Abstract
Regulatory T cells play an essential role in preventing fetal rejection by the maternal immune system. Here we show that, based on the expression of CCR5, regulatory T cells can be divided into a highly suppressive CCR5+ and a far less suppressive CCR5− subpopulation, suggesting that the former represent the effector arm of regulatory T cells. Although regulatory T cells from CCR5−/− gene deletion mutants still suppress, they are less effective mediators of maternal–fetal tolerance. The accumulation of CCR5+ regulatory T cells at this site appears to be enhanced by alloantigen. This finding is in stark contrast to the systemic expansion of regulatory T cells during pregnancy, which appears to be alloantigen-independent. The fact that CCR5+ regulatory T cells preferentially accumulate in the gravid uterus and that expression of CCR5 on regulatory T cells can be induced by activation lead us to propose that CCR5 is responsible for the accumulation of those regulatory T cells that have been activated by paternal antigens.
Keywords: effector T cells, pregnancy, tolerance, chemokine receptor
Regulatory T cells (TR cells) play an important role in the maintenance of peripheral tolerance and the prevention of autoimmunity (1). Where TR cells exert their suppressive function and what attracts them to and retains them at their site of action is poorly understood. Inducible TR cells (Tr1-like cells) have a preference for skin homing (2), whereas naturally occurring Foxp3+ TR cells can be found in all lymphoid organs (3, 4). The CD103+ subpopulation of TR cells has been shown to home to the site of inflammation (5); however, the mechanism by which this occurs remains elusive.
Previously, we have demonstrated that naturally occurring TR cells mediate maternal tolerance to the fetus and can be found in the uterus during pregnancy (6). Although the uterine accumulation of macrophages (7), natural killer cells (8, 9), and eosinophils (10) has been extensively studied, there has been no insight on how TR cells find their way to the gravid uterus.
Upon activation, professional antigen-presenting cells express the chemokine CCL4, which leads to the recruitment and/or retention of TR cells (11). Although it remains unclear which chemokine receptor is responsible for the CCL4-mediated effects on TR cells, biochemical studies have shown CCL4 to bind to the chemokine receptor CCR5 (12). Expression of CCR5 on T cells has been associated with both proinflammatory and antiinflammatory T cell function in mouse and human. CCR5 is thought to be expressed on antigen-experienced, effector T cells that home toward sites of inflammation outside the secondary lymphoid organs (13–15). CCR5+ T cells have been shown to infiltrate inflamed sites such as the synovium of rheumatoid arthritis patients (16, 17) and the central nervous system of mice with experimental autoimmune encephalomyelitis (18). Expression of CCR5 in pancreatic islets correlates with increased severity of diabetes in mice (19), and CCR5 is thought to mediate T cell migration to the islets (20). Deficiency in CCR5 leads to a reduced T cell infiltration to sites of Trypanosoma cruzei (21), Toxoplasma gondii (22), and viral infections (23). CCR5 also mediates infiltration of allografts (24–27) by proinflammatory, IFNγ-producing T helper (TH) 1-biased cells (17, 26) and macrophage infiltration at sites of inflammation (28, 29).
However, under certain conditions, mice receiving allografts of CCR5-deficient cells displayed accelerated and more severe graft-versus-host disease (30, 31). This suggests an antiinflammatory role of CCR5+ cells. Support for this notion comes from the fact that mice lacking CCR5 show increased delayed-type hypersensitivity responses (32). Indeed, CCR5 have been shown to be up-regulated upon activation on TR cells (33). Thus, it appears that CCR5 is present on both antiinflammatory and proinflammatory T cells.
Here we show that expression of CCR5 defines the effector arm of TR cells carrying most of the suppressive activity. Lack of CCR5 on TR cells leads to an impairment of maternal–fetal tolerance despite their finding their way to the uterus. We present data suggesting that this is due to a lack of CCL4-mediated accumulation of those TR cells that have been activated by paternal alloantigen.
Results
CCR5+ Effector TR Cells.
