Abstract
Recent studies have shown for birds that females sometimes choose mates on the basis of condition-dependent variation in ultraviolet (UV, less than 400 nm) ornamentation, but there have been few comparable studies on invertebrates. Yet many invertebrates have UV structural coloration. Here, we investigate Cosmophasis umbratica, a jumping spider (Araneae: Salticidae) that has sexually dimorphic UV-iridescent ornamentation, and we provide evidence that male UV coloration is condition dependent in this species. Spectral-reflection patterns change with male age and prior feeding history. The position of the UV band (i.e. UV hue) of the carapaces of younger (field-collected as subadults and matured as adults in laboratory) males shifted, relative to older (field-collected as adults) males, significantly towards longer wavelengths. Food deprivation significantly decreased the spectral intensity of the abdomen, but not the carapace. Questions concerning the mechanisms by which UV ornaments change are highlighted, as are hypotheses concerning the role of condition-dependent UV variation in male–male competition and as a criterion used by females when making mate-choice decisions.
Keywords: UV vision, UV reflectance, body conditions, jumping spiders, structural colour, Cosmophasis umbratica
1. Introduction
Pigment-based ornaments have long been known to be important in intraspecific communication (reviewed by Hill 1999), but recent work is showing that optical interference of biological microstructural characteristics may also play an important role in animal communication. Many animals may have colour vision based in part on sensitivity to ultraviolet (UV) and sometimes these animals also have UV-reflecting ornaments, with these ornaments appearing to function in communication (e.g. Jacobs 1992; Bennett & Cuthill 1994; Tovée 1995; Losey et al. 1999; Cuthill et al. 2000a,b; Shi et al. 2001). Research on birds, in particular, has shown that structure-based ornaments reflecting in the UV may reveal an individual's state or condition (Keyser & Hill 1999, 2000; Mougeot et al. 2005; Doucet et al. 2005, 2006; Delhey et al. 2006) and function in the context of male–male competition for access to females (Alonso-Alvarez et al. 2004; Siefferman & Hill 2005) and in the context of female choice of males with which to mate (Cuthill et al. 2000a).
Variation in the UV reflectance of bird ornaments may be influenced by both biotic and abiotic factors, including age (e.g. red grouse Lagopus lagopus scoticus: Mougeot et al. 2005; eastern bluebirds Sialia sialis: Siefferman et al. 2005; blue tits Parus caeruleus: Delhey & Kempenaers 2006; Peters et al. 2006), nutritional condition (blue grosbeaks Guiraca caerulea: Keyser & Hill 1999; McGraw et al. 2002), season (blue tits P. caeruleus: Örnborg et al. 2002; Delhey et al. 2006), parasite load (red grouse, L. l. scoticus: Mougeot et al. 2005) and feather-degrading bacteria (Burtt & Ichida 1999). However, despite UV vision and UV-based ornamentation being widespread in invertebrates (Fox 1976; Parker 1999, 2000; Briscoe & Chittka 2001), our understanding of invertebrate UV signalling lags behind avian UV signalling. For example, although feeding regimes of caterpillars are known to affect the ultimate UV-structural ornaments of butterflies (Kemp et al. 2006), there have been no studies that test hypotheses about how age or prior feeding might influence these ornaments in the adult stage.
Here, we address the imbalance in our understanding of invertebrate versus vertebrate reliance on UV by presenting findings from a study on a sexually dichromatic jumping spider (Araneae: Salticidae). Salticids are known to have UV-sensitive receptors in the retinas of their principal eyes (Land 1969, 1985; DeVoe 1975; Yamashita & Tateda 1976; Blest et al. 1981; Peaslee & Wilson 1989) and male salticids are well known for their often extravagant coloration, and there has been a strong emphasis in the salticid literature on the hypothesis that the mate-choice behaviour of females has driven the evolution of elaborate salticid male ornamentation (Peckham & Peckham 1889, 1890, 1894; Crane 1949a,b). Besides pigmentary coloration (e.g. red, yellow or black; Townsend & Felgenhauer 1999), some salticids have structural coloration (e.g. blue, green, white or UV; Parker & Hegedus 2003).
