Abstract
The access to libraries of molecules with interesting biomolecular properties is a limiting step in the drug discovery process. By virtue of a long molecular evolution process, natural products are recognized as biologically validated starting points in structural space for library development. We introduce here a strategy to generate natural product-like libraries. A semisynthetic mixture of compounds was produced by diversification of a natural product extract through the chemical transformation of common chemical functionalities in natural products into chemical functionalities rarely found in nature. The resulting mixture showed antifungal activity against Candida albicans, whereas the starting extract did not show such activity. Bioguided fractionation led to the isolation of a previously undescribed active semisynthetic pyrazole. The result illustrates how biological activity can be generated by designed chemical diversification of a natural product mixture, and represents the proof of principle of an alternative strategy for producing natural product-like libraries from natural products libraries.
Keywords: diversity, natural products
Natural products have been invaluable as platforms for developing front-line drugs (1–3). This value is in part due to the fact that their chemical diversity is complementary to the diversity found in synthetic libraries (4). Natural products are sterically more complex and have broader diversity of ring systems compared with synthetic and combinatorial counterparts (4, 5). Those structural features are the result of a long evolutionary selection process (6). Less than one-fifth of the ring systems found in natural products are represented in current trade drugs (7). On the other hand, drug and combinatorial molecules tend to include a higher number of nitrogen-, sulfur-, and halogen-containing groups (4, 5, 8).
Because the identification of new chemotypes for drug development remains an urgent need in many therapeutic areas, innovative strategies for natural products to contribute their full range of chemical diversity are being developed. Such strategies range from the exploration of unconventional sources of natural compounds (9, 10) to the development of synthetic methodologies for the preparation of natural product-like libraries (11–15) through the diversification of natural product mixtures by combinatorial biosynthesis and related techniques (16–21).
We report here a strategy to generate bioactive compounds through chemical diversification of inactive natural product mixtures (i.e., natural extracts). Natural product extracts are natural libraries of complex composition, mostly uncharacterized, that usually contain a high number of molecules with different scaffolds and functionalities.
Provided that a significant proportion of the different molecules present in a given extract is chemically altered, a considerable number of compounds will be generated; hence, changes in the biological properties of the mixture could be expected (22, 23).
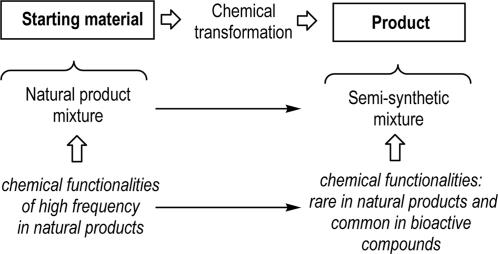
Our approach to modify as many compounds as possible in complex natural mixtures of unknown composition is to focus into the transformation of chemical functionalities that are very common in natural products and thus expected to be present in a substantial proportion of the members of the mixture (Fig. 1).
Fig. 1.
Schematic representation of the approach to transform natural products mixtures without previous knowledge of their composition.
If those common chemical functionalities are transformed into chemical functionalities that are rarely produced by nature, we could complement nature's chemical capabilities. To increase the chances of generating libraries with interesting biomolecular properties, the approach also points to incorporating some of the characteristics of biologically active compounds into the natural mixtures (i.e., average heteroatom content).
Results and Discussion
Carbonyl Group as the Target.
