Abstract
A prey animal may have the alternative of flying away or feigning death when it encounters predators. These alternatives have a genetic base as anti-predator strategies in the adzuki bean beetle, Callosobruchus chinensis. A negative genetic correlation between death-feigning intensity and flying ability was found in C. chinensis, i.e. lower flying ability is genetically connected to escaping by dropping from a perch and then feigning death, whereas higher flying ability does not correspond to death-feigning behaviour. Two bidirectional artificial selections for death-feigning duration and flying ability were conducted independently in C. chinensis. The strains selected for shorter (longer) duration of death-feigning had higher (lower) flying ability, while the strains selected for lower (higher) flying ability showed longer (shorter) duration of death-feigning. When the two traits were compared in 21 populations of C. chinensis derived from different geographical regions, a significant negative correlation was found between death-feigning intensity and flying ability. Based on these results, the choice between alternative escaping behaviours in animals is discussed from two points of view: phenotypic plasticity, an individual with two tactics; and pleiotropic genetic correlation, different individuals with opposite strategies.
Keywords: Callosobruchus chinensis, heritability, selection experiment, thanatosis, tonic immobility
1. Introduction
At the moment a predator attempts to grasp an insect perched on a branch, the insect often falls from the branch and goes out of the predator's view (Fabre 1900; Frost 1959; Edmunds 1974). The dropped insect frequently freezes tonically, which is often called death-feigning (Miyatake et al. 2004; Ruxton et al. 2004; Ruxton 2006), on the ground. The dark body colour of the insect blends into the ground, and thus the predator may lose sight of it. After a few minutes of searching, the predator's interest may move to other objects when other insects come into view, or the predator may lose interest in the insect and give up searching. In another case, an insect on a branch may fly away from the perch just before a predator catches it, and the predator does not capture the insect (Edmunds 1974). The predator then searches for other insects.
What is the difference between the insect that drops and the insect that flies away when it is in danger? In other words, do all individuals have two alternative tactics, drop or fly, or do different individuals use opposite strategies?
Predation is a key selection pressure in shaping prey behaviour (Lima & Dill 1990; Lima 1998). Since natural selection favours individuals that successfully avoid predators, prey have adjusted their anti-predator behaviour (Lima & Dill 1990; Sih 1992). When it perceives danger, an insect on a perch must decide between alternative tactics to survive, fly away or drop-and-feign-death, it cannot adopt both simultaneously. Alternative behavioural choices to escape from predators are known for various prey behaviours in a wide range of taxonomic groups, for example, swimming away or hiding in a refuge for fishes (Lehtiniemi 2005), running or hiding for lizards (Martin & Lopez 2000), and autotomy or fighting for crabs (Wasson & Lyon 2005). Mainly in aquatic organisms, alternative choices for anti-predatory behaviours are considered to be a tactic of an individual with phenotypic plasticity (e.g. Harvell 1984; Boyero et al. 2006). However, there is a possibility that the alternative behaviours are two different strategies against predators that genetically diverged (Weisser et al. 1999; Agrawal et al. 2000; Abjornsson et al. 2004; Mondor et al. 2005). Under the situation of drop-and-feign-death, alternative strategies, in which the individuals with lower flying ability escape from a predator by dropping and feigning death and the individuals with higher flying ability flee by flying, may be adaptive.
We found a negative genetic trade-off between death-feigning intensity and flying ability in the adzuki bean beetle, Callosobruchus chinensis. Death-feigning has sometimes been called animal hypnosis, playing dead, thanatosis or tonic immobility (Ruxton et al. 2004). Although the definition and adaptive value of death-feigning behaviour are debatable (see Miyatake et al. 2004; Honma et al. 2006; Ruxton 2006), we define death-feigning as the dropping and subsequent tonic immobilizing behaviours of folding up the legs and antennae when adult beetles are stimulated.
