romero & Wikelski 2001; Wikelski et al. 2001).
Figure 1.
Corticosterone (CORT) increase in captured marine iguanas during restraint in an opaque bag. CORT concentrations 2 min after capture (mean±s.e.m.=5.29±0.98 ng ml−1) reflect undisturbed baseline levels. 15 min after capture concentrations significantly increased to 20.22±2.91 ng ml−1.
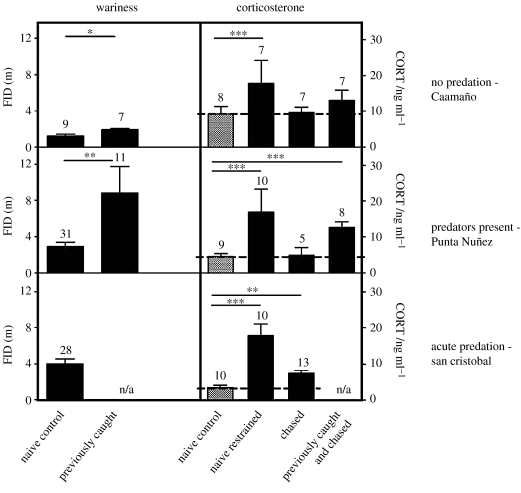
To test the animals' ability to detect and respond to a novel threat, we simulated predation attempts directed at marine iguanas from three islands with contrasting predation pressure (figure 2).
Figure 2.
Wariness and corticosterone (CORT) concentrations at three sites differing in intensity of predation risk. Blood samples from naive control animals were collected on an average 2 min after capture (i.e. CORT baselines), from naive restrained animals 30 min after capture (i.e. CORT response to restraint in a bag). Chased and previously caught and chased animals were subjected to 15 min of experimental harassment prior to capture. In contrast to naive chased individuals, the latter group had been caught and handled once, three to four weeks before. Dashed lines mark CORT baseline levels (average of the naive control group). All values in means±s.e.m.
(a) No predation
On Caamaño Island, CORT concentrations in animals chased for the first time (chased) were indistinguishable from baselines in un-chased naive controls (t=−0.639, d.f.=13, p=0.534, figure 2, top right). We then tested for a learned response to new predators in animals that were previously caught and subsequently chased (three to four weeks after initial capture). Similar to the naive animals, there was no significant CORT increase after chasing (t=−0.004, d.f.=13, p=0.334). Only naive control animals, when put in a bag after capture without prior chasing (naive restrained), significantly elevated CORT concentrations at all the three sites (F1,23=42.575, p<0.001).
In contrast to the CORT results, FIDs on Caamaño were significantly higher in the previously caught group as compared with naive control animals (t=−2.935, d.f.=14, p=0.011, figure 2, top left). Thus a one-time prior experience was enough to increase FID in subsequent tests.
(b) Intermediate predation risk
As on Caamaño, CORT in chased animals was not significantly different from naive controls (t=0.230, d.f.=12, p=0.822) and far below CORT concentrations in naive restrained animals. However, the previously caught and chased animals at this site mounted a significant CORT response to chasing (t=−4.819, d.f.=15, p<0.001). Furthermore, iguanas at Punta Nuñez also had significantly higher FIDs than iguanas on predator-free Caamaño (site differences in FIDs, F2,81=18.501, p<0.001 with Caamaño versus Punta Nuñez p<0.001), especially previously caught individuals (naive controls versus previously caught at Punta Nuñez, t=−3.271, d.f.=40, p=0.002).
(c) Acute, heavy predation risk
On San Cristobal, we observed numerous corpses and bite marks on the tails of live individuals. In contrast to the other sites, this heavy acute predation threat resulted in significant CORT increases to the threat of experimental chasing by a human even in chased animals without prior capture experience (t=−3.678, d.f.=21, p<0.001). Furthermore, CORT responses to restraint were highest at this site and naive iguanas had the highest FIDs on this island.