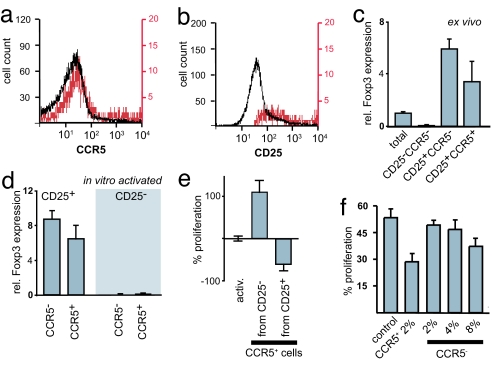
CCR5 appears to be the only cognate receptor for CCL4 (12). Given the previously shown role of CCL4 in the trafficking of TR cells (11), we examined their expression of CCR5. We purified the cells from nonimmunized mice kept under specific pathogen-free conditions; thus, the CD25+ subpopulation of CD4+ cells consists almost entirely of TR cells (34). Our analysis revealed that CCR5 is expressed on ≈20% of all CD4+CD25+ cells in the spleen (Fig. 1a). Because virtually all CD4+CCR5+ cells were CD25+, the CCR5+ cells represent a subpopulation fully contained within the pool of CD25+ cells (Fig. 1b). Similar results were obtained for cells from blood and lymph nodes (data not shown). Both the CCR5− and CCR5+ subpopulations of TR cells express Foxp3, although the latter do so at a slightly lower level (Fig. 1c).
Fig. 1.
CCR5+ effector TR cells. (a) CD4+ cells were gated for the expression of CD25. CCR5 staining on total CD4+ cells (black) and on CD4+CD25+ cells (red) is shown. (b) CD4+ cells were gated for the expression of CCR5. CD25 staining on total CD4+ cells (black) and CD4+CCR5+ cells (red) is shown. (c) Relative expression of Foxp3 in CD4+ cells comparing the total population with the CD25−, CD25+CCR5−, and CD25+CCR5+ subpopulations. (d) Relative expression of Foxp3 normalized to HPRT in CD4+CCR5− and CD4+CCR5+ cells originating from CD25+ or CD25− cells by activation. (e) Effect of CCR5+ cells originating from CD25+ or CD25− on the proliferation of CD4+CD25− target cells. (f) CFSE-labeled CD4+CD25− target cells were cocultured with CD4+CD25+ CCR5+ (CCR5+) or with increasing numbers of CCR5-depleted CD4+CD25− cells (CCR5−) and activated by anti-CD3 cross-linking. The proliferation of target cells (increase in the number of CFSE-labeled cells) was analyzed by FACS on day 3.
Expression of CCR5 has been mostly associated with proinflammatory effector memory T cells (13). Upon activation, naïve CD4+ T cells start to express CD25, a subpopulation of which also expresses CCR5. The conversion from CCR5− to CCR5+ TH cell is enhanced in the presence of proinflammatory cytokines, in particular IL-12 (35). Similarly, expression of CCR5 can be induced in CCR5−CD4+CD25+ TR cells by activation, in particular in the presence of IL-2 (33). However, unlike the CCR5+ cells present in the pool of CD4+CD25+ TR cells, the CCR5+ cells purified from activated TH cells [CD4+CD25− activated for 48 h with plate-bound anti-CD3 (2 μg/ml) in the presence of IL-12 (10 ng/ml) and then sorted into CCR5-enriched and CCR5-depleted populations] do not express Foxp3 (Fig. 1d).
Given the distinct origin of these two CCR5+ cell populations, one would expect them to have a diametrically opposed effect on the proliferation of naïve T cells. To address this we performed coculturing experiments (Fig. 1e). CD4+CD25− cells were activated by cross-linking with anti-CD3. Addition of CCR5+ cells (at a 1:10 ratio) purified from activated CD4+CD25− cells resulted in a 110% increase in proliferation of the target cells, confirming their proinflammatory effector cell status. In contrast, addition of the same number of CCR5+ cells purified from CD4+CD25+ TR cells resulted in a 62% inhibition of proliferation. These results confirmed that the two CCR5+ cell populations are functionally opposed.