In the present study, first we artificially selected for the two traits, the duration of death-feigning and flying ability, independently, for more than eight generations, and then observed either trait as correlated responses for every generation to confirm a genetic correlation (Falconer & Mackay 1996). In this experiment, the negative correlation was shown by the result that beetles with higher flying ability showed weaker intensity of feigning death, whereas beetles with lower flying ability showed stronger intensity of death-feigning. The negative correlations between flying ability and death-feigning intensity were also found in a comparison of C. chinensis populations collected from different geographical regions, suggesting that the negative genetic correlation may be exposed under natural selection.
2. Material and methods
(a) Insects and culture
Cultures collected from different geographical regions were reared on adzuki beans, Vigna angulalis (Willd.). All rearing and subsequent experiments were conducted in a chamber (3.70 m wide, 5.00 m deep, 2.56 m high; Sanyo, Tokyo, Japan) maintained at 25°C and 60% relative humidity under a photoperiod cycle of 16 : 8 h light: dark. To avoid a density effect, larval density was limited to one larva per bean (excess eggs were scraped off) for two generations before starting the following experiments. Each bean was transferred to a microcentrifuge tube (5 ml) before the larva emerged from the bean, and then virgin females and males were collected. Within 24 h of emerging, each individual was placed in an Eppendorf tube (1.5 ml) labelled with its identification number. Within 48 h, death-feigning intensity and flying ability were measured as described below. It is known that the intensity of death-feigning in other beetle species is affected by starvation (Acheampong & Mitchell 1997; Miyatake 2001a), and thus all C. chinensis adults were used without feeding just after emergence to ensure the uniform physiological condition of the beetles in the present study. The two selection experiments for duration of death-feigning and flying ability were independently replicated. We used a mixed population as the base population with the following four strains, some of which have been reared long term in a laboratory, as the grandparents. The strain name, collection year, locality of population, the name of collector, the number of founder females and the references are: jC-S, 1936, Kyoto City, S. Utida, unknown and Utida (1941); mC, 1960, Morioka City, H. Nakamura, unknown and Nakamura (1969); isC, 1997, Ishigaki City, K. Kohno, more than 100 and Yanagi & Miyatake (2003); and yoCO2, 2002, Akoh City, T. Miyatake, 9 and Harano & Miyatake (2005).
(b) Artificial selection for duration of death-feigning
One day before observation, each beetle was placed in a well of a 48-well tissue culture plate (Falcon, Becton Dickinson and Company, Lincoln Park, NJ, USA) to avoid disturbance by other beetles. Each beetle was gently placed on its back in a white china saucer (140 mm diameter, 15 mm deep). Death-feigning behaviour was induced by dropping an approximately 5 g fragment of a plastic rubber (MONO PE04A, Tombow, Tokyo, Japan) on the abdomen of the beetle from 1 cm height to provide a uniform stimulus to all beetles. A trial consisted of provoking the death-feigning behaviour and recording its duration with a stopwatch. The behaviour duration was specified as the length of time between the rubber touching the beetle and detecting its first visible movement. If the beetle did not respond, the same stimulus was provided once more or even a third time. When the beetle feigned death, the duration was recorded. If the beetle failed to respond to all three stimuli, the value for the duration of death-feigning was recorded as zero. Two trials were conducted for each beetle at more than a 1 h interval to reduce the effect of disturbance, and the mean value was noted as the death-feigning intensity of each beetle. All the trials were conducted between 12.00 and 18.00 in the chamber previously described.
A random selection of 50 males and 50 females was made from the stock culture, and their death-feigning behaviour was observed (F0 generation). The males and females (7 each) with the shortest duration of death-feigning were selected to propagate short-duration lines (SD lines); similarly, the males and females (7 each) with the longest duration were selected to propagate long-duration lines (LD lines). As a correlated response, the flying ability of all males and females was measured 1 h after the observation of death-feigning by the method described below. Then, the males and females (14 beetles for each line) were placed in a plastic cup (9.1 cm diameter and 4.0 cm height) with 150 adzuki beans and allowed to copulate and lay eggs for 24 h. On the next day, all the adults were removed from the cup, and larval density was controlled at one larva per bean (excess eggs were scraped off) to avoid a density effect. When the beetles that emerged were 1-day old, 50 males and 50 females were randomly selected from each line, and their death-feigning intensity and flying ability (as a correlated response) were measured again (F1 generation). The same procedure was carried out in each generation. Two selection replicates for the short and long lines (SD-1 and LD-1; SD-2 and LD-2) initiated at the same time were tested and maintained in the chamber. The selection regimes were continued for eight generations for each line, and the flying ability was measured for SD and LD lines at every generation as a correlated response.
