Abstract
We previously described unique features of the IL-15 receptor (IL-15R)α. IL-15Rα by itself forms stable complexes with IL-15 on cell surfaces and presents IL-15 in trans to neighboring natural killer/T cells. Moreover, the membrane IL-15/IL-15Rα complexes (membIL-15) undergo endosomal internalization but survive lysosomal degradation, allowing the complexes to recycle back to the cell surface. Here, we show that membIL-15+ cells act as a persistent source of IL-15 for the surrounding microenvironment (intercellular reservoir effect). Additionally, membIL-15+ cells give rise to augmented retention of IL-15 in the circulation as well as in tissues. Curiously, IL-15 retention was particularly associated with lungs, rather than with lymph nodes, in normal unstimulated mice, which correlated with the preferential homing of antigen-specific CD8 T cells to lungs during their contraction phase in an IL-15Rα-dependent manner. Furthermore, membIL-15, unlike soluble IL-15, caused sustained IL-15 signal transduction in the target cells. Collectively, these characteristics define IL-15 as a unique cytokine with prolonged in vivo survival and sustained biological action on the target cells, which may account for the proposed persistent action of IL-15 that helps the long-term survival of functional CD8 memory T cells in vivo.
Keywords: cytokine retention in vivo, memory CD8 homing
Despite the sharing of critical signaling components between IL-2 and IL-15 (1, 2), these two cytokines exert fundamentally distinct functions (reviewed by Waldmann and Tagaya in ref. 3). Mice deficient in these cytokines or their private receptor components show nonoverlapping phenotypes (3–9). In our previous study (10) we demonstrated the fundamental differences in the way these two cytokines act. Briefly, IL-15 exists as a membrane-associated cytokine acting on cell-to-cell contact as part of an immunological synapse. In contrast, IL-2 remains a soluble factor. Other groups have provided compelling evidence that the transpresentation may be the major style of IL-15 action in vivo (11–18). IL-15 is produced by activated dendritic cells (DCs), monocytes, and stromal cells (3), whereas IL-2 is produced by antigen (Ag)-stimulated T cells. Thus, it is reasonable that IL-15 that bridges accessory and T cells requires presentation in trans whereas IL-2 activates T cells in an autonomous manner.
IL-15 may be persistently needed in vivo, especially in the context of the IL-15-dependent prolonged survival of functional memory CD8 T cells (19, 20) as well as in the developmental requirement for IL-15 in the ontogeny of natural killer cells (7, 21) and γδ T cells (22–24). Based on RT-PCR, IL-15 expression was considered rather constitutive. However, IL-15 protein is not easily detectable by biochemical means in normal humans and mice (3). Thus, the mechanism underlying such persistent IL-15 action in vivo remains elusive. In our previous study (10) we demonstrated that a cytokine-dependent cell line, CTLL-2, when cultured with IL-15, became resistant to apoptotic death upon IL-15 withdrawal (remained viable >72 h), whereas the same cells cultured with IL-2 underwent prompt apoptosis within 12 h of IL-2 withdrawal (10). Because IL-15/IL-15Rα complexes recycled after endosomal internalization, cells could continue to use IL-15 after the removal of soluble IL-15 from the culture media (10). Thus, the IL-15/IL-15Rα system has an intrinsic characteristic that makes this cytokine act stably in vivo. We tried to exploit this persistence of IL-15 in the context of biological function and found that indeed IL-15 is a well retained cytokine in vivo because of recycling. In addition, we show here that IL-15Rα-expressing cells are constantly present in select tissues in normal mice, thereby contributing to the retention and persistent presence of IL-15 in these tissues that maintain IL-15 activity in the absence of constitutive production of the IL-15 protein. We also show evidence that links this observation with the IL-15-mdiated homing of Ag-primed CD8 T cells in the contraction phase.
Furthermore, we demonstrate the unique nature of growth signals provided by the cell-surface membrane-associated IL-15/IL-15Rα complex (membIL-15). In short, membIL-15 presented in trans to target cells transduces signals that are qualitatively distinct from those provided by soluble IL-15, which lead to more prolonged and persistent activation of the target cells. These distinct characteristics of IL-15 may provide a mechanistic explanation for the persistent action of IL-15 in vivo and makes this cytokine a special factor among those γc-using cytokines (IL-15, IL-2, and IL-7) (25, 26) that are known to contribute to the generation and survival of the Ag-specific memory CD8 T cells.
Results
IL-15Rα-Positive Cells Can Provide a Persistent IL-15 Reservoir to the Surrounding Microenvironment.