Cosmophasis umbratica, a salticid species that is common in sunlit vegetation in Singapore, is particularly appropriate for investigating UV signals. Males of this species have complex iridescent markings, some of which are structural, on various parts of their bodies, but especially on the top and sides of the carapace and on the sides of leg femora (Lim & Li 2006), but females do not reflect UV (Lim & Li 2006). The males' abdomens are mostly black, but with iridescent white lines running anterior to posterior. Earlier work has also revealed that UV reflectance varies considerably among males (Lim & Li 2006), with these findings being the rationale for two questions that we investigate here: (i) does spectral reflectance of structure-based UV ornaments depend on age? and (ii) does prior feeding history affects structure-base UV ornaments?
2. Material and methods
All spiders were kept individually in transparent cylindrical cages (diameter×height: 6.5×8.5 cm) with opaque sides to prevent contact among individuals, and maintained in a laboratory under controlled environmental conditions (relative humidity, 80–85%; temperature, 25±1°C; light regime, 12 : 12 h; lights on at 08.00 h), with the provision, for 4 h daily (09.00–11.00 h; 16.00–18.00 h), of additional lighting (Arcadia Natural Sunlight Lamp) that simulated natural sunlight.
To test whether structure-based spectral reflectance is affected by post-maturity male age (i.e. time elapsing in the natural environment after the male's final moult), we used two groups: ‘old’ (collected as adults in the field; n=25) and ‘young’ (collected as subadults from the field and maintained until they matured in the laboratory; n=25). Spiders for the two groups were collected from the same location at the same time. The precise post-maturity age of the old males was unknown, but the important variable in this initial study was simply that we knew they were mature when collected in the field and, therefore, their post-maturity age when we used them in the laboratory was greater than that of the young males and that only old males had spent any time as adults in the field.
Upon collection, these spiders were maintained on a standardized diet of house flies (Musca domestica), fruit flies (Drosophila melanogaster) and small instars of crickets (Acheta domestica) twice per week (Lim & Li 2006). The period in the laboratory before reflectance spectra measurements were obtained was 10 days post-maturity (i.e. 10 days after the final moult) for young males and 10 days for field-collected males (i.e. the post-maturity age of these males was 10 days plus their unknown time as adults in the field).
To explore whether prior feeding history affects the spectral reflectance of males, a separate group of laboratory-matured males (i.e. collected as subadults; n=10) were initially maintained for 10 days, then starved for 10 days and subsequently fed for 5 days, as earlier studies have shown that salticids have low metabolic rate (e.g. Anderson 1996) and are typically maintained in the laboratory by feeding with a few prey items once a week (e.g. Jackson & Hallas 1986). All spiders were first fed fruit flies (D. melanogaster) until satiation at day 0, and were then kept without food (only water via a cotton roll was provided ad libitum) from day 1 for 10 days. At day 11, the reflectance spectra of all the spiders were measured (for measurements, see below). From day 12, each individual was fed with 10 fruit flies every day for 5 days, and the reflectance spectra of these same individuals were then measured again at day 16. Abdominal dimensions (e.g. widths of abdomens and dorsal iridescent line) were not measured as these changes, after 10 days of food deprivation, were too subtle. Therefore, to confirm that starved individuals had successfully fed on provided prey, individual masses were recorded on the same day the reflectance spectra were obtained (i.e. day 11 and day 16). Owing to the extensibility of a spider's abdomen, but not its carapace, here we could assume that an increase in mass should result from abdomen distension.
The spectral reflectance of two body parts, the dorsal cephalothorax (hereafter carapace) and the dorsal abdomen (hereafter abdomen), which are extensively displayed during intraspecific interaction in C. umbratica (Lim & Li 2004), was measured. Spectral measurement followed that of Lim & Li 2006, which was modified from previous well-established standard protocols (Endler 1990; Endler & Théry 1996; Andersson & Amundsen 1997; Cuthill et al. 1999). To facilitate accurate measurement of reflectance, spiders were temporarily immobilized by exposure to carbon dioxide (ca 5 min). A stable and continuous source of full-spectrum light (215–1700 nm) was provided by a DH-2000 deuterium tungsten halogen light source (Ocean Optics Inc., Dunedin, FL, USA) that illuminated a round area of approximately 1 mm in diameter, at a distance of 2 mm, from the reflection probe to the body region. A reflection probe that housed a single fibre, surrounded by six hexagonally arranged illuminating fibres, recorded reflected spectra from the surface under study. The distance between the specific body region and the probe was set at 2 mm by clamping the probe to a vertical adjustable translation stage (Creative Stars Electro-Optics Inc., Redmond, WA, USA); this allowed the intended distance to be accurately achieved (with a resolution of 0.01 mm) while maintaining a fixed perpendicular angle between the probe and the plane of the intended area on the spider. All of the spectral reflectance data were captured using a USB2000 UV/VIS miniature fibre-optic spectrometer (Ocean Optic Inc.) and processed using OOIBase32 spectrometer operating software (v. 2.0.1.3), with a Spectralon white standard (Labsphere, North Sutton, NH, USA), an almost perfect diffuser (more than 95% reflectance from 250 to 2000 nm), and a dark reference (lights off in a dark room) being employed for comparison. Five spectra were repeatedly taken from each body part through a process of resetting the probe's position and obtaining a spectral reading, and the average was used for statistical analyses.