(c) Artificial selection for flying ability
A hand-made cuboid (50 cm wide, 50 cm deep and 30 cm high) was prepared with a ceiling made of an acrylic acid resin, sides supported by four wooden pillars, and covered with nylon cloth. Five circles each with radii of 5, 10, 15, 20 and 25 cm from the centre were drawn on a sheet of paper laid on the bottom of the cuboid. Each adult beetle was dropped through a hole (7 mm diameter) in the ceiling of the cuboid. We scored the flying ability as 0–6 points as follows. If a beetle's wings did not flutter before landing and thus it fell within the circle with a radius of 5 cm, we scored it as zero points. If a beetle flew, but landed within the circle with a radius of 5 cm, we scored it as 1 point. If a beetle flew and landed on any spot between 5 and 10 cm, 10 and 15 cm, 15 and 20 cm or 20 and 25 cm, we gave it 2, 3, 4 or 5 points, respectively. When a beetle landed on the bottom outside the largest circle or landed on any side, it was scored as 6 points. The test was replicated at 1 h intervals for each beetle, and the mean score was used as the flying ability. Measurements of flying ability were conducted between 12.00 and 18.00 (light phase).
Fifty males and 50 females were randomly collected from the stock culture and their flying ability was evaluated (F0 generation). Males and females (7 each) with the lowest scores of flying ability (almost zero) were selected to propagate the low-score lines (LS lines); similarly, males and females (7 each) with the highest scores were selected to propagate the high-score lines (HS lines). The duration of death-feigning was measured 1 h after the test of flying ability by the method described above for all males and females. The propagation methods were similar to those for selection for death-feigning described above. Two selection replicates for the low- and high-scored lines (LS-1 and HS-1; LS-2 and HS-2) initiated at the same time were tested. The selection regimes were continued for 13 generations for each line, and the duration of death-feigning was measured for LS and HS lines at every generation as a correlated response.
(d) Inter-population comparison
Death-feigning intensity and flying ability were measured for adults of 21 different populations of C. chinensis derived from different geographical regions. All beetles used for the experiments were reared with one larva per one bean before the experiment. For each population, 30 males and 30 females emerging from beans were randomly collected, and both traits were measured within 1 day after emergence. All measurements were conducted between 12.00 and 18.00 in the chamber described above. To evaluate the correlation between death-feigning intensity and flying ability, Kendall's coefficients of rank correlation were used.
Four of the 21 populations were jC-S, mC, isC and yoC02, described above. The strain name, collection year, locality of population, the name of collector, the number of founder females and the references of the other 17 populations are as follows: jC-F, 1936, Kyoto City, S. Utida, unknown and Utida (1941); pC, 1980, Punjab City, India, S. Utida, unknown and K. Fujii (2006, personal communication); TajC, 1993, Saitama City, Y. Toquenaga, 1 and Y. Toquenaga (2006, personal communication); akC02, 2002, Sanyo Town, F. Nakasuji, more than 50 and Harano & Miyatake (2005); and smC02, Izumo City, Y. Narai, more than 10 and Harano & Miyatake (2005). For the other 12 populations, we used two of each of the isolated-female lines (6×2 lines): kkC98, mrC98, mgC98, tsC98, skC98 and kzC98 strains, all of which were collected in 1998, and see Kondo et al. (1999) for their collection localities.