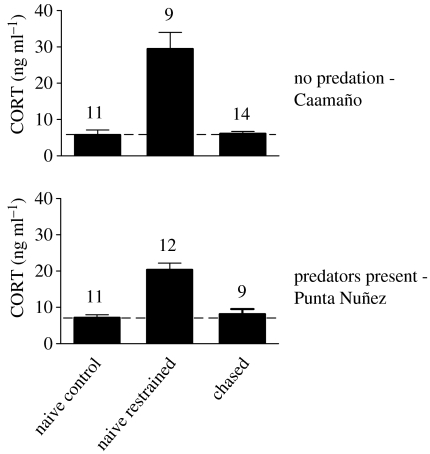
We supplemented our results of adult males by replicating the comparisons of CORT concentrations between naive controls and chased groups with young animals between 2 and 3 years of age (figure 3). Similar to adult males, young iguanas did not respond to experimental chasing with a CORT increase (Caamaño t=−1.095, d.f.=23, p=0.285, Punta Nuñez t=−0231, d.f.=18, p=0.820). However, restraint caused a significant CORT increase at both sites (Caamaño t=−6.137, d.f.=18, p<0.001, and Punta Nuñez t=−7.408, d.f.=21, p<0.001).
Figure 3.
CORT concentrations of young animals (2–3 years of age) at two sites differing in intensity of predation risk. Blood samples from naive control animals were collected on an average 2 min after capture (i.e. CORT baselines), from naive restrained animals 30 min after capture (i.e. CORT response to restraint in a bag). Chased animals were subjected to 15 min of experimental harassment prior to capture. Dashed lines mark CORT baseline levels (average of the naive control group). All values in means±s.e.m.
4. Discussion
Predator-naive marine iguanas tolerate close human approach and fail to mount a stress response during sustained chasing, thus are naive in both behaviour and in endocrine control systems. When iguanas live with predators such as on Punta Nuñez, animals chased by humans still did not activate their HPA axis, even though a recent capture experience primed marine iguanas to elicit a CORT response. At the same time FIDs were significantly higher in previously captured animals than in naive controls. Marine iguanas at the predator-exposed site on San Cristobal elicited a CORT response to chasing even without previous capture events by a human experimenter.
Our results demonstrate for the first time that low wariness in a naive insular organism is accompanied by the failure to activate the HPA-axis, suggesting deficient predator recognition. A one-time experience with a novel predation attempt (capture), however, was enough to increase FID and, in combination with predator presence, to activate a CORT response to chasing to a similar degree as restraint. On Caamaño, CORT baseline concentrations were higher than at the other sites, perhaps resulting from a higher population density (Romero & Wikelski 2001). This increased baseline reduced our power to detect a potential increase of CORT resulting from chasing previously caught iguanas. Nevertheless, overall we suggest that living among predators may prime the HPA system, enabling a physiological response (CORT secretion) to a novel stimulus (the human experimenter). Because the HPA system is arguably important for surviving common metabolic stressors in marine iguanas (Romero & Wikelski 2001), these island endemic reptiles appear to retain the classic HPA function through long evolutionary periods without predators. Thus the HPA system can apparently quickly regain its activity if predation resumes.
But, are the observed FID changes sufficient to allow marine iguanas to survive and thrive in the face of introduced predators? The answer is most likely negative. Although marine iguanas increased FID after a one-time experience, the magnitude of change was small and insufficient (c.f. Blazquez et al. 1997). We could recapture even previously experienced animals with a noose on a 3 m pole repeatedly up to six times in four weeks, as has been done for a previous experiment (Berger et al. 2005). Accordingly, introduced cats and dogs are known to cause large scale depredations among marine iguana populations (Kruuk & Snell 1981; Laurie 1983). On San Cristobal near the town Puerto Baquerizo Moreno, dogs drove the local study population to near extinction within a short time.
Since changes in FID were insufficient in absolute terms, it becomes counterintuitive that a behavioural trait is more conservative than a corresponding physiological control system. One possible interpretation is that experience with predators adjusted CORT responses within the reaction norm for other, e.g. metabolic, stressors. A similar pleiotropy of genetically linked different functions is currently being proposed to explain the maintenance of anti-predator traits under relaxed selection (Blumstein 2006). In contrast, the behavioural responses of marine iguanas are never stimulated by anything that would resemble a mammalian predator. In other words, marine iguanas occasionally need to walk away from sea lions that stumble through their colonies, and escape from hawks by hiding under rocks. But, none of these responses can easily be scaled up to a response against a mammalian predator. In other words, the reaction norm for CORT responses is broad enough to encompass the necessary adjustments to a novel predator, but the reaction norm for behavioural responses is too narrow to encompass an effective response. In support of this idea, the magnitude of FID differences between naive and previously caught animals is comparable to the magnitude of differences based on age and sex (Berger et al. in preparation). Therefore, narrow reaction norms of FIDs could be a result of selection in a social context and reflect the fact that exaggerated behavioural responses (such as fast and long-lasting escape flights) would be too costly (Blumstein 2006).