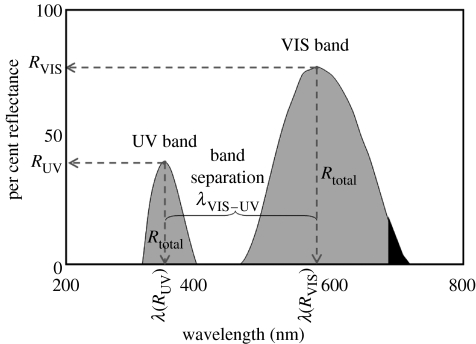
Three standard descriptions of reflectance spectra are commonly used in colour analysis (Hailman 1977), namely brightness (spectral intensity, R), hue (spectral location, λ) and chroma (saturation or spectral purity). For this study, we used only brightness and hue to analyse the recorded spectral data. Measurement of chroma (or spectral purity) was not attempted here owing to the lack of a distinctive trough in the reflectance curve of male C. umbratica (Lim & Li 2006) that greatly hindered accurate chroma estimation. Instead, we introduced the use of relative spectral position, termed ‘band separation’, of both UV and VIS bands to represent spectral purity. This is because the proximity (spectral position) of the UV band relates to the bandwidth of the VIS band in a multilayered reflector system (Land 1972), one type of structural colour mechanism which is proposed in C. umbratica, based on the prominence of the VIS and UV reflection bands as those of a main band and oscillating sidebands, respectively (Land 1972). To objectively describe variation in spectral shape (‘colour’: hue) and intensity (‘brightness’), we described seven colorimetrics based on the reflectance spectra recorded from C. umbratica adult males (figure 1 and for definitions see table 1). For convenience, we used brightness and intensity interchangeably.
Figure 1.
Spectral variables of typical spectral reflectance curve of adult male C. umbratica. RUV, UV intensity (%); RVIS, VIS intensity (%); λ(RUV), UV hue, the spectral position of UV peak (nm); λ(RVIS), VIS hue, the spectral position of VIS peak (nm); Rtotal, total intensity of reflectance spectra (300–700 nm); λVIS–UV, band separation (nm), wavelength difference between the peaks of UV and VIS bands.
Table 1.
Definition of spectral variables in the reflectance spectra of a C. umbratica male.
| variables | definition |
|---|---|
| UV intensity (RUV) | percentage reflectance (%) of UV reflection band |
| VIS intensity (RVIS) | percentage reflectance (%) of VIS reflection band |
| total intensity (Rtotal) | area under reflectance graph (from 300 to 700 nm) |
| UV hue (λ(RUV)) | spectral position of UV band peak: wavelength (nm) of maximal reflectance in UV range (300–400 nm) |
| VIS hue (λ(RVIS)) | spectral position of VIS band peak: wavelength (nm) of maximal reflectance in VIS range (400–700 nm) |
| band separation (λVIS–UV) | wavelength difference (in nm) between UV hue and VIS hue |
3. Results
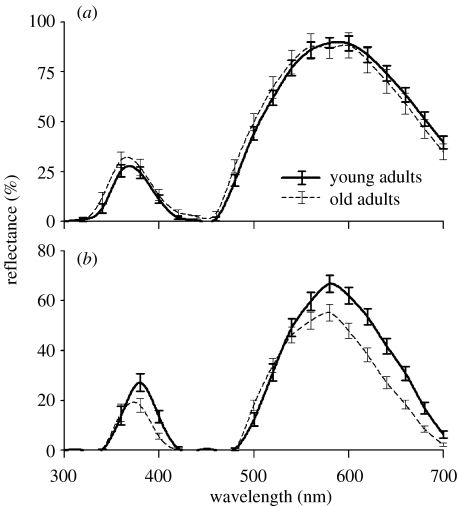
For abdominal spectral reflectance traits, young males were not significantly different from old males in brightness (UV, VIS and total intensity), UV and VIS hues or band separation (λVIS–UV; figure 2a and table 2). However, old males' carapace UV hue shifted, relative to young males, significantly towards shorter wavelengths, and young males had brighter carapaces in the VIS range and greater overall intensity than old males. There was a suggestion of a small shift in the VIS hue of the carapace towards shorter wavelengths in old males, but this was not significant. Carapace band separation (λVIS–UV) of young was not significantly different from that of old males (figure 2b and table 2).