3. Results
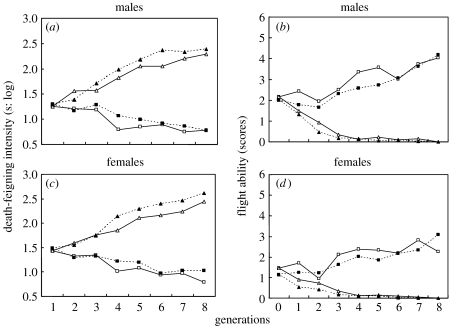
Death-feigning intensity, measured as the duration of death-feigning, showed a clear direct response to selection and a steady divergence between the two selection regimes (figure 1). After eight generations of selection, LD strains had durations in males and females of about 35 and 45 times as long as SD strains, respectively. Flying ability as a correlated response was the direct opposite, i.e. LD strains had lower flying ability, whereas SD strains showed higher flying ability in the two replications.
Figure 1.
(a,c) Death-feigning intensity as the direct response and (b,d) flying ability as the correlated response to selection in (a,b) male and (c,d) female C. chinensis on short duration (SD; circles) and long duration (LD; triangles) death-feigning. Open symbols, first replicate; filled symbols, second replicate; log, log translated.
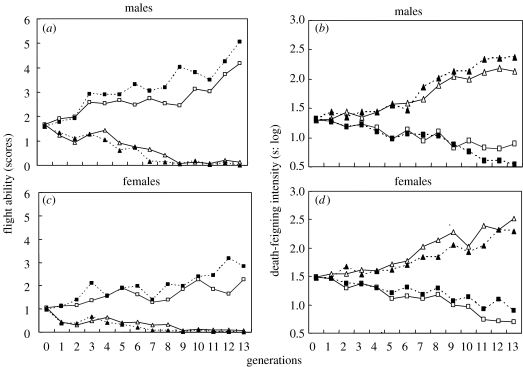
The flying ability also showed a clear direct response to selection regimes with a diverged nature (figure 2). After 13 generations of selection, the beetles derived from HS strains earned about 5 points in males and about 3 for females, whereas the LS strains showed almost no flying ability. Correlated responses were the direct opposite, i.e. HS and LS strains had shorter and longer durations of death-feigning, respectively.
Figure 2.
(a,c) Flying ability as the direct response and (b,d) duration of death-feigning intensity as the correlated response to selection in (a,b) male and (c,d) female C. chinensis on high scores (HS; circles) and low scores (LS; triangles) for flying ability. Open symbols, first replicate; filled symbols, second replicate; log, log translated.
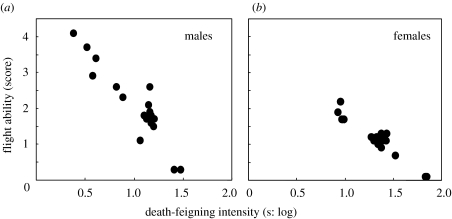
Inter-population comparison showed significant negative correlations between death-feigning intensity and flying ability in males (figure 3a: n=21, τ=−0.657, p<0.001) and females (figure 3b: n=21, τ=−0.519, p=0.001).
Figure 3.
Relationship between duration of death-feigning and scores for flying ability for 21 populations of C. chinensis. (a) And (b) graphs show male and female results, respectively; log, log translated.
4. Discussion
Negative phenotypic relationships between death-feigning and activity have been observed in some insects. In the parasitoid species Nasonia vitripennis, the females that were least active had the greatest tendency to exhibit thanatosis (King & Leaich 2006). In the sweet potato weevil Cylas formicarius, frequency and duration of death-feigning are affected by the behaviour before the weevil was startled: almost all resting individuals feigned death, whereas walking ones seldom feigned death (Miyatake 2001b). Adults of C. formicarius, a nocturnal species, seldom showed death-feigning behaviour during the nighttime, when they are active for reproduction, whereas they showed a higher frequency and longer duration of death-feigning in the daytime (Miyatake 2001b). Among other beetles, starved adults have a lower frequency of death-feigning (Acheampong & Mitchell 1997; Miyatake 2001a). High temperatures, in which insects are usually activated, suppress death-feigning behaviour in a water bug (Holmes 1906). These studies suggest the existence of two behavioural modes, active and resting, in which less and more death feigning, respectively, occurs.