Our results suggest that the estimated 5–15 Myr of release from predation (Rassmann 1997) narrowed the reaction norm for FID too far for marine iguanas to adjust their behaviour fast enough to an unknown stimulus. Thus, we predict that the current FIDs will not serve to minimize predation by introduced mammals, which have been present for no longer than 150 years. We suggest a similar pattern in other insular vertebrates with long periods of evolutionary isolation from predators: high phenotypic plasticity of the physiological stress response to novel stressors, but restricted plasticity in flight behaviour. There is an urgent need for further studies on the generality of limited reaction norms in predator-naive organisms. Such studies will improve our general knowledge about the interactions between wariness and its physiological control mechanisms, and such investigations will help wildlife managers to predict the impact of introduced predators on natural island populations as well as to improve the ability to modify behavioural responses of captive-bred animals prior to re-introductions (Beck et al. 1994; Maloney & MacLean 1995; Griffin et al. 2000).
Acknowledgments
We thank the Alexander von Humboldt Foundation, the Gottlieb Daimler and Karl Benz Foundation, the National Science Foundation (grant DEB-0545592), and the Arthur v. Gwinner Foundation for funding, the Charles Darwin Research Station and the Galápagos National Park Service, and the Galápagos Genetics, Epidemiology and Pathology Laboratory for logistic aid and permits, the late E. Gwinner for continuous support and the Max-Planck Society for the use of its research platform. Thanks also to our field assistants and to S. Thomas and S. Darling for cooperating with their trained dogs, and to Dan Blumstein and an anonymous reviewer for their helpful comments. Experimental procedures adhere to the guidelines set forth by the American Society of Ichthyologists and Herpetologists, were approved by the Princeton Institutional Animal Care and Use Committees and conducted with permission by the Galápagos National Park Service. This is publication no. 1046 of the Charles Darwin Foundation.
Supplementary Material
Online supplementary figures demonstrating (a) the use of a dog for the estimation of flight initiation distances and (b) acute dog predation at our study site on the island of San Cristobal.
References
- Beck B.B, Rapaport L.G, Stanley Price M.R, Wilson A.C. Reintroduction of captive-born animals. In: Olney P.J.S, Mace G.M, Feistner A.T.C, editors. Creative conservation: interactive management of wild and captive animals. Chapman & Hall; London, UK: 1994. pp. 265–286. [Google Scholar]
- Berger J, Swenson J.E, Persson I.-L. Recolonizing carnivores and naive prey: conservation lessons from pleistocene extinctions. Science. 2001;291:1036. doi: 10.1126/science.1056466. doi:10.1126/science.1056466 [DOI] [PubMed] [Google Scholar]
- Berger S, Martin L.B, II, Wikelski M, Romero L.M, Kalko E.K.V, Vitousek M.N, Rödl T. Corticosterone suppresses immune activity in territorial Galápagos marine iguanas during reproduction. Horm. Behav. 2005;47:419–429. doi: 10.1016/j.yhbeh.2004.11.011. doi:10.1016/j.yhbeh.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Berger, S., Wikelski, M., Romero, L. M., Kalko, E. K. V. & Rödl, T. In preparation. Behavioral and physiological adjustments to new predators in an endemic island species, the Galápagos marine iguana. [DOI] [PubMed]
- Blanchard D.C, Spencer R.L, Weiss S.M, Blanchard R.J, McEwen B, Sakai R.R. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. doi:10.1016/0306-4530(94)E0045-B [DOI] [PubMed] [Google Scholar]
- Blazquez M.C, Rodriguez-Estrella R, Delibes M. Escape behavior and predation risk of mainland and island spinytailed iguanas (Ctenosaura hemilopha) Ethology. 1997;103:990–998. [Google Scholar]
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. doi:10.1046/j.1365-2699.2002.00717.x [Google Scholar]
- Blumstein D.T. The multipredator hypothesis and the evolutionary persistence of anti-predator behaviour. Ethology. 2006;112:209–217. doi:10.1111/j.1439-0310.2006.01209.x [Google Scholar]