Figure 2.
Reflectance spectra for (a) dorsal abdomen and (b) dorsal carapace of young (solid line) and old (dashed line) C. umbratica. Error bars represent standard errors, mean±s.e.
Table 2.
Effects of age and prior feeding history on the reflectance spectral traits of male C. umbratica. (Data are presented as mean±s.e. and are analysed by independent t-tests for age effects or paired t-tests for effects of prior feeding history. Boldfaced cells show statistically significant results. *Mann–Whitney U-test.)
| spectral variables | body part | age (see figure 2) | prior feeding history (see figure 3) | |||||
|---|---|---|---|---|---|---|---|---|
| young | old | statistics | comments | change | statistics | comments | ||
| UV intensity (%) | abdomen | 31.67±2.83; n=25 | 38.69±3.74; n=23 | t46=−1.511, p=0.138 | no difference | 10.79±2.17 | t9=−4.977, p<0.001 | satiated males are UV brighter |
| carapace | 32.44±3.42; n=24 | 25.86±3.17; n=22 | t44=1.405, p=0.167 | no difference | −0.74±1.56 | t9=0.472, p=0.648 | no change | |
| VIS intensity (%) | abdomen | 89.50±3.52; n=25 | 88.18±6.09; n=25 | t48=0.191, p=0.850 | no difference | 16.93±2.61 | t9=−6.486, p<0.001 | satiated males are VIS brighter |
| carapace | 64.38±3.29; n=25 | 53.20±3.23; n=25 | t48=2.451, p=0.018 | young males brighter | −1.14±1.75 | t9=0.650, p=0.532 | no change | |
| normalized total intensity | abdomen | 0.61±0.03; n=25 | 0.60±0.05; n=25 | t48=0.088, p=0.930 | no difference | 0.14±0.02 | t9=−6.717, p<0.001 | satiated males are brighter |
| carapace | 0.57±0.04; n=25 | 0.44±0.04; n=25 | t48=2.305, p=0.026 | young males brighter | 0.00±0.01 | t9=0.076, p=0.941 | no change | |
| UV peak (nm) | abdomen | 369.15±2.18; n=25 | 366.77±2.29; n=25 | t48=0.753, p=0.455 | no difference | −0.17±4.34 | t9=0.121, p=0.907 | no change |
| carapace | 376.62±1.64; n=25 | 370.57±1.46; n=25 | t48=2.765, p=0.008 | UV band shift towards shorter wavelengths in old males | −0.89±3.48 | t9=0.813, p=0.437 | no change | |
| VIS peak (nm) | abdomen | 584.20±2.89; n=25 | 579.23±3.24; n=25 | t48=1.144, p=0.258 | no difference | −3.38±8.82 | t9=1.211, p=0.257 | no change |
| carapace | 580.64±1.72; n=25 | 574.33±2.19; n=25 | Z50=−0.805, p=0.071* | no difference | −0.57±5.30 | t9=0.337, p=0.744 | no change | |
| band separation (nm) | abdomen | 215.05±1.57; n=25 | 212.46±1.75; n=25 | t48=1.133, p=0.263 | no difference | −3.21±9.33 | t9=1.088, p=0.305 | no change |
| carapace | 204.02±1.11; n=25 | 203.76±1.39; n=25 | t48=0.179, p=0.858 | no difference | 0.33±6.61 | t9=−0.158, p=0.878 | no change | |
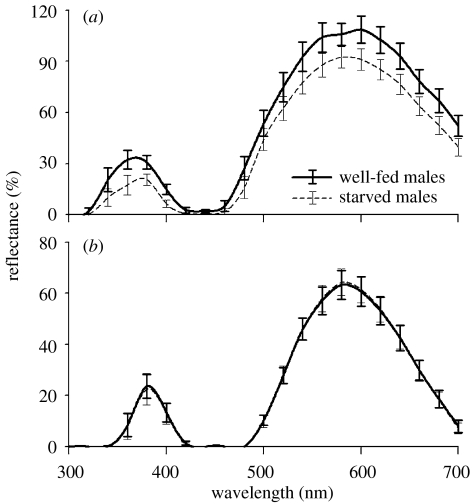
When males were fed again after a fast, there was a significant increase in mass (0.0127±0.0003 g; paired t-test: t=−3.836, p=0.003) and prior feeding history had a pronounced effect on abdomen, but not carapace, spectral reflectance (figure 3a and table 2). There was a significant increase in the intensities of UV and VIS bands, as well as the overall abdomen brightness, when spiders were fed again after starvation. However, the abdominal hues (i.e. UV, VIS and band separation) did not change with starvation. Nor did prior feeding history affect carapace spectral reflectance variables (figure 3b and table 2).