The present study shows a genetic base for the relationship between activity and death-feigning, i.e. selection for higher flying ability caused lower intensity of death-feigning, and selection for lower flying ability caused longer duration of death-feigning. On the other hand, selection for longer duration of death-feigning was correlated with lower flying ability, and selection for shorter duration of death-feigning was correlated with higher flying ability. The two opposite correlated responses to selection clearly indicate a negative genetic correlation, namely a genetic trade-off, between death-feigning intensity and flying ability in C. chinensis.
The genetic cause of the correlation is chiefly pleiotropy, which is a common property of major genes (Falconer & Mackay 1996). It might be considered that a genetic factor pleiotropically controls death-feigning and flying ability. Death-feigning behaviour may be controlled by the level of activity (Erhard et al. 1999; Miyatake 2001b), and flying may be a sign of an insect with high activity levels. Neurotransmitters or biogenic amines, including dopamine and octopamine, derived from the amino acid tyrosine, may participate in insect behaviours, such as aggressiveness, flying ability and escape (e.g. Sombati & Hoyle 1984; Bicker & Menzel 1989; Goldstein & Camhi 1991; Stevenson et al. 2000; Libersat & Pfluger 2004). Thus, we propose the hypothesis that artificial selection for death-feigning or flying ability can cause an elevated or decreased level of the expression of biogenic amines in insects. Although neuro-ethological work is needed to prove this, if it is true, the negative relationship between activity and death-feigning may be explained by a pleiotropic gene controlling the expression levels of amines, and thus controlling death-feigning and flying ability. We cannot, however, rule out linkage as a cause of the negative genetic correlation (Falconer & Mackay 1996). Since it is difficult to examine the recombination rate of these two traits to evaluate linkage disequilibrium in C. chinensis, it will be practical to look for a molecular factor controlling insect activity and to compare the expression levels of neurotransmitters between strains in beetles selected in the future.
The negative relationship between death-feigning intensity and flying ability was also found in 21 populations derived from fields. This supports the hypothesis that the negative genetic trade-off between both traits has been selected in nature. Possible heritable variations of death-feigning behaviour in nature have been reported in a damselfly larva in which larvae collected from a pond without predatory fishes entered thanatosis more frequently and had longer durations of death-feigning than larvae collected from a pond with predators (Gyssels & Stokc 2005). Heritable variation of death-feigning intensity has also been reported in Tribolium castaneum (Prohammer & Wade 1981; Miyatake et al. 2004). In the present study, however, some of the populations used have been reared for many successive generations in laboratories, and thus the negative relation might be influenced by the rearing history of each population. For example, in a long-term laboratory-reared strain, the energy allocation may be biased towards reproduction rather than flying ability with an assignment of energy towards laying eggs. In other words, there must be a trade-off between dispersal and reproduction (Roff 1986, 1990). In such cases, a third trait, such as reproduction, may correlate with death-feigning, and thus the relation between death-feigning and flying ability might be a pseudo-correlation. To examine this possibility, a comparison of fecundity in strains artificially selected for death-feigning duration and flying ability may be appropriate, although this is a subject for future study.
Different genetic disposition, death feigning or flying away, might evolve in different natural populations through the following scenarios. The most probable explanation is that the relative effectiveness of death feigning and flying away varies between different predatory species, and the relative frequencies of different predatory species vary between the localities inhabited by the 21 populations. Alternatively or additionally, it might be that it is intensity of total predation risk that varies between locations, and the two alternative strategies vary in their costs. For example, perhaps investment in the muscles required for fast flight are more attractive only if predation risk is relatively high, so in low-risk environments death feigning is more strongly selected.