We previously reported that IL-15/IL-15Rα complexes formed on the cell surfaces undergo internalization but survive this intracellular endosomal processing to recycle back to the cell surface as a biologically active cytokine (10). To determine whether the recycled IL-15/IL-15Rα complexes release free IL-15 into the environment, we transfected 293T cells with an IL-15Rα expression construct and added recombinant human (rh) IL-15 to the culture for a 6-h incubation. The cells were then washed with acidic PBS (pH 3.5) to completely strip the cell-bound IL-15 (completion of which was confirmed by flow cytometry; data not shown). These cells containing intracellular IL-15/IL-15Rα complexes were transferred to a cytokine-free environment to determine whether the intracellularly stored IL-15 was released after recycling. An ELISA (Fig. 1A) and a biological CTLL-2 assay (Fig. 1B) allowed us to confirm that biologically active IL-15 was released in a time-dependent manner. During the recycling of IL-15/IL-15Rα, cell surface of these cells become positive for membIL-15 (10). Even after the release of some IL-15 molecules into the supernatant, the cell surfaces of the treated 293T cells remained positive for recycled IL-15/IL-15Rα complexes (data not shown). At this time, the majority of the IL-15 was still associated with cells because IL-15 recovered from plasma-membrane-associated fractions after sucrose-gradient ultracentrifugation contained five times more IL-15 protein than was released (Fig. 1C). These results suggest that the IL-15Rα-positive cells (note that these are not necessarily IL-15-producing cells) can capture and stably store IL-15 molecules as intracellular reservoir through recycling, which then provides an environment with released biologically active IL-15. Nevertheless, the majority of the IL-15 was retained in association with the plasma membrane, presumably as IL-15/IL-15Rα complexes because of the high binding affinity between these two molecules, thus allowing these cells to behave as an IL-15 reservoir for a prolonged time. We propose to call this function an “intercellular reservoir effect.”
Fig. 1.
IL-15Rα-positive cells provide a persistent IL-15 reservoir. (A) Progressive release of the soluble IL-15 after recycling of the IL-15/IL-15Rα complexes. Human 293T cells (negative for IL-15 and IL-15Rα expression) were transfected with a plasmid encoding IL-15Rα (10) and incubated with 1 nM rhIL-15 (PeproTech) for 6 h to facilitate the recycling of IL-15/IL-15Rα complexes. Cells were then stripped of IL-15 (10) from the surface IL-15/IL-15Rα complexes and transferred to a cytokine-free culture. Aliquots were removed from the supernatant at the indicated time points, and the amounts of soluble IL-15 were determined by a specific ELISA. (B) Biological activity of the released IL-15. IL-15 concentrations in the aliquots collected above were determined by a specific ELISA. Biological activities of the released IL-15 and rhIL-15 (matched in concentrations) were compared by using a CTLL-2 proliferation assay. (C) The intercellular reservoir effect. The majority of the recycled IL-15 molecules remain associated with the plasma membrane fraction while a small fraction of the recycled IL-15 is released. Forty-eight hours after the initiation of the recycling experiment, 2 ml of the 293T culture was separated into supernatant and cellular pellets. Cells were homogenized and fractionated over a continuous sucrose-gradient by ultracentrifugation. The plasma-membrane fractions were identified by the migration of biotinylated cell-surface proteins, and the endosomal fractions were identified by the distribution of early endosomal protein 1 (10). Shown is the amount of IL-15 released back into the culture supernatant and those of IL-15 recovered in membrane-associated and endosomal fractions. The IL-15 in the plasma-membrane fractions was considered as cell-retained.
IL-15 Is an Effectively Retained Cytokine in Vivo.
Levels of a cytokine in in vivo environments are determined by various factors including their production, consumption, and excretion. We postulated that the intercellular reservoir effect might prolong the biological availability of IL-15 in vivo. To test this hypothesis, we administered the γc-cytokines IL-2, IL-7, and IL-15 into mice and examined the rate of the clearance of these proteins from the circulation. Because of the lack of a sensitive ELISA for mouse IL-15, we injected (i.p.) 5 μg of human cytokines into mice (27). As described, IL-2 and IL-7 levels declined to undetectable levels by 48 h after the injection (28). However, the serum levels of IL-15 showed different kinetics. There was a rapid decay during the first 24 h (28), followed by a second retention phase, and IL-15 levels remain detectable (>10 pg/ml) even 120 h after the injection, demonstrating that the administered IL-15 was retained longer than other γc-cytokines in WT mice (Fig. 2A).
Fig. 2.
IL-15 retention in the circulation of WT, IL-15Rα−/−, and IL-15Rα Tg mice. (A) Unique prolonged retention of IL-15 in the circulation of WT mice. Mice were injected with 5 μg of rhIL-15, rhIL-2, and rhIL-7 (i.p.). Serum samples were collected at the indicated timings, and their cytokine concentrations were determined by specific ELISAs (R & D Systems). (B) Rapid clearance of administered IL-15 from IL-15Rα−/− mice. Five micrograms of rhIL-15 was injected (i.p.) into WT or IL-15Rα−/− mice. (C) Augmented IL-15 retention in IL-15Rα Tg mice. Five micrograms of rhIL-15 was injected (i.p.) into IL-15Rα Tg mice or WT mice. Because the serum IL-15 concentrations from WT mice were too compressed to visualize at the given scale, an expanded graph with a readable scale is inserted on the right. (D) Concomitant prolonged expansion of CD44hiCD8 T cells in IL-15-injected IL-15Rα Tg mice. Peripheral blood mononuclear cells from IL-15-injected WT or IL-15Rα Tg mice were analyzed by flow cytometry, and percentages of CD44hi population in the entire CD8 subset are shown.