Figure 3.
Reflectance spectra of (a) dorsal abdomen and (b) dorsal carapace of starved (dashed line) and satiated (solid line) C. umbratica. Error bars represent standard errors, mean±s.e.
4. Discussion
Our findings show that the UV structural coloration of C. umbratica males varies significantly with male age and male prior feeding history. This is the first demonstration of the expression of structure-based coloration in the jumping spider being condition dependent, but there are studies showing comparable condition dependence of UV coloration in birds (Andersson & Amundsen 1997; Siitari & Huhta 2002; Mougeot et al. 2005; Siefferman et al. 2005).
The mechanisms responsible for variation in the structural colours of C. umbratica are currently unknown. However, as salticid scales are basically chitinous, cumulative periods of exposure to UV radiation may cause changes in the refractive indexes (as related to optical thickness) of the chitin layers and thereby cause the spectral characteristics of old males to diverge from the spectral characteristics of young males. It is of interest that photodegradation has been suggested as a basis of coloration change in birds (Smith 1995; Blanco et al. 2005). A particularly interesting area for future research on salticids might be to identify, measure and compare the optical traits of the cuticular scales of young and old males using transmission microscopy techniques (Fitzstephens & Getty 2000).
Male–male interactions of C. umbratica often entail considerable contact, with the spiders rolling about and biting each other (Lim & Li 2004). Perhaps, these intensive interactions cause abrasive loss of scales, with this possibly accounting, at least in part, for old males having spectral characteristics that differ from the spectral characteristics of young males. Physical contact between interacting males tends to involve especially the anterior region of the spiders' bodies, suggesting that this is a mechanism that may account especially for loss of cuticular scales on the carapace.
It will be of interest to investigate whether, for C. umbratica, condition dependence of male UV spectral characteristics functions in the context of sexual selection. Other research (M. L. M. Lim & D. Li unpublished) has shown that C. umbratica females rely on cues from male UV spectral characteristics when making mate-choice decisions. However, we do not currently know whether the female gains a fitness advantage by choosing young instead of old males or by choosing well-fed instead of fasted males.
Having found significant increase in the UV and VIS reflectance of the abdomen, but not the carapace, after feeding suggests that abdomen stretching is a mechanism by which feeding changes a male's UV spectral characteristics. The pattern of packing and overlapping of numerous iridescent cuticular scales adorning the abdomens of C. umbratica males (Lim & Li 2004) may be sensitive to the level of abdomen distension, which in turn is sensitive to feeding history. It is of interest that C. umbratica males actively move their abdomens not only during intraspecific interactions but also during normal locomotion, with these movement patterns most probably making abdomen coloration conspicuous to other conspecific individuals (Lim & Li 2004), suggesting that the effects of feeding history on abdomen UV spectral characteristics may function as an indicator of male quality, or as an amplifier of a male's physical condition (Taylor et al. 2000).
Acknowledgements
We thank Poh Moi Goh for rearing the fruit flies. Comments and suggestions from Robert R. Jackson, Lian Pin Koh and Tien Ming Lee greatly helped to improve the manuscript. This work was supported by grants to D.L. from the National University of Singapore ARC (R-154-000-140-112). The experiments comply with the ‘Principles of Animal Care’, publication No. 86-23 (revised 1985) of the National Institute of Health, and also with the current laws of Singapore.