Prey must choose one or more of many options as anti-predator tactics when they face predators (Edmunds 1974; Lima & Dill 1990; Sih 1992). In some cases, a species may have a phenotypic plasticity in which each individual adopts the optimal anti-predator tactic in accordance with its situation (Harvell 1984; Boyero et al. 2006). The present results, however, suggest that two alternative anti-predator behaviours, such as flying away and dropping-and-feigning-death, which cannot be adopted simultaneously, may be selected as different strategies, and that these are controlled by a genetic factor concerning some chemical materials pleiotropically controlling the two behaviours. Considering the source of the variation in anti-predator behaviours as a phenotypic plasticity or as different strategies based on genetic factors may lead to different evolutionary consequences in prey–predator relationships. Phylogenetic research in flying ability and death-feigning behaviours is intriguing for examining the generality of the pleiotropic effects for death-feigning intensity and flying ability.
In a closely related species, Callosobruchus maculatus, flying and flightless forms have been observed: the flying adult beetles tended to fly when they were dropped, whereas the flightless beetles frequently feigned death by folding up their legs and antennae for a moment (Utida 1954), a behaviour similar to that of C. chinensis. Utida (1972) found that the phase dimorphism of C. maculatus was a density-dependent polymorphism in which the larvae grow in a bean, and he considered that the dimorphism was not genetically based, in contrast to the present result, which found genetic differences in flying and death-feigning lines. Although the present results demonstrate that there is a strong genetic component to anti-predatory strategies, this does not completely rule out individuals retaining some behavioural flexibility in which response they adopt in a given situation. A similar phase dimorphism for flying forms has been observed in Callosobruchus beetles (Southgate et al. 1957; Silim Nahdy et al. 1999), and thus it will be interesting to examine the relationship between phase dimorphism and death-feigning behaviours in bean beetles. In practical terms, more complex relationships between phenotypic plasticity and a genetic base might be detected for anti-predator behaviours including death-feigning and flying ability in this group of beetles.
Acknowledgments
The authors thank K. Fujii, K. Kohno, N. Kondo, F. Nakasuji, Y. Narai, M. Shimada and Y. Toquenaga for kindly providing the insect cultures of the bean beetles. This study was supported by a grant-in-aid for Scientific Research (KAKENHI 16370013) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Present address: Toyo Sangyo Corporation, 3-19-20 Shinyashiki-cho, Okayama City, Okayama 700-0986, Japan.
References
- Abjornsson K, Hansson L.A, Bronmark C. Responses of prey from habitats with different predator regimes: local adaptation and heritability. Ecology. 2004;85:1859–1866. [Google Scholar]
- Acheampong S, Mitchell B.K. Quiescence in the Colorado potato beetle, Leptinotarsa decemlimeata. Entomol. Exp. Appl. 1997;82:83–89. doi:10.1023/A:1002948008807 [Google Scholar]
- Agrawal A.A, Laforsch C, Tollrian R. Transgenerational induction of defenses in animals and plants. Nature. 2000;401:60–63. doi:10.1038/43425 [Google Scholar]
- Bicker G, Menzel R. Chemical codes for the control of behaviour in arthropods. Nature. 1989;337:33–39. doi: 10.1038/337033a0. doi:10.1038/337033a0 [DOI] [PubMed] [Google Scholar]