IL-15Rα Mediates the Retention of IL-15 in Mice.
To determine whether the IL-15 retention was mediated by a specific mechanism involving IL-15Rα, 5 μg of human cytokines was injected into IL-15Rα−/− mice. IL-15 failed to display the retention phase in IL-15Rα −/− mice (Fig. 2B). Notably, the rapid clearance pattern of IL-15 in IL-15Rα−/− mice resembled that of IL-2 or IL-7 in WT mice (Fig. 2A and B), suggesting that IL-15 behaved like other γc-cytokines in the absence of IL-15Rα.
Augmentation of IL-15Rα Expression Led to Enhanced Retention of IL-15 in Vivo.
In general, the internalization-mediated processing facilitates the catabolism of the soluble ligands, contributing to terminating the signal initiated by the ligands. Because of the unique intercellular reservoir effect, we speculated that IL-15 might have an intrinsically accumulating nature. To test this possibility, we administered rhIL-15 (5 μg, i.p.) into human IL-15Rα transgenic (IL-15Rα Tg) mice, in which the promoter of the elongation factor 1α drove the ubiquitous expression of the human IL-15Rα molecule. At 24 h after injection, we observed ≈100-fold more IL-15 retention in the sera of the IL-15Rα Tg mice than the WT mice. The serum IL-15 stayed above detectable levels (>10 pg/ml) for ≈16–20 days (Fig. 2C). No such prolonged retention was observed with IL-2 and IL-7 in any mice (data not shown). Concomitantly, we observed ≈50-day prolonged expansion of CD44hiCD8 T cells (Fig. 2D), a hallmark of augmented in vivo IL-15 action that was also seen in IL-15 Tg mice (29) or in WT mice repeatedly infused with IL-15 (27) for 24–30 consecutive days.
Systemic Challenge of Mice with Toll-Like Receptor Ligands That Stimulate IL-15Rα Expression Resulted in Augmented Retention of IL-15.
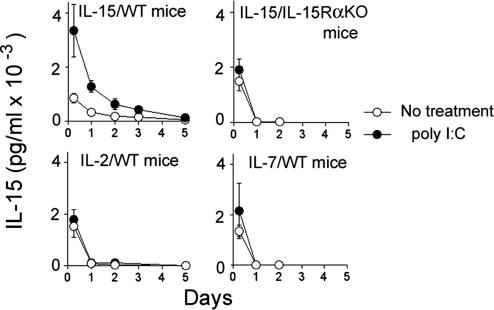
Microbial invasion induces IL-15Rα on accessory cells. To test whether IL-15Rα induction on these cells augments the IL-15 retention in vivo, we challenged mice with Toll-like receptor ligands such as poly inosinic poly cytidylic acid (poly I:C) or LPS to induce IL-15Rα expression (30) and observed a prolonged IL-15 retention in WT, but not in IL-15Rα−/−, mice (Fig. 3). No such prolonged retention was observed with IL-2 or IL-7 under the same circumstances. These data collectively demonstrate that the expression of IL-15Rα regulates the extent of IL-15 retention in mice.
Fig. 3.
Increased IL-15 retention in mice challenged by poly I:C. Mice were injected with 5 μg of rhIL-15 (i.p.) after treatment with or without poly I:C (100 μg, i.p. injected 24 h before the IL-15 injection). The IL-15 concentrations in serum samples were determined by a specific ELISA (n = 5).
Tissue-Specific Retention of IL-15 in Steady-State WT Mice.
The IL-15 retention in the circulation seems to be a consequence of the intercellular reservoir effect caused by IL-15Rα-positive cells, which prompted us to examine whether IL-15 is found associated with some tissues in mice. Because of the lack of a reliable antibody to detect mouse IL-15, we again injected rhIL-15 into mice and homogenized their tissues to detect associated human IL-15. Among the tissues examined [the liver, spleen, kidney, thymus, lymph nodes (LNs), and lungs], only the lungs and LNs gave a significant signal when assessed with a specific ELISA at 9 days after the initial injection of rhIL-15 (Fig. 4). In the spleen, IL-15 was barely detectable as early as at day 3. It is noteworthy that IL-15 was no longer detectable in the circulation of these mice, suggesting that the rhIL-15 in tissues did not reflect that left in the circulation, but rather IL-15 was retained by a specific mechanism.