Because CCR5+ proinflammatory T cells are effector cells we wondered whether the same applies to CCR5+ TR cells. Indeed, direct comparison of the CCR5+ and CCR5− subpopulations of TR cells revealed that the vast majority of the regulatory activity resides in the CCR5+ cells. In coculturing experiments using CD4+CD25− target cells, CCR5+ cells led to marked reduction (P = 0.02; t test) of the target cell proliferation at a 1:50 ratio (Fig. 1f), even in the absence of antigen-presenting cells. The same number of CCR5− cells had no inhibitory effect. Only when we substantially increased the number of CCR5-depleted TR cells, we observed a slight increase in inhibition; however, this was not statistically significant. Notably, most “coculturing suppression experiments” described in the literature include irradiated antigen-presenting cells (36). These cells are likely to provide the costimulatory signals that facilitate the conversion of TR cells into effector cells. The presence of CCR5+ TR cells in naïve animals may be an indication of a baseline activation in response to self-antigens.
Accumulation of CCR5+ TR Cells in the Gravid Uterus.
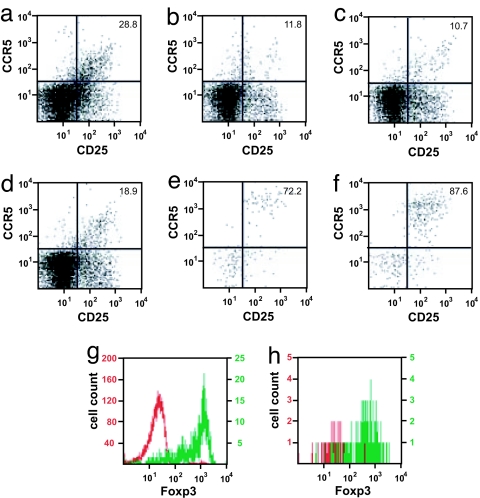
Proinflammatory effector T cells home to sites of infection outside the secondary lymph organs (21). In analogy one might expect a similar directed accumulation of CCR5+ effector TR cells to the sites where their action is required. In the case of pregnancy, the site of implantation poses clearly a main target of effector TH cells (35). Similarly, we have shown that significant number of TR cells can be detected in the gravid uterus at the maternal–fetal interface (6). We therefore reexamined the accumulation of TR cells that migrate to the gravid uterus with respect to their expression of CCR5 (Fig. 2). We found that >70% of CD4+CD25+ cells in the gravid uterus and placenta expressed CCR5 (Fig. 2 e and f). In contrast, the frequency of CCR5+ cells in the spleen, blood, and other secondary lymphoid organs was significantly lower (P < 0.005; t tests for individual tissues) and in all cases <30% of all TR cells within the tissue (Fig. 2 a–d). Virtually all of these CD4+CD25+ cells were Foxp3+ (Fig. 2 g and h). Interestingly, the iliac and lumbar lymph nodes (Fig. 2c), which drain the uterus, do not show the same elevation of CCR5 expression as the uterus itself.
Fig. 2.
Distribution of CCR5+ TR cells in pregnant mice. Lymphocytes were isolated from allogeneically mated C57BL/6 mice at E10.5 (mid-gestation) and analyzed by FACS for the surface expression of CD4, CD25, and CCR5. Plots show CD25 and CCR5 surface expression for CD4+ gated cells from spleen (a), blood (b), iliac and lumbar lymph nodes (c), inguinal lymph nodes (d), uterus (e), and placenta (f). A representative sample is shown (n = 3). The number in the top right corner of each plot denotes the percentage of CCR5+ cells among CD4+CD25+ cells. (g and h) Intracellular Foxp3 stain of CD4+CD25+ (green) or CD4+CD25− (red) cells from spleen (g) and uterus (h) at E10.5.
Chemokine Expression in the Gravid Uterus.