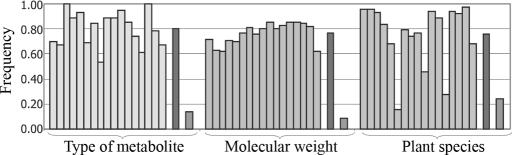
One chemical functionality commonly found in natural products is the carbonyl group. According to the Dictionary of Natural Products (24), ≈80% of the 147,852 structures within the database contain at least one carbonyl group in their structure. To gain insight into the distribution of those structures within the database, the number of carbonyl containing molecules was analyzed in three sets of 17 groups of compounds or “virtual extracts” selected from the database through the use of three different filters. In set one, each group of compounds contained every molecule within the database found in one particular plant species.¶ In the second set, each of the 17 “virtual extracts” included only one type of secondary metabolite (i.e., flavonoids, alkaloids, etc.). Finally, in set three, each virtual extract included all of the molecules in the database with molecular weights starting with arbitrarily defined values (i.e., 10, 20, etc.). The average frequency of carbonyl-containing molecules was between 0.76 and 0.80 for all of the sets of virtual extracts (Fig. 2) and, perhaps more importantly, there is >80% probability that at least 65% of structures included in the virtual extracts contain one or more carbonyl groups, regardless of the filter used. This result suggests that chemical transformation of the carbonyl group could be an interesting entry point to the chemical transformation of a significant number of the component molecules of natural extracts.
Fig. 2.
Frequencies of carbonyl containing structures in three sets of “virtual extracts” selected from the database through the filters, showing type of metabolite, molecular weight, and plant species. Darker bars represent the mean value and standard deviation for each set.
To select reagents and conditions suitable to transform carbonyl groups, the database (24) was searched for common forms of carbonyl groups in natural product structures, and we discovered that carbonyl groups are mainly found as esters (50%) and ketones (40%), followed by carboxylic acids (27%), amides (13%), and aldehydes (5%).
Hydrazine as the Reagent.
Aiming at the diversification of the components of natural extracts through carbonyl group transformation, we first tested the reaction with hydrazine monohydrate. This reagent can react with ketones, aldehydes, esters, and amides to form either hydrazones or acyl hydrazides. These reactions result in the exchange of one oxygen atom with two nitrogen atoms, which is interesting considering that different reports indicate that the average nitrogen content per molecule in natural products is lower than in drug molecules, and the opposite is observed for oxygen content (4, 5, 8).
In addition, the presence of a second nitrogen atom in the reagent results is attractive because of the following reasons: (i) it increases the nucleophilic character, (ii) it can give further reactions increasing the potential number of products, and (iii) the N–N moiety is uncommon in natural products.‖
Alteration of Composition.
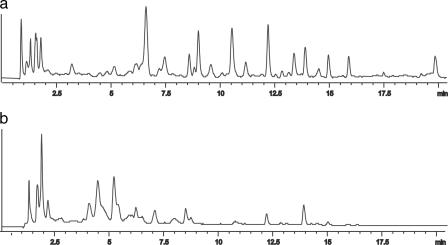
When a n-butanol extract of the plant Polygonum ferrugineum Wed (Polygonaceae) (25) was treated with hydrazine monohydrate in refluxing ethanol, interesting changes were observed by high-performance liquid chromatography (HPLC) and NMR analysis. The HPLC profile of the mixture was affected by the reaction: the modified mixture showed a major number of peaks with retention times >6 min, whereas the most prominent peaks in the chromatogram of the unmodified extract concentrated within a range of retention times <7 min (Fig. 3).
Fig. 3.
HPLC trace of BuOH extract of P. ferrugineum after reaction with hydrazine monohydrate (a) and BuOH extract of P. ferrugineum (b).