References
- Alonso-Alvarez C, Doutrelant C, Sorci G. Ultraviolet reflectance affects male-male interactions in the blue tit (Parus caeruleus ultramarinus) Behav. Ecol. 2004;15:805–809. doi:10.1093/beheco/arh083 [Google Scholar]
- Anderson J.F. Metabolic rates of resting salticid and thomisid spiders. J. Arachnol. 1996;24:129–134. [Google Scholar]
- Andersson S, Amundsen T. Ultraviolet colour vision and ornamentation in bluethroats. Proc. R. Soc. B. 1997;264:1587–1591. doi:10.1098/rspb.1997.0200 [Google Scholar]
- Bennett A.T.D, Cuthill I.C. Ultraviolet vision in birds—what is its function. Vision Res. 1994;34:1471–1478. doi: 10.1016/0042-6989(94)90149-x. doi:10.1016/0042-6989(94)90149-X [DOI] [PubMed] [Google Scholar]
- Blanco G, Frias O, Garrido-Fernandez J, Hornero-Mendez D. Environmental-induced acquisition of nuptial plumage expression: a role of denaturation of feather carotenoproteins? Proc. R. Soc. B. 2005;272:1893–1900. doi: 10.1098/rspb.2005.3157. doi:10.1098/rspb.2005.3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest A.D, Hardie R.C, McIntyre P, Williams D.S. The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. J. Comp. Physiol. A. 1981;145:227–239. doi:10.1007/BF00605035 [Google Scholar]
- Briscoe A.D, Chittka L. The evolution of colour vision in insects. Ann. Rev. Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. doi:10.1146/annurev.ento.46.1.471 [DOI] [PubMed] [Google Scholar]
- Burtt E.H, Ichida J.M. Occurrence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116:364–372. [Google Scholar]
- Crane J. Comparative biology of salticid spiders at Rancho Grande, Venezuela. Part III. Systematics and behavior in representative species. Zoologica. 1949;34:31–52. [Google Scholar]
- Crane J. Comparative biology of salticid spiders at Rancho Grande, Venezuela. Part IV. An analysis of display. Zoologica. 1949b;34:159–215. [Google Scholar]
- Cuthill I.C, Bennett A.T.D, Partridge J.C, Maier E.J. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 1999;153:183–200. doi: 10.1086/303160. doi:10.1086/303160 [DOI] [PubMed] [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D. Avian UV vision and sexual selection. In: Espmark Y, Amundsen T, Rosenqvist G, editors. Animal signals: signalling and signal design in animal communication. Tapir Academic Press; Trondheim, Norway: 2000. pp. 61–82. [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D, Church S.C, Hart N.S, Hunt S. Ultraviolet vision in birds. Adv. Stud. Behav. 2000b;29:159–214. [Google Scholar]
- Delhey K, Kempenaers B. Age differences in blue tit Parus caeruleus plumage colour: within-individual changes or colour-biased survival? J. Avian Biol. 2006;37:339–348. doi:10.1111/j.2006.0908-8857.03655.x [Google Scholar]
- Delhey K, Peters A, Hojnson A, Kempenaers B. Seasonal changes in blue tit crown color: do they signal individual quality? Behav. Ecol. 2006;17:790–798. doi: 10.1093/beheco/ar1012 [Google Scholar]
- DeVoe R.D. Ultraviolet and green receptors in principal eyes of jumping spiders. J. Gen. Physiol. 1975;66:193–207. doi: 10.1085/jgp.66.2.193. doi:10.1085/jgp.66.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet S.M, Mennill D.J, Montgomerie R, Boag P.T, Ratcliffe L.M. Achromatic plumage reflectance predicts reproductive success in male black-capped chickadees. Behav. Ecol. 2005;16:218–222. doi:10.1093/beheco/arh154 [Google Scholar]
- Doucet S.M, Shawkey M.D, Hill G.E, Montgomerie R. Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 2006;209:380–390. doi: 10.1242/jeb.01988. doi:10.1242/jeb.01988 [DOI] [PubMed] [Google Scholar]