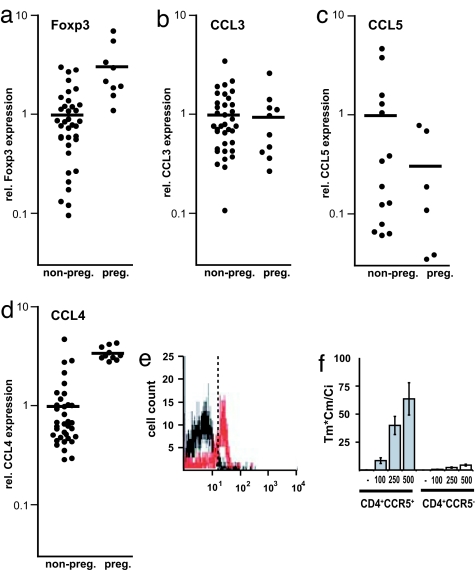
CCR5 is a promiscuous chemokine receptor binding several chemokines. Its ligands CCL3 (37–39), CCL4 (8), and CCL5 (40) have been reported to be expressed by a variety of cells in the human endometrium. To assess their role in the accumulation of TR cells to the gravid uterus, we compared the levels of Foxp3 mRNA with those of CCL3, CCL4, and CCL5 in whole uterine tissue in the pregnant and nonpregnant state by quantitative RT-PCR (Fig. 3a–d). The uterine levels of Foxp3 mRNA (Fig. 3a) were significantly elevated in pregnant mice (P < 0.0001; t test). Similarly, CCL4 mRNA levels (Fig. 3d) were significantly elevated in the gravid uteri (P < 0.0001; t test), whereas CCL3 and CCL5 mRNA levels (Fig. 3 b and c) did not show any statistically significant change (P = 0.85 and P = 0.28 respectively; t test). These results show a strong correlation between uterine CCL4 expression and the number of TR cells present in the tissue.
Fig. 3.
Elevated CCL4 expression in the gravid uterus and CCL4-mediated recruitment of CCR5+ TR cells. (a–d) Relative levels of Foxp3 (a), CCL3 (b), CCL5 (c), and CCL4 (d) in total uterine mRNA normalized to HPRT. Uteri were isolated from nonpregnant (non-preg.) mice or pregnant (preg.) mice at E10.5. Each point corresponds to one animal. Horizontal bars represent the mean of each data set. (e) Transwell migration assay of lymphocytes to CCL4. Input and migrated cells were analyzed by FACS for the expression of CD4 and CCR5. Histograms show CD4-gated cells. CCR5+ cells in the input cell population (black) versus the CCL4-recruited population (red). (f) Histogram comparing the migration indexes of CD4+CCR5+ and CD4+CCR5− cells recruited by CCL4 (representative experiment, n = 3), for a range of CCL4 concentrations (0, 100, 250, and 500 ng/ml). The migration index was calculated based on the number of migrated cells (Tm), migrated cells expressing the cognate marker (Cm), and input cells expressing the marker (Ci).
CCL4 Preferentially Attracts CCR5+ TR Cells.
The fact that naïve TR cells can be distinguished from activated, effector TR cells based on the expression of CCR5 suggests a difference in their migratory behavior. To study this difference in an unbiased fashion, we examined the migration of total splenocytes toward a range of CCL4 concentrations (0–500 ng/ml) in transwell migration assays (Fig. 3 e and f). The number of CD4+CCR5+ cells in the input and migrated cell populations was determined by FACS. We found that the vast majority of cells that migrated toward CCL4 expressed CCR5. Only 1.8% of CD4+ cells (Fig. 3e, black) in the input population expressed CCR5. In contrast, 61.8% of CD4+ cells (Fig. 3e, red) that had migrated toward CCL4 were CCR5+. The migration index (Tm × Cm/Ci, where Tm is the number of migrated cells, Cm is the number of migrated cells expressing the cognate marker, and Ci is the number of input cells expressing the marker) is an accurate indicator of both the potency and specificity of a chemokine. CD4+CCR5+ cells exhibited a migration index of 64 for migrations to 500 ng/ml CCL4 (Fig. 3f). These results demonstrate that, at least in vitro, CCL4 preferentially accumulates CCR5+ effector TR cells.
The Role of CCR5 on TR Cells in Pregnancy.