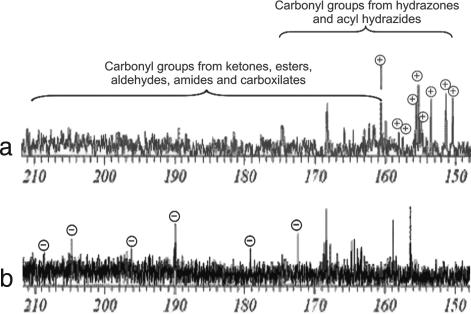
Comparison of the 13C NMR spectra of treated and untreated mixtures showed interesting changes in the signals corresponding to carbonyl carbons. Considering that NMR signals for carbonyl carbons of ketones, esters, aldehydes, and amides appear between 160 and 210 ppm, and signals for C N carbons of hydrazones and C
N carbons of hydrazones and C O carbons of acyl hydrazides usually appear within the range of 150–175 ppm, three zones of expected signal changes could be defined as follows: (i) 175–210 ppm where signals are expected to disappear, (ii) 150–160 ppm where new signals are expected to show up, and (iii) 160–175 ppm where signals of starting material as well as products can be found so that signals are expected to either disappear or appear. Fig. 3 shows that the main signals that disappear from the 13C NMR spectrum of the original mixture because of the reaction (Fig. 4b, marked as ⊝) were located between 170 and 210 ppm. In addition, several signals emerge in the expected range of chemical shift of the 13C NMR spectrum of the modified mixture (Fig. 4a, marked as ⊕).
O carbons of acyl hydrazides usually appear within the range of 150–175 ppm, three zones of expected signal changes could be defined as follows: (i) 175–210 ppm where signals are expected to disappear, (ii) 150–160 ppm where new signals are expected to show up, and (iii) 160–175 ppm where signals of starting material as well as products can be found so that signals are expected to either disappear or appear. Fig. 3 shows that the main signals that disappear from the 13C NMR spectrum of the original mixture because of the reaction (Fig. 4b, marked as ⊝) were located between 170 and 210 ppm. In addition, several signals emerge in the expected range of chemical shift of the 13C NMR spectrum of the modified mixture (Fig. 4a, marked as ⊕).
Fig. 4.
Carbon carbonyl region of the 13C NMR spectra of BuOH extract of P. ferrugineum after reaction with hydrazine monohydrate (a) and BuOH extract of P. ferrugineum (b).
Alteration of Biological Properties.
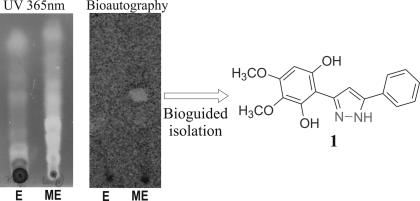
Differences between the biological properties of the modified and unmodified extracts were studied by TLC bioautography, a technique that combines chromatographic separation with in situ activity determination (26). Bioautography was carried out with Candida albicans, the most frequently isolated human fungal pathogen, which causes a wide variety of mucosal and systemic infections as an opportunistic organism in immunocompromised patients (27, 28). When a developed TLC of both mixtures was stained by use of a viability dye for the fungus (29), a clear inhibition zone was detected in the modified extract, whereas no inhibition could be detected in the original extract (Fig. 5).
Fig. 5.
TLC traces of unmodified BuOH extract (E) and modified BuOH extract of P. ferrugineum by reaction with hydrazine (ME).
Bioactivity-guided fractionation of the semisynthetic mixture through normal and reversed-phase chromatography led to the isolation of pyrazole 1. Compound 1 contains the expected N–N moiety in its structure, indicating that it was produced during the diversification step. This conclusion is further supported by the fact that pyrazoles are very uncommon secondary metabolites in plants (30).
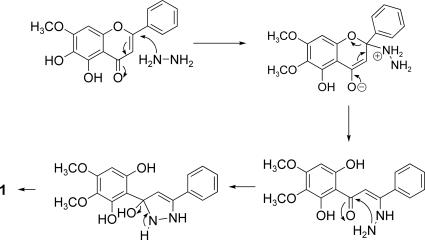
Pyrazole 1 could be formed by reaction of hydrazine with an inactive flavone present in the unmodified extract (31, 32). Nucleophilic attack of hydrazine at C-2 of the flavone followed by ring-opening and further nucleophilic attack of the second nitrogen atom at the carbonyl carbon and subsequent dehydration could have led to the formation of the pyrazole ring (Fig. 6).
Fig. 6.
Possible reaction pathway for the formation of 1.