- Endler J.A. On the measurement and classification of color in studies of animal color patterns. Biol. J. Linn. Soc. 1990;41:315–352. [Google Scholar]
- Endler J.A, Théry M. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am. Nat. 1996;148:421–452. doi:10.1086/285934 [Google Scholar]
- Fitzstephens D.M, Getty T. Colour, fat and social status in male damselflies, Calopteryx maculata. Anim. Behav. 2000;60:851–855. doi: 10.1006/anbe.2000.1548. doi:10.1006/anbe.2000.1548 [DOI] [PubMed] [Google Scholar]
- Fox D.L. University of California Press; Berkeley, CA: 1976. Animal biochromes and structural colours: physical, chemical, distributional and physiological features of coloured bodies in the animal world. [Google Scholar]
- Hailman J.P. Indiana University Press; Bloomington, IN: 1977. Optical signals: animal communication and light. [Google Scholar]
- Hill G.E. Mate choice, male quality, and carotenoid-based plumage coloration. In: Adams N, Slowtow R, editors. Proceedings of the 22nd International Ornithological Congress. University of Natal; Durban, South Africa: 1999. pp. 1654–1668. [Google Scholar]
- Jackson R.R, Hallas S.E.A. Comparative biology of Portia africana, P. albimana, P. fimbriata, P. labiata, and P. schultzi, araneophagic web-building jumping spiders (Araneae: Salticidae): utilisation of silk, predatory versatility, and intraspecific interactions. New Zeal. J. Zool. 1986;13:423–489. [Google Scholar]
- Jacobs G.H. Ultraviolet vision in vertebrates. Am. Zool. 1992;32:544–554. [Google Scholar]
- Kemp D.J, Vukusic P, Rutowski R.L. Stress-mediated covariance between nano-structural architecture and ultraviolet butterfly coloration. Func. Ecol. 2006;20:282–289. doi:10.1111/j.1365-2435.2006.01100.x [Google Scholar]
- Keyser A.J, Hill G.E. Condition-dependent variation in the blue-ultraviolet coloration of a structurally based plumage ornament. Proc. R. Soc. B. 1999;266:771–777. doi:10.1098/rspb.1999.0704 [Google Scholar]
- Keyser A.J, Hill G.E. Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav. Ecol. 2000;11:202–209. doi:10.1093/beheco/11.2.202 [Google Scholar]
- Land M.F. Structure of the retinae of the eyes of jumping spiders (Salticidae: Dendryphantinae) in relation to visual optics. J. Exp. Biol. 1969;51:433–470. doi: 10.1242/jeb.51.2.443. [DOI] [PubMed] [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophy. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. doi:10.1016/0079-6107(72)90004-1 [DOI] [PubMed] [Google Scholar]
- Land M.F. The morphology and optics of spider eyes. In: Barth F.G, editor. Neurobiology of arachnids. Springer; Berlin Heidelberg, NY: 1985. pp. 53–78. [Google Scholar]
- Lim M.L.M, Li D. Courtship and male–male agonistic behaviour of Cosmophasis umbratica Simon, an ornate jumping spider (Araneae: Salticidae) from Singapore. Raffles B. Zool. 2004;52:435–448. [Google Scholar]
- Lim M.L.M, Li D. Extreme ultraviolet sexual dimorphism in jumping spiders (Araneae: Salticidae) Biol. J. Linn. Soc. 2006;89:397–406. doi:10.1111/j.1095-8312.2006.00704.x [Google Scholar]
- Losey G.S, Cronin T.W, Goldsmith T.H, Hyde D, Marshall N.J, McFarland W.N. The UV visual world of fishes: a review. J. Fish Biol. 1999;54:921–943. doi:10.1111/j.1095-8649.1999.tb00848.x [Google Scholar]
- McGraw K.J, Mackillop E.A, Dale J, Hauber M.E. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 2002;205:3747–3755. doi: 10.1242/jeb.205.23.3747. [DOI] [PubMed] [Google Scholar]
- Mougeot F, Redpath S.M, Leckie F. Ultra-violet reflectance of male and female red grouse, Lagopus lagopus scoticus: sexual ornaments reflect nematode parasite intensity. J. Avian Biol. 2005;36:203–209. doi:10.1111/j.0908-8857.2005.03424.x [Google Scholar]