CCR5−/− gene deletion mutant mice as such have an unremarkable phenotype (25) and display a normal pregnancy outcome in allogeneic matings. Both proinflammatory and antiinflammatory effects are reported when using T cells from these mice, depending on the experimental model used (30, 41). These observations may be due to the fact that both proinflammatory effector TH cells and effector TR cells express CCR5. Thus, any impairment of TR cells caused by the lack of CCR5 (30) might be compensated by an opposing effect caused by lack of CCR5 on activated TH cells (41), resulting in an immunological stalemate. Indeed, both CCR5-deficient TR (33) and TH (25, 33) cells are impaired in their migration toward CCL4. This could explain why successful allogeneic pregnancies are possible in these mice, because the lack of CCR5 will affect both aggressors and regulators.
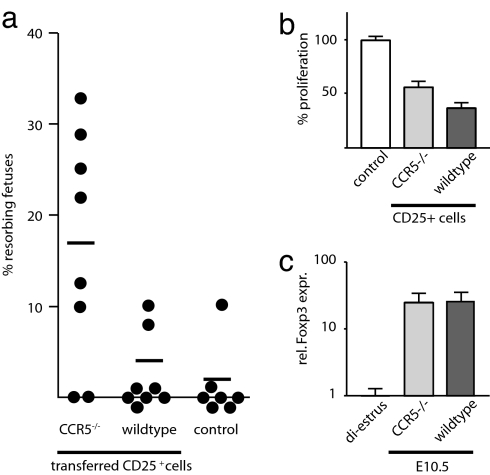
Thus, it is necessary to examine the mutant cells in the context of a wild-type immune system to reveal the effect of CCR5 deficiency on TR cells in pregnancy. We adoptively transferred wild-type CD25-depleted splenocytes reconstituted with CD25+ cells prepared from either CCR5-deficient or wild-type mice at a 15:1 ratio into F5×Rag1−/− female recipients (semilymphopenic, lacking CD4+ T cells). Both donor mice and recipient mice were of the same genetic background (H-2b). The mice were then allogeneically mated (BALB/c males, H-2d). The homeostatic expansion after transfer of 1.5 × 107 cells into lymphopenic animals is minimal (42) and further minimized by using semilymphopenic recipients. Intact and resorbing fetuses were scored blindly at mid-gestation (Fig. 4a). C57BL/6 control females had an average litter size of 8 (n = 8) and 1% resorbing fetuses. F5×Rag1−/− recipients that had received CD25-depleted cells reconstituted with wild-type CD25+ cells had an average litter size of 7 (n = 8) and 2% resorbing fetuses. Neither the average litter size (number of intact fetuses, P = 0.33; t test), nor the number of resorbing fetuses (P = 0.72; nonparametric test) between the control and the experimental group that had received CD25+ cells from wild-type mice shows a statistically significant difference. In contrast, F5×Rag1−/− recipients, which had received CD25-depleted cells reconstituted with CCR5-deficient CD25+ cells, had an average litter size of 6.8 (n = 8) and 17% resorbing fetuses. The percentage of resorbing fetuses was significantly higher than that observed in both the control (P = 0.01; nonparametric test) and the experimental group that received wild-type CD25+ T cells (P = 0.02; nonparametric test). These data demonstrate an impairment of CCR5-deficient TR cells in the sustenance of maternal–fetal tolerance, albeit not a complete loss of function. The inability of CCR5-deficient TR cells to fully protect against fetal loss could be due to impaired function and/or accumulation of these cells. In the case of proinflammatory effector T cells, CCR5 alters the length of their interaction with antigen-presenting cells, leading to enhanced T cell activation (43). To assess whether CCR5 is required for the suppressive activity of TR cells, we performed coculturing experiments with CD25+ cells prepared from either wild-type mice or CCR5−/− gene deletion mutants. We found that CD25+ cells prepared from CCR5-deficient mice clearly suppressed the proliferation of CD4+CD25− target cells in vitro (P = 0.009; nonparametric test). However, they were slightly less (P = 0.04; nonparametric test) suppressive than wild-type TR cells (Fig. 4b).
Fig. 4.