Fig. 4.
Tissue distribution of injected IL-15. Bars are as follows: A, serum; B, liver; C, kidney; D, spleen; E, lung; F, LN; G, thymus. WT mice were injected with 5 μg of rhIL-15 (i.p.). Nine days after the administration, various tissues were collected from these mice and homogenized (except blood samples), and the IL-15 associated with each tissue was determined by a specific ELISA. Serum, liver, and spleen showed no meaningful levels of IL-15 (<10 pg/ml) whereas values from lungs and LNs appeared significantly positive (n = 4). ∗, P < 0.0001; †, P = 0.0001 compared with serum level.
Demonstration of IL-15Rα-Expressing Cells in Select Tissues in Mice.
Based on the previous results, we postulated a constitutive existence of IL-15Rα-positive cells in some tissues such as the lungs and LNs. Unfortunately, we found the commercial anti-IL-15Rα antibodies not reliable for tissue staining, because they nonspecifically stained cells from IL-15Rα−/− mice. Therefore, we chose to first incubate the tissue cells with rhIL-15, followed by a staining using anti-IL-15 Abs. In fact, we could detect membIL-15+ cells in the lungs (Fig. 5A). To confirm the specificity of such a staining scheme, similar experiments were performed by using IL-15Rα−/− mice with which we detected no meaningful staining for membIL-15 (Fig. 5A, R3) despite the presence of a similar cell population in the CD45/class II dot plot (data not shown). In light of a previous report by Schluns et al. (16), we distinguished hematopoietic and nonhematopoietic cells by virtue of CD45 expression, and IL-15Rα expression was associated with CD45-negative cells. After a systemic challenge of mice with poly I:C, we observed an augmentation of IL-15 retention in the LNs and spleen, but not in the lungs (Fig. 5B), consistent with the prediction that IL-15Rα+ cells in the lymphoid organs may be predominantly myeloid/monocytic. By contrast, the failure of poly I:C to induce IL-15 retention in lung cells seems to support the flow-cytometric assessment that the majority of pulmonary IL-15Rα+ cells are of nonhematopoietic lineage. As a negative control, tissues from IL-15Rα−/− mice injected with IL-15 were examined, and they showed no IL-15 retention even at 24 h (data not shown) after IL-15 administration.
Fig. 5.
Detection of nonhematopoietic IL-15Rα positive cells in lung tissue. (A) IL-15Rα expression in lung cell subsets demonstrated by membIL-15 staining. For the detection of IL-15Rα, cells from the homogenized lung tissue were incubated with 1 nM rhIL-15 for 30 min on ice, followed by staining with anti-IL-15 (10) (R & D Systems), antiCD45, and anti-I-Ab antibodies for 30 min on ice. Four major populations were defined and analyzed by separate gates as shown (Left). From each gate, the morphological information (forward light scatter vs. side light scatter) and IL-15 staining are shown (Right). In addition, mean fluorescence intensity of the surface IL-15 staining is shown for each cell population. Cells from R3 (CD45−) showed significant surface IL-15 staining. (B) Increase of IL-15 retention after poly I:C injection into WT mice. Bars are as follows: A, serum; B, liver; C, kidney; D, spleen; E, lung; F, LN; G, thymus. After systemic poly I:C and 5 μg of rhIL-15 injections, tissue samples were collected and homogenized from mice. The amounts of tissue-associated IL-15 were determined by a specific ELISA, and the fold increase after the poly I:C challenge is shown.
Preferential Homing of Ag-Specific CD8 T Cells in the Lungs During the Contraction Phase.
Because the lungs may have a potential to provide an efficient IL-15-retaining environment, we determined whether Ag-specific CD8 T cells demonstrate preferential homing to the lungs, in particular during their contraction phase, when they critically depend on IL-15. WT and IL-15Rα−/− mice were immunized by injecting DCs loaded with ovalbumin (OVA) peptide (257–264) twice with a 3-week interval. At day 45 after the initial immunization, Ag-specific CD8 T cells were identified by the OVA-tetramer staining. Percentages of tetramer-reactive cells in the whole CD8 subset demonstrated more preferential distribution of OVA-specific CD8 T cells in lungs in WT mice [Table 1 and supporting information (SI) Fig. 7]. The OVA-specific CD8 T cells showed poor survival in IL-15Rα−/− mice in general. Interestingly, the lungs of IL-15Rα−/− mice showed extremely poor homing of the tetramer-reactive cells, suggesting that the loss of the IL-15 intercellular reservoir effect was most demonstrable in the lungs among all tissues examined in this regard.
Table 1.