Conclusion
We have described the generation of biological activity in an inactive natural extract by means of chemical diversification of their components. The process focuses on the transformation of chemical groups highly common in natural products into chemical groups that are rarely produced by the secondary metabolism. In this way nature's biosynthetic machinery can be complemented to produce a whole range of new semisynthetic compounds in one step. Chemically engineered extracts could become an alternative source of compounds to feed the discovery process for new molecules with interesting biomolecular activities.
Materials and Methods
NMR analyses were carried out on an AC-200 spectrometer (200 MHz; Bruker, Billerica, MA) in CDCl3. Tetramethylsilane (TMS) was used as the internal standard for 1H and 13C NMR. Chemical shifts are reported in parts per million downfield from TMS, and J values are reported in hertz.
HPLC analysis was carried out by using a 1050 instrument (Hewlett–Packard, Palo Alto, CA), coupled to a Hewlett–Packard 1050 DAD; data were analyzed by using Hewlett–Packard ChemStation. Reversed-phase HPLC separations were carried out by using a 15 cm × 4.6 mm i.d., 3-μm particle size ABZ+ C18 alkylamide column (Supelco, Bellefonte, PA) using acetonitrile and water gradients.
High-resolution fast atom bombardment (HRFAB) mass spectra were recorded on a VG-ZAB (100-2600) apparatus in the Mass Spectrometry Laboratory at the University of California at Riverside (Riverside, CA).
“Virtual” Extracts.
The plant species selected were those with higher number of compounds described in the database.
Selected plant species (frequency of carbonyl containing molecules) are as follows: Withania somnifera (L.) Dunal (0.95), Tripterygium wilfordii Hook. f. (0.96), Taxus mairei (Lemée & H. Lév.) S. Y. Hu ex T. S. Liu (0.93), Taxus baccata L. (0.93), Buxus sempervirens L. (0.68), Foeniculum vulgare Mill. (0.16), Glycyrrhiza uralensis Fisch. ex DC. (0.79), Halimium viscosum (Willk.) P. Silva (0.74), Glycyrrhiza glabra L. (0.76), Cannabis sativa L. (0.46), Azadirachta indica A. Juss. (0.94), Helianthus annuus L. (0.88), Panax ginseng C. A. Mey. (0.28), Lupinus albus L. (0.97), Clausena excavata Burm. f. (0.95), Melia azedarach L. (0.98), and Cryptomeria japonica (Thunb. ex L. f.) D. Don (0.68).
Selected types of metabolites (frequency of carbonyl containing molecules) are as follows: aliphatics (0.70), alkaloids 0,67 aa and peptides (1.00), benzofuranoids (0.88) benzopyranoids (0.93), carbohydrates (0.69), flavonoids (0.84), lignans (0.54), oxygen heterocycles (0.89), polycyclic aromatics (0.89), polyketides (0.95) polypyrroles (0.85), simple aromatics (0.74), steroids (0.61), tannins (1.00), terpenoids (0.78), and monoterpenoids (0.68).
Two initial numbers molecular weight (frequency of carbonyl containing molecules) are as follows: 10*(0.72), 15*(0.63), 20*(0.62), 25*(0.71), 30*(0.70), 35*(0.77), 40*(0.81), 45*(0.75), 50*(0.80), 55*(0.86), 60*(0.80), 65*(0.83), 70*(0.86), 75*(0.85), 80*(0.85), 85*(0.82), 90*(0.63).
Reaction with Hydrazine Monohydrate.
An EtOH solution of the dry extract (2% wt/vol) containing hydrazine monohydrate (1% wt/vol) was stirred under reflux for 7 h, and the solvent was removed under reduced pressure. The residue was dissolved in dichloromethane (1.5 times the volume of the reaction) and washed twice with water. The organic fraction was dried (sodium sulfate) and gravity filtered, and the solvent was removed by rotary evaporation. Reactions were carried out in 100-mg, 5-g, and 35-g scale obtaining good reproducibility for chromatographic and bioautographic profiles.