CCR5 deficiency in TR cells increases fetal loss. (a) CD25-depleted cells from C57BL/6 (H-2b) donors in combination with CD25+ cells from either CCR5−/− (H-2b) mice (CCR5−/−) or wild-type animals were injected into F5×Rag1−/− (H-2b) females. Twenty-four hours later, the mice were mated with BALB/c (H-2d) males. Allogeneic matings of wild-type animals are shown as a control. The number of intact and resorbing fetuses in all successfully mated females was scored blindly at E10.5. The percentage of resorbing over total fetuses in each animal is shown. (b) CD4+CD25− target cells were cocultured with CD25+ cells from wild-type or CCR5-deficient mice and activated with anti-CD3 cross-linking. At day 3 the proliferation of target cells was analyzed by [3H]thymidine incorporation (n = 8, representative example). (c) Relative levels of Foxp3 in total uterine RNA from wild-type or CCR5−/− pregnant mice at E10.5 normalized to HPRT. Foxp3 levels are normalized to nonpregnant wild-type mice in diestrus
CCR5-Mediated Accumulation of TR Cells.
To test whether CCR5 deficiency leads to an impairment in the accumulation of TR cells in the gravid uterus, we compared uterine Foxp3 mRNA levels in wild-type and CCR5−/− gene deletion mutant mice at embryonic day 10.5 (E10.5) (Fig. 4c). We detected equivalent levels of Foxp3 in both lines of mice (P = 1; nonparametric test) revealing that CCR5-deficient TR cells still find their way to the gravid uterus. This suggests that the recruitment of TR cells to the gravid uterus itself can be achieved independent of CCR5. However, this does not rule out a role for CCR5 in the preferential accumulation of activated effector TR cells, competing against nonactivated TR cells for the same niche. To test this hypothesis we performed in vivo competition experiments between wild-type TR cells, CCR5-deficient TR cells, and TR cells expressing CCR5 ectopically.
TR cells prepared from either wild-type mice or CCR5 gene deletion mutant mice were transduced with a retroviral vector carrying a constitutively expressed GFP gene. This allowed us to follow these cells after adoptive transfer into wild-type females. The recipients were allogeneically mated. At E10.5 we determined the number of GFP-tagged TR cells in the uterus (Fig. 5a) and spleen (Fig. 5b).
Fig. 5.
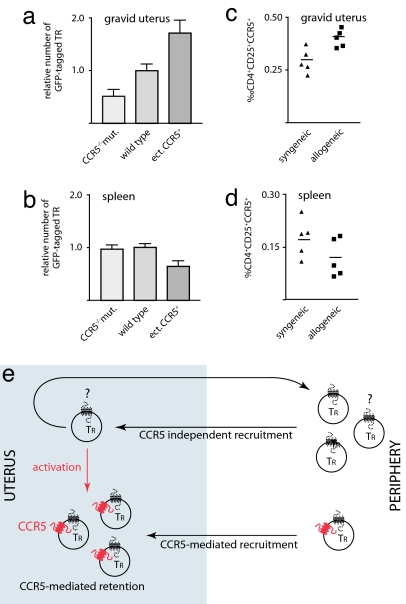
The role of CCR5 in recruitment and retention of TR cells during pregnancy. Wild-type female recipients were injected with CD25+ cells prepared from CCR5-deficient mice (CCR5−/−mut., n = 4) or wild-type mice (n = 11) transduced with a retroviral vector constitutively expressing GFP. Alternatively, wild-type CD25+ cells were transduced with a retroviral vector ectopically expressing CCR5 and GFP (ect.CCR5+, n = 7). Successfully mated females were analyzed by FACS at E10.5 for the number of GFP+ cells among CD4+ cells in the uterus (a) and spleen (b). (c and d) Cells from C57BL/6 females at E10.5 of gestation after syngeneic or allogeneic mating were analyzed by FACS. Graphs show the percentage of CD4+CD25+CCR5+ cells among all cells in the uterus (c) and spleen (d). Each point corresponds to one animal. (e) Model of the recruitment and retention of TR cells in the gravid uterus.