Poor homing of Ag-specific CD8 T cells in the lungs in the absence of membIL-15
| Recipient | % OVA-tetramer+ cells per CD8 subset |
|||
|---|---|---|---|---|
| PBMC | Spleen | LN | Lungs | |
| WT | 2.3 | 2.3 | 0.13 | 5.1 |
| IL-15Rα−/− | 0.3 | 0.8 | 0.06 | Below detection |
Bone marrow-derived DCs of WT and IL-15Rα−/− mice were stimulated with LPS and loaded with OVA peptide (257–264; SIINFEKL), then were injected into WT and IL-15Rα−/− mice, respectively. Three weeks later the mice underwent a similar boosting. OVA-tetramer staining was performed on day 45 after the initial immunization, and the percentages of Ag-specific cells in the total CD8 subset are shown. PBMC, peripheral blood mononuclear cells.
Prolonged Cellular Signaling by Membrane-Bound, but Not by Soluble IL-15 in Target T Cells.
We finally asked whether the membIL-15, with its persistent nature, indeed enables sustained signaling in the target cells. To this end, we monitored the time-dependent phosphorylation of the ribosomal S6 protein in primed T cell receptor (TCR) Tg (F5; see Materials and Methods) CD8 T cells in response to human IL-15 because the S6 phosphorylation reflects the status of ongoing mitogenic signal in various cells (31). Fig. 6(and more in detail in SI Fig. 8) depicts S6 phosphorylation monitored by flow cytometry using an anti-pS6 antibody. Soluble IL-15 (1 nM) showed a transient increase and subsequent decay by 24 h. In contrast, when DCs expressing IL-15 Rα were present, much lower doses of IL-15 (as low as 0.1 nM) were sufficient to sustain the S6 phosphorylation until 120 h after stimulation. To confirm that the sustained S6 phosphorylation was mediated by membIL-15, we used IL-15Rα−/− DCs, which are incapable of transpresenting IL-15. Even high doses of IL-15 (as high as 5 nM) failed to sustain S6 phosphorylation in CD8 T cells under these conditions, suggesting that the transpresented IL-15 enabled a more sustained signal in the responder CD8 T cells than did soluble IL-15.
Fig. 6.
Prolonged IL-15 signaling in CD8 T cells by the membIL-15. Spleen cells from F5 TCR Tg mice were stimulated with the nominal peptide (NP68) in the presence of H-2b Ag-presenting cells for 2 days, followed by an 18-h cytokine-free culture to arrest them in a quiescent status. MACS-purified CD8 T cells were incubated with soluble rhIL-15 (A), rhIL-15 in the presence of IL-15Rα Tg DCs (B), or rhIL-15 with IL-15Rα−/− DCs (C). The cells were then permeabilized/fixed and analyzed for S6 phosphorylation by two-color flow cytometry. (A) Time kinetics of S6 phosphorylation to various doses of transpresented IL-15 (by membIL-15) in F5 CD8 T cells. (B) S6 phosphorylation to various doses of soluble IL-15 (soluble IL-15 with IL-15Rα−/− DCs). (C) S6 phosphorylation to various doses of soluble IL-15 (IL-15 without any DCs). (D) Kinetic comparison of the S6 phosphorylation pattern in CD8 T cells caused by soluble IL-15 and membIL-15.
Discussion
In this report we described novel characteristics of IL-15 that are not shared with other cytokines, which may make this factor a unique contributor to the long-term survival of memory CD8 T cells. Despite numerous reports suggesting the critical involvement of IL-15 in the longevity of memory CD8 T cells (32), previous reports failed to explain why IL-15, among all cytokines, is unique in this context. By exploring the potential of our previous proposal (10), we hereby demonstrated a mechanism that may help us understand the phenomenon. The constitutive existence of membIL-15+ (or IL-15Rα+) cells enables IL-15 to be stably retained in select tissues (lungs and LNs), which would help the survival of memory CD8 T cells beyond the contraction phase. Notably, these tissues are portals for foreign Ag entry and hence the residential sites for long-lived memory CD8 T cells (32). Although the nature of these membIL-15+ cells needs further clarification, they do not seem to be of hematopoietic origin. This finding agrees with a previous proposal made by Schluns et al. (16), whereby they showed that IL-15 transpresentation in the lungs needs to be mediated by both hematopoietic and parenchymal cells.
Immunological challenges transiently cause IL-15Rα expression on accessory cells. Treatment of mice with Toll-like receptor ligands (LPS or poly I:C) increased the number of IL-15Rα+ cells in LNs, spleen, and peripheral blood mononuclear cells, which are positive for CD45 and CD11c. These effects may contribute to supporting the clonal expansion of Ag-specific CD8 T cells. The constitutive existence of nonhematopoietic IL-15Rα+ cells in tissues like lungs may provide the minimum but prolonged IL-15 signal required for the turnover and long survival of CD8 memory T cells outside of the primary or secondary lymphoid organs. In accordance with this hypothesis, the homing of tetramer-reactive CD8 T cells during their contraction phase was extremely poor in the lungs of IL-15Rα−/− mice. Reciprocally, long-living tetramer-reactive CD8 T cells show preferential accumulation in the lungs of WT mice. The poor but meaningful homing of Ag-specific CD8 T cells in spleen, LNs, and peripheral blood of IL-15Rα−/− mice suggests that various factors other than IL-15 may also contribute to the survival of memory CD8 T cells in these tissues. Interestingly, these other factors appear to play relatively limited roles in the lungs, emphasizing the relevance of the unexpected connection between the pulmonary CD8 memory homing and IL-15 retention.
