Abstract
An exceptionally preserved new ostracod crustacean from the Silurian of Herefordshire, England, preserves eggs and possible juveniles within its carapace, providing an unequivocal and unique view of parental brood care in the invertebrate fossil record. The female fossil is assigned to a new family and superfamily of myodocopids based on its soft-part anatomy. It demonstrates a remarkably conserved egg-brooding reproductive strategy within these ostracods over 425 Myr. The soft-tissue anatomy urges extreme caution in classifying ‘straight-hinged’ Palaeozoic ostracods based on the carapace alone and fundamentally questions the nature of the shell-based Palaeozoic ostracod record.
Keywords: brood care, exceptional preservation, Herefordshire Lagerstätte, Myodocopa, Ostracoda, Silurian
1. Introduction
Ostracod bivalved crustaceans are known from an estimated more than 20 000 living species (Podocopa and Myodocopa; Horne et al. 2002) and have colonized marine, non-marine and even semi-terrestrial habitats. They are by far the most abundant fossil arthropods, occurring as millions of tiny fossil valves from at least the Ordovician onwards (Hou et al. 1996), and are valuable palaeoenvironmental and biostratigraphic indicators. However, their fossilized soft parts are extremely rare (Smith 2000; Siveter et al. 2003).
Supposed examples of fossil invertebrate eggs are few and the only known candidate for fossil ostracod eggs are isolated spheres from the Cretaceous (Smith 1999). Possible examples of brooding in fossil ostracods are also very rare. Fossils of the Silurian Herefordshire Konservat-Lagerstätte in England (Briggs et al. 1996), a deposit dated approximately 425 Myr BP, furnish unrivalled insights into the palaeobiology of a range of invertebrates. They include a polychaete worm (Sutton et al. 2001c), an aplacophoran-like mollusc (Sutton et al. 2001a,b, 2004), a gastropod (Sutton et al. 2006), a pycnogonid (Siveter et al. 2004), a stem-group chelicerate (Orr et al. 2000b; Sutton et al. 2002), an ostracod (Siveter et al. 2003), a barnacle (Briggs et al. 2005), a phyllocarid (Briggs et al. 2004), a brachiopod (Sutton et al. 2005a), a stem-group asteroid (Sutton et al. 2005b) and a variety of unpublished forms. Here, we report the discovery of an ostracod specimen from this Lagerstätte, which preserves soft-part anatomy that includes eggs and possible juveniles in a brood space. This female ostracod is assigned to a new family and superfamily of Myodocopa. Myodocopes typically have at best weakly calcified valves and their scant fossil record begins with a single species documented from the late Ordovician Ashgill Series (Gabbott et al. 2003).
2. Material and methods
As with other exceptionally preserved fossils of the Herefordshire Konservat-Lagerstätte, the ostracod described here is preserved as a three-dimensional calcitic void infill in carbonate concretions within nodules in a volcaniclastic deposit (Orr et al. 2000a). The ostracod was reconstructed as a ‘virtual fossil’ by serial grinding and capturing images of the fossil at 20 μm intervals, digitally removing extraneous material, resolving fossil–matrix ambiguities and rendering to produce a colour-coded reconstruction (Sutton et al. 2001d).
Datasets from serial grinding are housed in the University Museum of Natural History, Oxford (OUM).
3. Systematic palaeontology
Phylum: Arthropoda, Subphylum: Crustacea, Class: Ostracoda.
Subclass: Myodocopa Sars, 1866
Order: Myodocopida Sars, 1866
Superfamily: Nymphatelinoidea superfamily nov.
Diagnosis: Myodocopida with elongate, epipod-bearing second maxilla and sixth appendage.
Family: Nymphatelinidae family nov.
Diagnosis: as for the superfamily.
Genus: Nymphatelina gen. nov.
Derivation of name: latin Nympha (young woman of the sea)+tutelina (guardian).
Diagnosis: Nymphatelinidae with carapace with a long simple gape, posterodorsal spine and admarginal ridge.
Species: Nymphatelina gravida sp. nov.
Derivation of name: latin gravida (pregnant).
Diagnosis: as for the genus (monotypic).