- Örnborg J, Andersson S, Griffith S.C, Sheldon B.C. Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus. Biol. J. Linn. Soc. 2002;76:237–245. doi:10.1046/j.1095-8312.2002.00061.x [Google Scholar]
- Parker A.R. Invertebrate structure colours. In: Savazzi E, editor. Functional morphology of the invertebrate skeleton. Wiley; London, UK: 1999. pp. 65–90. [Google Scholar]
- Parker A.R. 515 million years of structural colour. J. Opt. A: Pure Appl. Opt. 2000;2:R15–R28. doi:10.1088/1464-4258/2/6/201 [Google Scholar]
- Parker A.R, Hegedus Z. Diffractive optics in spiders. J. Opt. A: Pure Appl. Opt. 2003;5:S111–S116. doi:10.1088/1464-4258/5/4/364 [Google Scholar]
- Peaslee A.G, Wilson G. Spectral sensitivity in jumping spiders (Araneae, Salticidae) J. Comp. Physiol. A. 1989;164:359–363. doi: 10.1007/BF00612995. doi:10.1007/BF00612995 [DOI] [PubMed] [Google Scholar]
- Peckham G.W, Peckham E.G. Observations on sexual selection in spiders of the family Attidae. Occas. Pap. Wisconsin Nat. Hist. Soc. 1889;1:3–60. [Google Scholar]
- Peckham G.W, Peckham E.G. Additional observations in sexual selection in spider of the family Attidae. Occas. Pap. Wisconsin Nat. Hist. Soc. 1890;1:117–151. [Google Scholar]
- Peckham G.W, Peckham E.G. The sense of sight in spiders with some observations of the color sense. Trans. Wisconsin Acad. Sci. Arts Lett. 1894;10:231–261. [Google Scholar]
- Peters A, Delhey K, Goymann W, Kempenaers B. Age-dependent association between testosterone and crown UV coloration in male blue tits (Parus caeruleus) Behav. Ecol. Sociobiol. 2006;59:666–673. doi:10.1007/s00265-005-0095-7 [Google Scholar]
- Shi Y.S, Radlwimmer F.B, Yokoyama S. Molecular genetics and the evolution of ultraviolet vision in vertebrates. Proc. Natl. Acad. Sci. USA. 2001;98:11731–11736. doi: 10.1073/pnas.201257398. doi:10.1073/pnas.201257398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefferman L, Hill G.E. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim. Behav. 2005;69:67–72. doi:10.1016/j.anbehav.2003.12.026 [Google Scholar]
- Siefferman L, Hill G.E, Dobson F.S. Ornamental plumage coloration and condition are dependent on age in eastern bluebirds Sialia sialis. J. Avian Biol. 2005;36:428–435. doi:10.1111/j.0908-8857.2005.03401.x [Google Scholar]
- Siitari H, Huhta E. Individual color variation and male quality in pied flycatchers (Ficedula hypoleuca): a role of ultraviolet reflectance. Behav. Ecol. 2002;13:737–741. doi:10.1093/beheco/13.6.737 [Google Scholar]
- Smith G.J. New trends in photobiology: photodegradation of keratin and other structural proteins. J. Photoch. Photobio. B. 1995;27:187–198. doi:10.1016/1011-1344(94)07104-V [Google Scholar]
- Taylor P.W, Hasson O, Clark D.L. Body postures and patterns as amplifiers of physical condition. Proc. R. Soc. B. 2000;267:917–922. doi: 10.1098/rspb.2000.1090. doi:10.1098/rspb.2000.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovée M.J. Ultraviolet photoreceptors in the animal kingdom: their distribution and function. Trends Ecol. Evol. 1995;10:455–460. doi: 10.1016/s0169-5347(00)89179-x. doi:10.1016/S0169-5347(00)89179-X [DOI] [PubMed] [Google Scholar]
- Townsend V.R, Felgenhauer B.E. Ultrastructure of the cuticular scales of lynx spiders (Araneae: Oxyopidae) and jumping spiders (Araneae: Salticidae) J. Morphol. 1999;240:77–92. doi: 10.1002/(SICI)1097-4687(199904)240:1<77::AID-JMOR6>3.0.CO;2-P. doi:10.1002/(SICI)1097-4687(199904)240:1<77::AID-JMOR6>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Yamashita S, Tateda H. Spectral sensitivities of jumping spider eyes. J. Comp. Physiol. A. 1976;105:29–41. doi:10.1007/BF01380051 [Google Scholar]