- Boyero L, Rincon P.A, Bosch J. Case selection by a limnephilid caddisfly [Potamophylax latipennis (Curtis)] in response to different predators. Behav. Ecol. Sociobiol. 2006;59:364–372. doi:10.1007/s00265-005-0059-y [Google Scholar]
- Edmunds M. Longman; Harlow, UK: 1974. Defense in animals: a survey of antipredator defences. [Google Scholar]
- Erhard H.W, Mendl M, Christianses S.B. Individual differences in tonic immobility may reflect behavioural strategies. Appl. Anim. Behav. Sci. 1999;64:31–46. doi:10.1016/S0168-1591(99)00028-3 [Google Scholar]
- Fabre J.H. Paris, France; Delgrave: 1900. Souvenirs entomologiques, 7eme Serie. [Google Scholar]
- Falconer D.S, Mackay T.F.C. 4th edn. Longman; Essex, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Frost S.W. 2nd edn. Dover Publication; New York, NY: 1959. Insect life and insect natural history. [Google Scholar]
- Goldstein R.S, Camhi J.M. Different effects of the biogenic amines dopamine, serotonin and octopamine on the thoratic and abdominal portions of the escape circuit in the cockroach. J. Comp. Physiol. A. 1991;168:103–112. doi: 10.1007/BF00217108. doi:10.1007/BF00217108 [DOI] [PubMed] [Google Scholar]
- Gyssels F.G.M, Stokc R. Threat-sensitive responses to predator attacks in a damselfly. Ethology. 2005;111:411–423. doi:10.1111/j.1439-0310.2005.01076.x [Google Scholar]
- Harano T, Miyatake T. Heritable variation in polyandry in Callosobruchus chinensis. Anim. Behav. 2005;70:299–304. doi:10.1016/j.anbehav.2004.10.023 [Google Scholar]
- Harvell C.D. Predator-induced defense in a marine bryozoan. Science. 1984;224:1357–1359. doi: 10.1126/science.224.4655.1357. doi:10.1126/science.224.4655.1357 [DOI] [PubMed] [Google Scholar]
- Holmes J.S. Death-feigning in Ranatra. J. Comp. Neurol. Psychol. 1906;16:200–216. doi:10.1002/cne.920160305 [Google Scholar]
- Honma A, Oku S, Nishida T. Adaptive significance of death feigning posture as a specialized inducible defence against gape-limited predators. Proc. R. Soc. B. 2006;273:1631–1636. doi: 10.1098/rspb.2006.3501. doi:10.1098/rspb.2006.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.H, Leaich H.R. Variation in propensity to exhibit thanatosis in Nasonia vitripennis (Hymenoptera: Pteromalidae) J. Insect Behav. 2006;19:241–249. doi:10.1007/s10905-006-9022-7 [Google Scholar]
- Kondo N, Shimada M, Fukatsu T. High prevalence of Wolbachia in the azuki bean beetle Callosobruchus chinensis (Coleoptera: Bruchidae) Zool. Sci. 1999;16:955–962. doi:10.2108/zsj.16.955 [Google Scholar]
- Lehtiniemi M. Swim or hide: predator cues cause species specific reactions in young fish larvae. J. Fish Biol. 2005;66:1285–1299. doi:10.1111/j.0022-1112.2005.00681.x [Google Scholar]
- Libersat F, Pfluger H. Monoamines and the orchestration of behavior. Bioscience. 2004;54:17–25. doi:10.1641/0006-3568(2004)054[0017:MATOOB]2.0.CO;2 [Google Scholar]
- Lima S.L. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 1998;27:215–290. [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Martin J, Lopez P. Costs of refuge use affect escape decisions of Iberian rock lizards Lacerta monticola. Ethology. 2000;106:483–492. doi:10.1046/j.1439-0310.2000.00568.x [Google Scholar]
- Miyatake T. Effects of starvation on death-feigning in adults of Cylas formicarius (Coleoptera: Brentidae) Ann. Entomol. Soc. Am. 2001a;94:612–616. doi:10.1603/0013-8746(2001)094[0612:EOSODF]2.0.CO;2 [Google Scholar]
- Miyatake T. Diurnal periodicity of death-feigning in Cylas formicarius (Coleoptera: Brentidae) J. Insect Behav. 2001b;14:421–432. doi:10.1023/A:1011196420147 [Google Scholar]