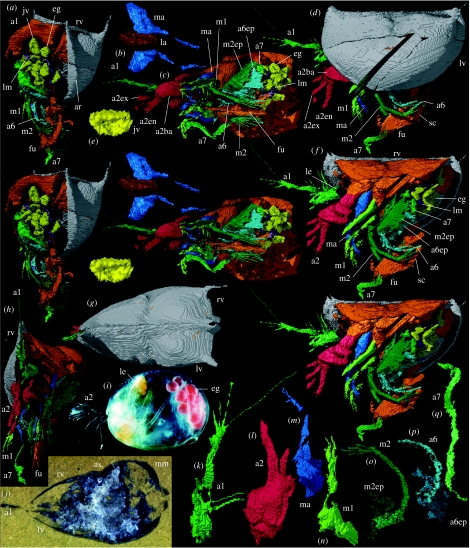
Holotype: a carapace (figure 1j) and soft parts, Oxford University Museum of Natural History OUM 29600; reconstructed in three-dimensions (figure 1a–h,k–q). No other material is known.
Figure 1.
(a–h,j–q) Holotype of Nymphatelina gravida: (a–h,k–q) ‘virtual’ reconstructions (the diagonal break across the valve, mandible and first maxilla represents lost data; and the exact boundary between structures such as body and appendages, as indicated by colour changes, is somewhat arbitrary); (j) specimen in rock. (a) Oblique posterior view with left valve omitted (stereo-pair), ×9. (b) Anteroventral view of labrum and mandibles (stereo-pair), ×13. (c) Ventral view with valves omitted (stereo-pair), ×9. (d) Left lateral view, ×9. (e) Internal lateral view of possible juvenile valve, ×27. (f) Left lateral view with left valve omitted (stereo-pair), ×9. (g) Dorsal view (anterior parts of first antennae omitted), ×9. (h) Oblique anterior view with left valve omitted, ×9. (j) Oblique anterior–posterior section, ×9. (k) Oblique posterior medial view of right first antenna, ×15. (l) Oblique posterior medial view of right second antenna, ×15. (m) Oblique posterior medial view of right mandible, ×15. (n) Oblique posterior medial view of right first maxilla, ×15. (o) Mirrored image of left second maxilla (epipod of right second maxilla is not preserved), with epipod picked out in a slightly darker colour, ×15. (p) Mirrored image of left of sixth appendage (epipod of right sixth appendage is poorly preserved), with epipod picked out in a slightly darker colour, ×15. (q) Oblique posterior medial view of right seventh appendage, ×15. (i) Myodocopid Gigantocypris dracontovalis, left lateral view of female with eggs; Recent, Atlantic Ocean, ×4. Abbreviations: a1, first antenna; a2ba, a2en, a2ex, basipod, endopod and exopod of second antenna; a6, sixth appendage; a6ep, epipod of sixth appendage; a7, seventh appendage; ar, admarginal ridge; as, adductorial sulcus; eg, egg; fu, furca; jv, possible juvenile; la, labrum; le, lateral eye; lm, lamella; lv, left valve; m1, first maxilla; m2, second maxilla; m2ep, epipod of second maxilla; ma, mandible; mm, marsupium; rv, right valve; sc, sclerosome.
Locality and stratigraphy: Herefordshire, England; Wenlock Series, Silurian.
The carapace is large (maximum length, height, width: 5900, 3200, 3500 μm), slightly gaping, with a straight dorsal margin and otherwise an almost evenly curved lateral outline (figure 1d). The carapace is inflated posteriorly and narrows evenly in front of mid-length (figure 1g). A narrow gape extends from above anterior mid-height to behind mid-length. At mid-length, a narrow shallow adductorial sulcus extends to mid-height. A faint short preadductorial sulcus outlines an indistinct preadductorial node. The postadductorial lobate area is gently curved dorsally, ending in a slender posterodorsal spine. Both cardinal corners have a small acroidal spine. The free margin is narrow, flat and defined abaxially by an admarginal ridge (figure 1a,d).
The first antenna (figure 1c,d,f,h,j,k) has an elongate, subtriangular-shaped, tapering proximal part (=a podomere?) bearing two slender subparallel setae distally. Its distal part (preserved only on the right antenna) is slightly longer, rod-like, geniculate at about mid-length (=a podomere boundary?), with pairs of long, fine, divergent setae subterminally and terminally. The second antenna (figure 1c,d,f,h,l) has a large almond-shaped basipod with an oblique lateral depression that accommodates the valve free edge and therefore is interpreted as a (‘post-mortem’) compression artefact. The endopod is short, slightly flexed in two places (podomere boundaries not discernible) and has at least four fine setae terminally (preserved on left limb only). Only a short slender proximal portion (=a podomere?) of the exopod is preserved.