Antifungal Evaluation.
For the bioautography, we used C. albicans ATCC 10231 (American Type Culture Collection, Rockville, MD). Chromatograms were placed in sterile Petri dishes with covers. Sabouraud growth medium with 0.6% agar/0.02% phenol red and an inoculum of cell suspensions at a final concentration of 1–5 × 105 cells, obtained according to reported procedures (33), was distributed over developed TLC plates (1 ml/cm2). After solidification of the medium, the TLC plates were incubated overnight at 28°C. Subsequently bioautograms were sprayed with an aqueous solution of methylthiazolyltetrazolium chloride (MTT; 1 mg/ml) and incubated for another 2 h at 28°C. Dark yellow inhibition zones appeared against a dark brown background (27). In accordance with literature, under these conditions the minimum amount detected of the known antifungal Amphotericin B was 1 μg (34).
Isolation of 1.
The modified extract was chromatographed on silica gel (230–400 mesh, hexane-ethyl acetate gradient) to obtain 19 fractions. The bioautography active fractions (14–18) were chromatographed on reversed-phase silica gel C-18 (water-methanol gradient) to obtain six fractions. Fraction 3 was further chromatographed onto preparative TLC on reversed-phase silica gel to obtain compound 1.
3(5)-(2,6-dihydroxy-4,5-dimethoxyphenyl)-5(3)-phenylpyrazole (1).
1H NMR (CDCl3) δ = 7.39 – 7.65 (5H, m, ArH), 7.22 (1H, s, ArH), 6.21 (1H, s, ArH), 3.94 (3H, s, OCH3), 3.90 (3H, s, OCH3). 13C NMR (CDCl3) δ = 154.47, 150.73 (Ar), 149.73, 143.41 (CN, Ar), 129.41, 129.20, 129.11, 128.79, 125.69, 103.83, 100.10, 90.27 (Ar), 60.97, 55.80 (OCH3). HRMS (EI) m/z: Calcd. 312.1110, observed 312.1114 (1.3 ppm)
Acknowledgments
We thank Prof. Susana Gattuso for collection of P. ferrugineum. This work was supported by Fundación Antorchas, Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo (CYTED), Universidad Nacional de Rosario, and Third World Academy of Sciences (TWAS). S.N.L. and I.A.R. received fellowships from CONICET.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
The selected plant species represent those containing a higher number of secondary metabolites reported.
Less than 1% of the 147,852 structures in the Dictionary of Natural Products (24) database contain the N–N moiety in their structure.
References
- 1.Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM, Snader K. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 3.Butler MS. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 4.Henkel T, Brunne RM, Müller H, Reichel R. Angew Chem Int Ed. 1999;38:643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Lee M-L, Schneider G. J Comb Chem. 2001;3:284–289. doi: 10.1021/cc000097l. [DOI] [PubMed] [Google Scholar]
- 6.Paterson I, Anderson EA. Science. 2005;310:451–453. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 7.Koehn FE, Carter GT. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 8.Feher M, Schmidt JM. J Chem Inf Comput Sci. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 9.Tulp M, Bohlin L. Drug Discov Today. 2004;9:450–458. doi: 10.1016/S1359-6446(04)03066-1. [DOI] [PubMed] [Google Scholar]