- Miyatake T, Katayama K, Takeda Y, Nakashima A, Sugita A, Mizumoto M. Is death-feigning adaptive? Heritable variation in fitness difference of death-feigning behaviour. Proc. R. Soc. B. 2004;271:2293–2296. doi: 10.1098/rspb.2004.2858. doi:10.1098/rspb.2004.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondor E.B, Rosenheim J.A, Addicott J.F. Predator-induced transgenerational phenotypic plasticity in the cotton aphid. Oecologia. 2005;142:104–108. doi: 10.1007/s00442-004-1710-4. doi:10.1007/s00442-004-1710-4 [DOI] [PubMed] [Google Scholar]
- Nakamura H. [Geographic variation of the ecological characters in Callosobruchus chinensis L.] Jpn. J. Ecol. 1969;19:127–131. [In Japanese with English summary.] [Google Scholar]
- Prohammer L.A, Wade M.J. Geographic and genetic variation in death-feigning behavior in the flour beetle, Tribolium castaneum. Behav. Genet. 1981;11:395–401. doi: 10.1007/BF01070822. doi:10.1007/BF01070822 [DOI] [PubMed] [Google Scholar]
- Roff D.A. The evolution of wing dimorphism in insects. Evolution. 1986;40:1009–1020. doi: 10.1111/j.1558-5646.1986.tb00568.x. doi:10.2307/2408759 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Antagonistic pleiotropy and the evolution of wing dimorphism in the sand cricket, Gryllus firmus. Heredity. 1990;65:169–177. [Google Scholar]
- Ruxton G. Grasshoppers don't play possum. Nature. 2006;440:880. doi: 10.1038/440880a. doi:10.1038/440880a [DOI] [PubMed] [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals & mimicry. [Google Scholar]
- Sih A. Prey uncertainty and the balancing of antipredator and foraging needs. Am. Nat. 1992;139:1052–1069. doi:10.1086/285372 [Google Scholar]
- Silim Nahdy M, Silim S.N, Ellis R.H. Effect of field infestations of immature pigeonpea (Cajanus cajanus (L.) Mill sp.) pods on production of active (flight) and sedentary (flightless) morphs of Callosobruchus chinensis (L.) J. Stored Prod. Res. 1999;35:339–354. doi:10.1016/S0022-474X(99)00017-X [Google Scholar]
- Sombati S, Hoyle G. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J. Neurobiol. 1984;15:481–506. doi: 10.1002/neu.480150607. doi:10.1002/neu.480150607 [DOI] [PubMed] [Google Scholar]
- Southgate B.J, Howe R.W, Brett G.A. The specific status of Callosobruchus maculatus (F.) and Callosobruchus analis (F.) Bull. Entomol. Res. 1957;48:79–89. [Google Scholar]
- Stevenson P.A, Hofmann H.A, Schoch K, Schildberger K. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 2000;43:107–120. doi:10.1002/(SICI)1097-4695(200005)43:2<107::AID-NEU1>3.0.CO;2-C [PubMed] [Google Scholar]
- Utida S. Studies on experimental population of the azuki bean weevil Callosobruchus chinensis (L.). I. The effect of population density on the progeny population. Mem. Coll. Agric. Kyoto Imperial Univ. 1941;48:1–31. [Google Scholar]
- Utida S. Phase dimorphism observed in the laboratory population of the cowpea weevil, Callosobruchus quadrimaculatus. Jpn J. Appl. Entomol. Zool. 1954;18:161–168. [Google Scholar]
- Utida S. Density dependent polymorphism in the adult of Callosobruchus maculatus (Coleoptera, Bruchidae) J. Stored Prod. Res. 1972;8:111–126. doi:10.1016/0022-474X(72)90028-8 [Google Scholar]
- Yanagi S, Miyatake T. Costs of mating and egg production in female Callosobruchus chinensis. J. Insect Physiol. 2003;49:823–827. doi: 10.1016/S0022-1910(03)00119-7. doi:10.1016/S0022-1910(03)00119-7 [DOI] [PubMed] [Google Scholar]
- Wasson K, Lyon B.E. Flight or fight: flexible antipredatory strategies in porcelain crabs. Behav. Ecol. 2005;16:1037–1041. doi:10.1093/beheco/ari086 [Google Scholar]
- Weisser W.W, Braendle C, Minoretti N. Predator-induced morphological shift in the pea aphid. Proc. R. Soc. B. 1999;266:1175–1181. doi:10.1098/rspb.1999.0760 [Google Scholar]