The limb stem (presumed basipod and coxa) of the mandible is subtriangular and bears several tapering enditic processes adaxially that rest against the posterior face of the labrum (figure 1b–d,f,m). The endopod is slender, tapered and is flexed backwards, forming two subequal parts and one slightly shorter distal part (=podomeres?) with two possible setae terminally. An exopod is not discernible. The first maxilla (figure 1a,c,d,f,h,n) has a limb stem (presumed basipod and coxa) with at least four long pointed enditic processes projecting adaxially in the outer part of the atrium oris. The ramus (presumed endopod) is slender, tapered and geniculate (angle about 40°) at mid-length; the proximal part (=a podomere?) possibly has a finely setose inner edge and the distal section is divided into two parts each with about three slender setae terminally.
The second maxilla (figure 1a,c,d,f,h,o) has an elongate presumed limb stem with 4–5 stout enditic processes adaxially. It is geniculate (angle about 75°) with a long slender presumed exopod that is flexed backwards a short distance from the limb stem and also at about mid-length, demarcating short, medium length and long sections (=podomeres?); terminally, there is a splay of about 10 mostly long slender setae. The base of the sixth appendage is broad, but details are obscure. The ramus is long, slender, tapering and projects backwards (figure 1a,c,d,f,h,p); flexures indicate at least four possible podomeres, the penultimate podomere bearing at least five long, posteriorly projecting, fine setae proximally (only two preserved on left limb) and the last podomere with two tiny setae proximally (preserved only on left limb). The second maxilla and sixth limbs each have a large, curved, outwardly flared epipod that projects postero-laterally from the basal part of the limb (epipod of right second maxilla is not preserved; figure 1c,f,o,p).
The vermiform seventh appendage arises near the base of the furca; the right limb projects ventrally (figure 1a,c,d,f,h,q) and the left limb is coiled within the carapace. A poorly preserved lamella of unknown affinity occurs adaxial to the left seventh limb. The furca is slender with a row of at least nine curved claws on each lamella (figure 1a,c,d,f,h). A finger-like process projecting from the anterodorsal part of the furca probably represents part of the sclerosome (figure 1c,d,f) and appears detached owing to the impersistent preservation of the connecting membrane. A lateral eye (presumed compound and preserved only on left side) lies adjacent to the bases of the first and second antenna (figure 1f,h). A median eye is not discernible. The labrum is elongate, has a curved axial ridge giving a broadly triangular cross-section, and a rounded margin at the atrium oris (figure 1b).
A remarkable discovery in this specimen is the presence of about 20 small (mean ‘length’: 558 μm) ovoid and two valve-shaped structures in the posterior domiciliar area that are interpreted as eggs and possible juveniles (=exuviae?: soft parts lacking), respectively, in a marsupium (figure 1a,c,e,f,j).
4. Discussion
Nymphatelina gravida inhabited waters 150–200 m deep (Briggs et al. 1996). Like most Recent myodocopes, it was probably nektobenthic and like some, it was possibly a predator, scavenger or detritivore. Unlike many myodocopes, N. gravida lacks a rostrum and rostral incisure; protrusion of its appendages from the carapace is facilitated by a long narrow gape. Its well-developed endites and furca indicate a probable ability to comminute food. Even though it was probably an adept swimmer, as implied by the substantial basipod of its second antenna, N. gravida is known from only one locality in the well-studied Welsh Basin and probably had limited dispersal capacity. An ecological shift in myodocopes in the late Silurian signals the origin of pelagic ostracods (Siveter 1984; Siveter et al. 1991).
Among ostracods, the presence of a lateral eye, vermiform seventh limb and a sclerosome (=area of hardened body wall just anterodorsal to the furca; Parker 1997) are features unique to the Myodocopida, to which N. gravida is therefore assigned. The elongate morphology of its second maxilla and sixth limb contrasts with their compact form in all other myodocopids and is like the supposed plesiomorphic condition (Kornicker & Sohn 1976; Horne et al. 2005) characteristic of halocyprid myodocopes, as is the presence of an epipod on the second maxilla and the sixth limb (the latter limb of myodocopids lacks an epipod).