The degradation-resistant nature of IL-15/IL-15Rα complexes during endosomal recycling (10) gave rise to another aspect of IL-15 characteristics that makes it a persistent in vivo factor through the intercellular reservoir effect. Small molecules like cytokines are rapidly lost from the body by the efficient renal filtration mechanism. IL-15 appears to be an exception. The clearance of IL-15 has two phases. In the first rapid clearance phase, IL-15 behaves like other cytokines (28), but detectable levels of IL-15 remain in circulation or in association with select tissues for more than a week after a bolus injection. This retention of IL-15 is mediated by IL-15Rα, because IL-15 disappears from IL-15Rα−/− mice without retention. Thus, we speculate that endogenous IL-15 can be captured and retained by IL-15Rα+ cells long after the cessation of its transient production. The rather exaggerated expansion of CD44hiCD8 T cells in IL-15-injected IL-15Rα Tg mice would be a proof of such an endogenous mechanism. Oh et al. (19) demonstrated a similar prolonged IL-15 action in vivo. They transiently produced IL-15 in mice by transducing a vaccinia–IL-15 construct. Nevertheless, the IL-15 effect was “imprinted” for several months after the cessation of IL-15 production. IL-2–vaccinia, on the other hand, failed to have such a sustained effect (19). Our observation may shed light on the mechanism underlying this intriguing observation.
It is plausible that in humans or mice kept in a nonclean facility, the survival of memory CD8 T populations might depend on minor bystander microbial infections. Periodical exposure to such pathogens would enable accessory cells to produce IL-15, which then would be captured by nonhematopoietic IL-15Rα+ cells in immunologic portals to constitute an intercellular reservoir maintained for an extended time thereafter. Thus, the turnover (intermittent division) of memory CD8 T cells may be maintained by preserving minute quantities of IL-15 produced upon periodic minor infections until the original pathogen invades the body to recall the memorized immune response.
Most extracellular ligands undergo receptor-mediated internalization (33–35). Ligands are then degraded whereas the receptor components are recycled back to the cell surface for another round of signal transduction. Failure in this process could lead to pathogenic conditions in humans. A mutation in the low-density lipoprotein receptor makes cells incapable of degrading the ligand through endosomal internalization, leading to the accumulation of low-density lipoprotein in the circulation, which eventually causes hypercholestremia (36), which appears similar to the remarkable IL-15 retention observed in IL-15Rα Tg mice. It would be interesting to design an IL-15 mutant that would retain full binding capability to IL-15Rα under neutral pH conditions but would sharply lose binding affinity as the pH shifts to acidic, because such an IL-15 would help distinguish long-term vs. short-term IL-15 effects.
Does soluble IL-15 act in vivo? Although naïve and activated T cells and natural killer cells do not express IL-15Rα, differentiated memory CD8 T cells may express IL-15Rα (19, 37) and respond to soluble IL-15. Thus, it becomes important to determine whether soluble IL-15 and membIL-15 could transduce qualitatively different signals. Our data suggest that the signal mediated by membIL-15 persists longer than that generated by soluble IL-15 at doses generating similar impacts on β/γc-expressing CD8 T cells standardized by short-term DNA synthesis. Interestingly, Cornish et al. (31) recently demonstrated that IL-2 and soluble IL-15 transduce qualitatively different signals, in that IL-2 signals are more persistent and result in proliferation and protein synthesis, thus making IL-2 act as a growth factor, whereas signals by soluble IL-15 decay quicker and primarily induce cellular proliferation, thus IL-15 acting as a pure mitogen. Our data suggest that membIL-15 may be a more comprehensive growth factor than soluble IL-15. It is possible that IL-2, soluble IL-15, and membIL-15 all have unique roles in vivo. We want to point out that IL-15 can still be presented to IL-15Rα+ target cells (10). How do soluble and membrane IL-15 transduce different signals? This distinction may arise from the spatial distribution and temporal nature of receptor engagements across the cellular surface. Alternatively, longer and intimate occupation of the receptor by membIL-15 enabled by persistent cell-to-cell contact could be responsible.