- Blumstein D.T, Daniel J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B. 2005;272:1663–1668. doi: 10.1098/rspb.2005.3147. doi:10.1098/rspb.2005.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Risk discrimination of direct versus tangential approach by basking black iguanas (Ctenosaura similis): variation as a function of human exposure. J. Comp. Psychol. 1990;104:388–394. doi:10.1037/0735-7036.104.4.388 [Google Scholar]
- Cockrem J.F, Silverin B. Sight of a predator can stimulate a corticosterone response in the great tit (Parus major) Gen. Comp. Endocrinol. 2002;125:248–255. doi: 10.1006/gcen.2001.7749. doi:10.1006/gcen.2001.7749 [DOI] [PubMed] [Google Scholar]
- Cooper W.E., Jr Risk factors affecting escape behavior by the desert iguana, Dipsosaurus dorsalis: speed and directness of predator approach, degree of cover, direction of turning by a predator, and temperature. Can. J. Zool. 2003;81:979–984. doi:10.1139/z03-079 [Google Scholar]
- Frid A, Dill L.M. Human caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002;6:11. http://www.consecol.org/vol6/iss1/art11 online. [Google Scholar]
- Griffin A.S, Blumstein D.T, Evans C.S. Training captive-bred or translocated animals to avoid predators. Conserv. Biol. 2000;14:1317–1326. doi:10.1046/j.1523-1739.2000.99326.x [Google Scholar]
- Hofer H, East M. Biological conservation and stress. In: Moeller A.P, Milinksi M, Slater P.J.B, editors. Biological conservation and stress. Academic Press; San Diego, CA: 1998. pp. 405–525. [Google Scholar]
- Kruuk H, Snell H. Prey selection by feral dogs from a population of marine iguanas. J. Appl. Ecol. 1981;18:197–204. doi:10.2307/2402489 [Google Scholar]
- Laurie A. Marine iguanas in Galápagos. Oryx. 1983;17:18–25. [Google Scholar]
- Maloney R.F, McLean I. Historical and experimental learned predator recognition in free-living New Zealand robins. Anim. Behav. 1995;50:1193–1201. doi:10.1016/0003-3472(95)80036-0 [Google Scholar]
- McNab B.K. Energy conservation and the evolution of flightlessness in birds. Am. Nat. 1994;144:628–642. doi:10.1086/285697 [Google Scholar]
- Romero L.M, Reed J.M. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol. A. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. doi:10.1016/j.cbpb.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Romero L.M, Wikelski M. Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA. 2001;98:7366–7370. doi: 10.1073/pnas.131091498. doi:10.1073/pnas.131091498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassmann K. Evolutionary age of the Galápagos iguanas predates the age of the present Galápagos Islands. Mol. Phylogenet. Evol. 1997;7:158–172. doi: 10.1006/mpev.1996.0386. doi:10.1006/mpev.1996.0386 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Scheuerlein A, Van't Hof T.J, Gwinner E. Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris) Proc. R. Soc. B. 2001;268:1575–1582. doi: 10.1098/rspb.2001.1691. doi:10.1098/rspb.2001.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankowich T, Blumstein D.T. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B. 2005;272:2627–2634. doi: 10.1098/rspb.2005.3251. doi:10.1098/rspb.2005.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P.A, Snell H.M, Snell H.L. Behavioural diversity as biological diversity: introduced cats and lava lizard wariness. Conserv. Biol. 1994;8:569–573. doi:10.1046/j.1523-1739.1994.08020569.x [Google Scholar]
- Wikelski M. Evolution of body size in Galápagos marine iguanas. Proc. R. Soc. B. 2005;272:1985–1993. doi: 10.1098/rspb.2005.3205. doi:10.1098/rspb.2005.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M, Romero L.M, Snell H.L. Marine iguanas oiled in the Galápagos. Science. 2001;292:437. doi: 10.1126/science.292.5516.437c. doi:10.1126/science.292.5516.437c [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Vleck C.M, Moore M.C. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran desert. J. Exp. Zool. 1992;264:419–428. doi: 10.1002/jez.1402640407. doi:10.1002/jez.1402640407 [DOI] [PubMed] [Google Scholar]
- Wright S.P. Adjusted P-values for simultaneous inference. Biometrics. 1992;48:1005–1013. doi:10.2307/2532694 [Google Scholar]
- Yacelgas, M. 1995 Metodologias para mejorar la sobrevivencia de iguanas marinas en poblaciones amanazadas. Ph.D. thesis, University Central, Quito, Ecuador.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplementary figures demonstrating (a) the use of a dog for the estimation of flight initiation distances and (b) acute dog predation at our study site on the island of San Cristobal.