Of living ostracods the vast majority reproduce sexually and females release their eggs directly to the water. Parthenogenesis is known in a few tens of species, of freshwater Podocopa, mostly Darwinuloidea. Species with both parthenogenetic and geographically separate sexual populations are even rarer (Horne et al. 1998). Some podocope species brood their eggs and up to three juvenile stages internally; internal brood care of only the eggs by the female is characteristic of platycope podocopes, all myodocopid myodocopes and an halocyprid myodocope species (Cohen & Morin 1990; Horne et al. 1998). Most supposed fossil invertebrate eggs are reported as spheres isolated from the supposed egg producer (e.g. Gall & Grauvogel 1966; Zhang & Pratt 1994). Putative in situ eggs or embryos have been documented from single fossils of a bradoriid arthropod (Shu et al. 1999), a tealliocaridid malacostracan (Briggs & Clarkson 1985), two branchiopod species (Gall & Grauvogel 1966; Vannier et al. 2003), and a few ammonoids (Davies et al. 1996) and specimens of a syncarid malacostracan species (Perrier et al. 2006). Direct demonstration of combined egg and juvenile brood care in fossil invertebrates is hitherto unknown. The only known possible fossil ostracod eggs are isolated spheres from the Cretaceous (Smith 1999). Previous records of possible brooding in fossil ostracods are very rare: specimens of a Silurian species and two Permian species, and single carapaces in the Devonian and Carboniferous, each containing a purported juvenile shell (see Lethiers et al. 1996). Evidence of reproductive strategies in fossil ostracods is otherwise indirect: presumed sexual dimorphism involving what is possibly brood space; or the supposed lack of males in the Darwinulidae, a post-Palaeozoic group, indicating asexual reproduction (Horne et al. 1998; but see Smith et al. 2006).
The size, number and position of the eggs in N. gravida, in a posterior and inflated part of the carapace, accord with those of living, sexually mature, female brooding myodocopes (Cohen & Morin 1990; figure 1i), allowing the gender of this specimen to be determined in an animal as old as Silurian. N. gravida proffers a rare example of fossil invertebrate eggs and the only unequivocal and in situ example in ostracods. The specimen offers, based on evidence unique in the invertebrate fossil record, direct evidence for an egg-brooding reproductive mode and one that has persisted in myodocopids for at least 425 Myr. In addition, it is the only record of possible brood care of juveniles in myodocopes. On the basis of a single known specimen, it is not possible to determine whether N. gravida reproduced sexually or parthenogenetically. However, given that parthenogenesis is unknown in Recent myodocopes, a sexual mode is perhaps more probable.
Although the soft parts of N. gravida clearly show a myodocopid affinity, the straight dorsal margin, lobes, spines and an admarginal ridge of the shell variously recall the morphology of halocyprids and especially palaeocopes (the dominant, extinct group of Palaeozoic ostracods, for which soft parts are unknown). The discovery of the soft parts of this Palaeozoic ostracod urges caution in interpreting the affinities of other so-called ‘straight-hinged’ examples based solely on shell morphology (with the inherent problem of homeomorphy; Horne et al. 2005). The taxonomic assignment of many of the hundreds of such genera of Palaeozoic ostracods, which are based on shells alone, may be incorrect.
Acknowledgments
We thank the Leverhulme Trust (F/08581/E), the Natural Environment Research Council (GR3/12053) and English Nature for support (grants to Derek S., DEGB and David S.); Kate Saunders for technical work; M. V. Angel and R. Smith for discussion and access to Recent material; Ann Cohen, David Horne and one other referee for their comments on the manuscript; and R. Fenn, T. Hall and J. Sinclair for general assistance.