Two recent publications (14, 18) have proposed that IL-15 and IL-15Rα need to be expressed by the same cell for the transpresentation to occur, which may seem to contradict our proposal in this article. However, the assessment by those articles was limited to homeostatic maintenance of natural killer cells and CD44hiCD8 T cells, in which IL-15 levels remain relatively low. After a dynamic production of IL-15 triggered by microbial intrusion, more IL-15 may be produced than just captured by the producer cells. It is also possible that these nonhematopoietic cells found in the lungs and other tissues may produce their own IL-15.
In this article we demonstrate unique features of IL-15 that are found with no other typical cytokine. These features enable a persistent in vivo activity of this cytokine as well as a prolonged signaling in CD8 T cells. These features may contribute to the long-term maintenance of memory CD8 T cells and control the homing of these cells to certain immunological portals.
Materials and Methods
Antibodies, Cytokines, and Related Reagents.
Human and mouse recombinant cytokines were purchased from PeproTech (Rocky Hill, NJ). Cytokine ELISAs were from R & D Systems (Minneapolis, MN). Antibodies for flow cytometry were purchased from eBioscience (San Diego, CA). Anti-mouse IL-15Rα polyclonal antibody (AF551) and anti-human IL-15 antibody (mAb 247) were from R & D Systems. AF551 was conjugated with fluorescein isothiocyanate (Sigma, St. Louis, MO) in house. Alexa Fluor 488 anti-phosphor S6 antibody was from Cell Signaling Technology (Danvers, MA). OVA (257–264; SIINFEKL)-tetramer was from Beckman-Coulter (Fullerton, CA). Poly I:C and LPS were from Sigma.
Cells.
Cells were maintained in 10% FCS/RPMI medium 1640 (Invitrogen, Carlsbad, CA) supplemented with 2 mM glutamine, 5 × 10−5 M 2-mercaptoethanol, and appropriate antibiotics. Ex vivo DCs were derived by culturing bone marrow precursor cells with 20 ng/ml mouse granulocyte–macrophage colony-stimulating factor for 8–10 days. For the proliferation assay, cells were seeded in the culture medium at 25,000 cells per well, cultured with the cytokine for 24 h (CTLL-2) or 72 h (ex vivo CD8 T cells), and pulsed with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine during the last 4 h.
Mice.
Human IL-15Rα Tg mice were generated in our facility by microinjecting a linearized plasmid fragment consisting of cDNAs encoding human IL-15Rα, a bovine preprolactin signal peptide (10), SV40 polyA signal, and a human EF-1α promoter into fertilized eggs of C57BL/6 mice.
IL-15Rα−/− mice (The Jackson Laboratory, Bar Harbor, ME) were backcrossed to the C57B6 strain for 10 additional generations in our facility. F5 TCR Tg mice (expressing TCR specific for influenza NP68 peptide) were from the Taconic Farm (Hudson, NY). All of the animal experiment protocols were approved by the National Institutes of Health animal care and use committee.
Determination of the Amount of IL-15 After Recycling.
293T cells were transfected with a human IL-15Rα plasmid (10). rhIL-15 (PeproTech) was added to 1 nM to their culture after 24 h for 6 h to allow cell-surface IL-15/15Rα complexes to form and internalize. Cell-surface IL-15 molecules were stripped by exposure to PBS/acetic acid (pH 3.5), leaving only internalized IL-15/IL-15Rα complexes retained (10). The cells were then transferred to a cytokine-free culture. Aliquots of supernatant were removed at the indicated time points for IL-15 quantification by a specific ELISA. The cells were then homogenized by passing through a 27-gauge needle and subjected to a sucrose-gradient (10–60%) ultracentrifugation fractionation (10). One-tenth of the cells were incubated with NHS-biotin (Pierce, Rockford, IL) before homogenization, and the biotinylated homogenates were added back to the remainder of the 293 homogenates and fractionated for tracking purposes. The gradient was recovered by 1-ml fractions from the top. Endosomal fractions were identified by the presence of early endosomal protein 1 (10). Additionally, the biological activity of the IL-15 rereleased into the culture supernatants was confirmed by using a CTLL-2 biological assay.
Determination of IL-15 Levels Associated with Tissues.
Serum and tissue samples were collected from WT mice 9 days after injecting 5 μg of rhIL-15. Tissues were homogenized to quantitate associated IL-15 by a specific ELISA. In the experiment assessing the effect of poly I:C (100 μg), rhIL-15 (5 μg) was injected 24 h after a systemic poly I:C challenge, and the IL-15 levels were measured 24 h after the IL-15 injection.
Detection of membIL-15-Positive Cells in Various Tissues.
To eliminate blood cell contamination in the tissues, mice were exsanguinated and tissue samples were homogenized by passing through a 70-μm mesh (BD Biosciences). They were incubated with 1 nM rhIL-15 (PeproTech) for 30 min on ice, followed by staining with an anti-human IL-15 mAb (R & D Systems), MHC class II, and CD45 antibodies (eBioscience).
Tracking of Ag-Specific CD8 T Cells.