- 10.Haefner B. Drug Discov Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 11.Koch MA, Schuffenhauer A, Scheck M, Wetzel S, Casaulta M, Odermatt A, Ertl P, Waldmann H. Proc Natl Acad Sci USA. 2005;102:17272–17277. doi: 10.1073/pnas.0503647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortholand J-Y, Ganesan A. Curr Opin Chem Biol. 2004;8:271–280. doi: 10.1016/j.cbpa.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan A. Curr Opin Chem Biol. 2004;15:584–590. [Google Scholar]
- 14.Chen C, Li X, Neumann CS, Lo MMC, Schreiber SL. Angew Chem Int Ed. 2005;44:2249–2252. doi: 10.1002/anie.200462798. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt EE, Fergus S, Galloway WRJD, Bender A, Fox DJ, Plowright AT, Jessiman AS, Welch M, Spring DR. Chem Commun. 2006:3296–3298. doi: 10.1039/b607710b. [DOI] [PubMed] [Google Scholar]
- 16.Knight V, Sanglier J-J, DiTullio D, Braccili S, Bonner O, Waters J, Hughes D, Zhang L. Appl Microbiol Biotechnol. 2003;62:446–458. doi: 10.1007/s00253-003-1381-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Weller RL, Thorson JS, Rajski SR. J Am Chem Soc. 2006;128:2760–2761. doi: 10.1021/ja056231t. [DOI] [PubMed] [Google Scholar]
- 18.Weist S, Süssmuth RD. Appl Microbiol Biotechnol. 2005;68:141–150. doi: 10.1007/s00253-005-1891-8. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez C, Zhu L, Braña AF, Salas AP, Rohr J, Méndez C, Salas JE. Proc Natl Acad Sci USA. 2005;102:461–466. doi: 10.1073/pnas.0407809102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floss HG. J Biotechnol. 2006;124:242–257. doi: 10.1016/j.jbiotec.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissman KJ, Leadlay PF. Nat Rev Microbiol. 2005;3:925–936. doi: 10.1038/nrmicro1287. [DOI] [PubMed] [Google Scholar]
- 22.Nefzi A, Ostresh JM, Yu J, Houghten RA. J Org Chem. 2004;69:3603–3609. doi: 10.1021/jo040114j. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaou KC, Pfefferkorn JA, Barluenga S, Mitchell HJ, Roecker AJ, Cao G-Q. J Am Chem Soc. 2000;122:9968–9976. [Google Scholar]
- 24.Buckingham J. Dictionary of Natural Products on CD-ROM. London: Chapman & Hall; 2001. [Google Scholar]
- 25.López SN, Gonzalez Sierra M, Gattuso SJ, Furlan RLE, Zacchino SA. Phytochemistry. 2006;67:2156–2158. doi: 10.1016/j.phytochem.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Ramallo IA, Zacchino SA, Furlan RLE. Phytochem Anal. 2006;17:15–19. doi: 10.1002/pca.874. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Garcia F, Sanchez M, Nombela C, Pla J. FEMS Microbiol Rev. 2001;25:245–268. doi: 10.1111/j.1574-6976.2001.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal K, Carbon J. Proc Natl Acad Sci USA. 2002;99:12969–12974. doi: 10.1073/pnas.162488299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena G, Farmer S, Towers GHN, Hancock REW. Phytochem Anal. 1995;6:125–129. [Google Scholar]
- 30.Chen JQ, Rahbe Y, Delobel B. Phytochemistry. 1999;50:1117–1122. [Google Scholar]
- 31.Pinto DCGA, Silva AMS, Almeida LMPM, Cavaleiro JAS, Elguero J. Eur J Org Chem. 2002:3807–3815. [Google Scholar]
- 32.Pinto DCGA, Silva AMS, Cavaleiro JAS, Foces-Foces C, Llmaz-Saiz AL. Tetrahedron. 1999;55:10187–10200. [Google Scholar]
- 33.National Committee for Clinical and Laboratory Standards. A Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. 2nd Ed. No 15. Vol 22. Wayne, PA: Natl Committee for Clin and Lab Standards; 2002. p. 6. NCCLS Document M 27-A2. [Google Scholar]
- 34.Rahalison L, Hamburger M, Monod M, Frenk E, Hostettmann K. Planta Med. 1994;60:41–44. doi: 10.1055/s-2006-959405. [DOI] [PubMed] [Google Scholar]