References
- Briggs D.E.G, Clarkson E.N.K. The Lower Carboniferous shrimp Tealliocaris from Gullane, East Lothian, Scotland. Trans. R. Soc. Edinb. Earth Sci. 1985;76:173–201. [Google Scholar]
- Briggs D.E.G, Siveter D.J, Siveter D.J. Soft-bodied fossils from a Silurian volcaniclastic deposit. Nature. 1996;382:248–250. doi:10.1038/382248a0 [Google Scholar]
- Briggs D.E.G, Sutton M.D, Siveter D.J, Siveter D.J. A new phyllocarid (Crustacea: Malacostraca) from the Silurian Fossil-Lagerstätte of Herefordshire, UK. Proc. R. Soc. B. 2004;271:131–138. doi: 10.1098/rspb.2003.2593. doi:10.1098/rspb.2003.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs D.E.G, Sutton M.D, Siveter D.J, Siveter D.J. Metamorphosis in a Silurian barnacle. Proc. R. Soc. B. 2005;272:2365–2369. doi: 10.1098/rspb.2005.3224. doi:10.1098/rspb.2005.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.C, Morin J.G. Patterns of reproduction in ostracodes: a review. J. Crust. Biol. 1990;10:184–211. doi:10.2307/1548480 [Google Scholar]
- Davies R.A, Landman N.H, Dommergues J.-L, Marchand D, Bucher H. Mature modifications and dimorphism in ammonoid shells. In: Landman N.H, Tanabe K, Davis R.A, editors. Ammonoid paleobiology. Topics in geobiology. Vol. 13. Plenum Press; New York, NY: 1996. pp. 463–539. [Google Scholar]
- Gabbott S.E, Siveter D.J, Aldridge R.J, Theron J.N. The earliest myodocopes: ostracodes from the late Ordovician Soom Shale Lagerstätte of South Africa. Lethaia. 2003;36:151–160. doi:10.1080/00241160310004620 [Google Scholar]
- Gall J.C, Grauvogel L. Pontes d'invertébrés du Buntsandstein Supérieur. Ann. Paléontol. (Invert.) 1966;52:155–161. [Google Scholar]
- Horne, D.J., Cohen, A. & Martens K. 2002 Taxonomy, morphology and biology of Quaternary and living Ostracoda In The Ostracoda: applications in Quaternary research (ed. J. A. Holmes & A. Chivas) Geophysical monograph, 131, 5–36
- Horne D.J, Danielopol D.L, Martens K. Reproductive behaviour. In: Martens K, editor. Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracods. Backhuys; Leiden, The Netherlands: 1998. pp. 157–195. [Google Scholar]
- Horne D.J, Schön I, Smith R.J, Martens K. What are Ostracoda? A cladistic analysis of the extant superfamilies of the subclasses Myodocopa and Podocopa (Crustacea: Ostracoda) In: Koenemann S, Jenner R.A, editors. Crustacea and arthropod relationships. Crustacean issues. Vol. 16. Taylor & Francis; Boca Raton, FL: 2005. pp. 249–273. [Google Scholar]
- Hou X.-G, Siveter D.J, Williams M, Bergström J. Appendages of the arthropod Kunmingella from the early Cambrian of China: its bearing on the systematic position of the Bradoriida and the fossil record of the Ostracoda. Phil. Trans. R. Soc. B. 1996;351:1131–1145. [Google Scholar]
- Kornicker L.S, Sohn I.G. Phylogeny, ontogeny, and morphology of living and fossil Thaumatocypridacea (Myodocopa: Ostracoda) Smithson. Contrib. Zool. 1976;219:1–124. [Google Scholar]
- Lethiers F, Damotte R, Whatley R. Evidence of brooding in Permian non-marine Ostracoda. Lethaia. 1996;29:219–223. [Google Scholar]
- Orr P.J, Briggs D.E.G, Siveter D.J, Siveter D.J. Three-dimensional preservation of a non-mineralized arthropod in concretions in Silurian volcaniclastic rocks from Herefordshire, England. J. Geol. Soc. Lond. 2000a;157:173–186. [Google Scholar]
- Orr P.J, Siveter D.J, Briggs D.E.G, Siveter D.J, Sutton M.D. A new arthropod from the Silurian Konservat-Lagerstätte of Herefordshire, England. Proc. R. Soc. B. 2000b;267:1497–1504. doi: 10.1098/rspb.2000.1170. doi:10.1098/rspb.2000.1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.R. Functional morphology of the Myodocopine (Ostracoda) furca and sclerotized body plate. J. Crust. Biol. 1997;17:632–653. doi:10.2307/1549366 [Google Scholar]
- Perrier V, Vannier J, Rachebouf P.R, Charbonnier S, Chabard D, Sotty D. Syncarid crustaceans from the Montceau Lagerstätte (Upper Carboniferous; France) Palaeontology. 2006;49:647–672. doi:10.1111/j.1475-4983.2006.00553.x [Google Scholar]
- Sars G.O. Oversigt af Norges marine ostracodes. Norske Vidensk.-Akad Forhandl. 1866;1865:1–130. [Google Scholar]