Bone marrow cells of WT and IL-15Rα−/− mice were cultured ex vivo for 5–6 days, then stimulated with 5 ng/ml LPS and 1 μg/ml OVA peptide (257–264; SIINFEKL; AnaSpec, San Jose, CA) overnight. WT DCs were injected (0.33 million s.c. plus 0.66 million i.p.) into WT mice, and IL-15Rα−/− DCs were injected into IL-15Rα−/− mice. Three weeks later, the mice underwent a boosting with the same protocol. OVA-specific CD8 T cells in the peripheral blood, spleen, LNs, and lungs were analyzed 45 days after the initial immunization by using flow cytometry, after staining with OVA-tetramer, and fluorochrome-conjugated anti-CD3 and anti-CD8 antibodies (eBioscience).
Analysis of the Phosphorylation of Ribosomal S6 Protein by Flow Cytometry.
Spleen cells from F5 TCR Tg mice were primed with 5 ng/ml of the nominal NP68 peptide (ASNENMDAM; NeoMPS, San Diego, CA) for 2 days. CD8 T cells were then purified by MACS (Miltenyi Biotec, Auburn, CA) and rested for 18 h in cytokine-free culture. One million CD8 T cells were cultured with rhIL-15 and/or DCs (1 million cells) grown from IL-15Rα−/− or IL-15Rα Tg mice. Those cells were stained with an anti-CD8 antibody, then treated with Fix/Perm buffer (eBioscience) according to the manufacturer's instructions, followed by staining with Alexa Fluor 488 anti-phosphor S6 antibody (Cell Signaling Technology) in permeabilization buffer (eBioscience) and analyzed by a flow cytometer (FACSCalibur; Becton Dickinson).
Supplementary Material
Acknowledgments
We thank Dr. Lionel Feigenbaum, head of the Transgenic Mouse Model Laboratory of National Cancer Institute-Frederick (Frederick, MD) for the generation of IL-15Rα Tg mice. We also greatly appreciate the contribution of Dr. Sigrid Dubois, who initially conceptualized the ideas from which this work developed. This work was supported by intramural research funding from the National Cancer Institute, National Institutes of Health.
Abbreviations
- rh
recombinant human
- membIL-15
cell-surface membrane-associated IL-15/IL-15Rα complex
- poly I:C
poly inosinic poly cytidylic acid
- DC
dendritic cell
- Tg
transgenic
- LN
lymph node
- Ag
antigen
- OVA
ovalbumin
- TCR
T cell receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610115104/DC1.
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldmann TA, Tagaya Y. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 5.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 6.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehniger TA, Caligiuri MA. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 9.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Cytokine Growth Factor Rev. 2002;13:429–439. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 10.Dubois S, Mariner J, Waldmann TA, Tagaya Y. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 11.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. Proc Natl Acad Sci USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 16.Schluns KS, Klonowski KD, Lefrancois L. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 17.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Proc Natl Acad Sci USA. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 19.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann TA, Taniguchi T, Taki S. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 22.Edelbaum D, Mohamadzadeh M, Bergstresser PR, Sugamura K, Takashima A. J Invest Dermatol. 1995;105:837–843. doi: 10.1111/1523-1747.ep12326630. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki-Ohara K, Nishimura H, Mitani A, Yoshikai Y. Eur J Immunol. 1997;27:2885–2891. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 24.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. J Immunol. 1998;160:4322–4329. [PubMed] [Google Scholar]
- 25.Williams MA, Tyznik AJ, Bevan MJ. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler T, Hammerling GJ, Arnold B. J Immunol. 2004;172:15–19. doi: 10.4049/jimmunol.172.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H, Carrasquillo JA, Paik CH, Waldmann TA, Tagaya Y. Cancer Res. 2000;60:3577–3583. [PubMed] [Google Scholar]
- 29.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois SP, Waldmann TA, Muller JR. Proc Natl Acad Sci USA. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornish GH, Sinclair LV, Cantrell DA. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Ahmed R. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein JL, Anderson RG, Brown MS. Ciba Found Symp. 1982:77–95. doi: 10.1002/9780470720745.ch5. [DOI] [PubMed] [Google Scholar]
- 34.Oka Y, Rozek LM, Czech MP. J Biol Chem. 1985;260:9435–9442. [PubMed] [Google Scholar]
- 35.Jackle S, Runquist EA, Miranda-Brady S, Havel RJ. J Biol Chem. 1991;266:1396–1402. [PubMed] [Google Scholar]
- 36.Gonzalez-Gaitan M, Stenmark H. Cell. 2003;115:513–521. doi: 10.1016/s0092-8674(03)00932-2. [DOI] [PubMed] [Google Scholar]
- 37.Schluns KS, Stoklasek T, Lefrancois L. Int J Biochem Cell Biol. 2005;37:1567–1571. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.