- Shu D.-G, Vannier J, Luo H.-L, Chen L.-Z, Zhang X.-L, Hu S.-X. Anatomy and lifestyle of Kunmingella (Arthropoda, Bradoriida) from the Chengjiang fossil Lagerstätte (Lower Cambrian, Southwest China) Lethaia. 1999;32:279–298. [Google Scholar]
- Siveter D.J. Habitats and modes of life of Silurian ostracodes. In: Bassett M.G, Lawson J.D, editors. The autecology of Silurian organisms. Palaeontology, special paper. Vol. 32. Palaeontological Association; London, UK: 1984. pp. 71–85. [Google Scholar]
- Siveter D.J, Sutton M.D, Briggs D.E.G, Siveter D.J. An ostracode crustacean with soft parts from the Lower Silurian. Science. 2003;302:1749–1751. doi: 10.1126/science.1091376. doi:10.1126/science.1091376 [DOI] [PubMed] [Google Scholar]
- Siveter D.J, Sutton M.D, Briggs D.E.G, Siveter D.J. A Silurian sea spider. Nature. 2004;431:978–980. doi: 10.1038/nature02928. doi:10.1038/nature02928 [DOI] [PubMed] [Google Scholar]
- Siveter D.J, Vannier J.M.C, Palmer D. Silurian myodocopes: pioneer pelagic ostracodes and the chronology of an ecological shift. J. Micropalaeont. 1991;10:151–173. [Google Scholar]
- Smith R.J. Possible fossil ostracod (Crustacea) eggs from the Cretaceous of Brazil. J. Micropalaeont. 1999;18:81–87. [Google Scholar]
- Smith R.J. Morphology and ontogeny of Cretaceous ostracods with preserved appendages from Brazil. Palaeontology. 2000;43:63–98. doi:10.1111/1475-4983.00119 [Google Scholar]
- Smith R.J, Kamiya T, Horne D.J. Living males of the ‘ancient asexual’ Darwinulidae (Ostracoda: Crustacea) Proc. R. Soc. B. 2006;273:1569–1578. doi: 10.1098/rspb.2005.3452. doi:10.1098/rspb.2005.3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. An exceptionally preserved vermiform mollusc from the Silurian of England. Nature. 2001a;410:461–463. doi: 10.1038/35068549. doi:10.1038/35068549 [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. Acaenoplax—polychaete or mollusc? Nature. 2001b;414:602. doi: 10.1038/414601a. doi:10.1038/414602a [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. A three-dimensionally preserved fossil polychaete worm from the Silurian of Herefordshire, England. Proc. R. Soc. B. 2001;268:2355–2363. doi: 10.1098/rspb.2001.1788. doi:10.1098/rspb.2001.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. Methodologies for the visualization and reconstruction of three-dimensional fossils from the Silurian Herefordshire Lagerstätte. Paleont. Electron. 2001;4:1–17. http://palaeo-electronica.org/2001_1/s2/issue1_01.htm art. 2. [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J, Orr P.J. The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. B. 2002;269:1195–1203. doi: 10.1098/rspb.2002.1986. doi:10.1098/rspb.2002.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. Computer reconstruction and analysis of the vermiform mollusc Acaenoplax hayae from the Herefordshire Lagerstätte (Silurian, England), and implications for molluscan phylogeny. Palaeontology. 2004;47:293–318. doi:10.1111/j.0031-0239.2004.00374.x [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. Silurian brachiopods with soft-tissue preservation. Nature. 2005a;436:1013–1015. doi: 10.1038/nature03846. doi:10.1038/nature03846 [DOI] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J, Gladwell D.J. A starfish with three-dimensionally preserved soft parts from the Silurian of England. Proc. R. Soc. B. 2005;272:1001–1006. doi: 10.1098/rspb.2004.2951. doi:10.1098/rspb.2004.2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.D, Briggs D.E.G, Siveter D.J, Siveter D.J. Fossilized soft tissues in a Silurian platyceratid gastropod. Proc. R. Soc. B. 2006;273:1039–1044. doi: 10.1098/rspb.2005.3403. doi:10.1098/rspb.2005.3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier J, Thiéry A, Rachebouf P.R. Spinicaudatans and ostracods (Crustacea) from the Montceau Lagerstätte (late Carboniferous, France): morphology and palaeoenvironmental significance. Palaeontology. 2003;46:999–1030. doi:10.1111/1475-4983.00330 [Google Scholar]
- Zhang X.-G, Pratt B. Middle Cambrian arthropod embryos with blastomeres. Science. 1994;266:637–639. doi: 10.1126/science.266.5185.637. doi:10.1126/science.266.5185.637 [DOI] [PubMed] [Google Scholar]