In the uterus we found only half as many CCR5-deficient TR cells as we did find wild-type TR cells (P = 0.048; t test). In contrast, there was no significant difference (P = 0.80; t test) in the number of GFP-tagged CCR5-deficient and wild-type cells in the spleen of the recipients. This indicates that CCR5 is not only a marker for TR cells that accumulate in the uterus, but is functionally involved in the accumulation itself. The TR cells used in the experiment were prepared from the spleens of nonpregnant mice, and only ≈20% of them expressed CCR5. In addition, paternal alloantigen and fetal antigens might lead to activation and thus CCR5 expression on some of the cells. However, it is clear that only a subpopulation of cells will express CCR5. One would predict that constitutive expression of CCR5 in the TR cells transferred would lead to a further increase in their uterine accumulation, because in this scenario all transferred cells have the potential to accumulate. Indeed, we found that TR cells transduced with a retroviral vector carrying a constitutively expressed CCR5 gene accumulated with almost twice the efficiency (P = 0.01; t test) in the uterus (Fig. 5a). Interestingly, we found significantly fewer (0.64×) of these cells in the spleen (P = 0.012; t test) (Fig. 5b). These results show that expression of CCR5 on TR cells has a direct effect on their selective accumulation in the gravid uterus.
Antigen-Induced Accumulation of TR Cells.
Not all TR cells will have antigen specificities suitable to the recognition of paternal/fetal antigens, and only the antigen-activated TR cells, which will express CCR5, will have to be retained. Obviously, in the case of the CCR5-deficient mice this selective retention would be irrelevant because the antipaternal/fetal proinflammatory TH cells would be likewise impaired by the CCR5 deficiency. If maternal–fetal tolerance requires the selective accumulation of alloantigen-specific effector TR cells one would expect fewer CCR5+ T cells in the uterus of syngeneic pregnancies. Because there is no paternal alloantigen, activation of TR cells is restricted to fetal antigens (e.g., male antigens, carcinoembryonic antigens, etc.) (44), resulting in fewer activated CCR5+ cells. As demonstrated above this would lead to a reduction in the number of CCR5+ cells accumulating in the uterus. To test this hypothesis, we compared the relative abundance of CCR5+ TR cells among total uterine cells recruited to the uterus of syngeneic versus allogeneic pregnancies at E10.5 (Fig. 5c). We found that the number of CD4+CD25+CCR5+ cells in the uteri of allogeneic pregnancies was significantly (P = 0.0096; t test) higher (0.041% of all uterine cells) than that in the uteri of syngeneic pregnancies (0.03% of all uterine cells). In contrast, there was no significant difference (P = 0.16; t test) in the spleens (Fig. 5d) of the animals compared. Therefore, the selective accumulation of CCR5+ TR cells in the gravid uterus is affected by antigen.
We conclude not only that TR cells migrate to the uterus, but that the antigen-activated CCR5+ effector TR population accumulates in this tissue. Given the concurrent expression of CCL4, this may be based on a CCL4-mediated mechanism and could be due to either preferential recruitment of CCR5+ cells or preferential retention of CCR5+ effector TR cells that have been recruited by a CCR5-independent mechanism.
Discussion
The effector arm of proinflammatory T cells is characterized by three main criteria (13–15, 26): (i) effector function manifested in the increased production of proinflammatory cytokines such as IFNγ, (ii) migration to inflamed tissue, and (iii) the expression of CCR5. Similarly, individual effector features have been ascribed to TR cells. Activation of TR cells has been shown to result in increased suppressive potential (45) and to lead to up-regulation of CCR5 (33). Furthermore, the CD103+ subpopulation of TR cells is thought to migrate into inflamed sites where it appears to exert its suppressive function (5). Here we show that the CCR5+ subpopulation of TR cells carries most of the suppressive capacity within the pool of TR cells. This effector function can be shown for both CCR5+ cells isolated from nonimmunized mice and those induced by de novo activation of CCR5− TR cells. We therefore propose that these CCR5+ cells constitute the effector arm of TR cells.
CCR5+ cells can be found at the site of antigenic insult such as infection (22) and organ transplants (46). Furthermore, they have been found at sites of organ-specific autoimmunity (16, 47) and in the proximity of tumors (48). In most cases this is thought to be an influx of proinflammatory effector T cells. However, at least in some cases, CCR5+ TR cells have been shown to contribute to this population (33). We propose that the equilibrium of these two effector cell populations with diametrically opposed functions can determine the outcome of immune responses. We show that during pregnancy CCR5+ TR cells accumulate in the uterus, interference with which causes a significant increase in fetal resorptions. A similar increase in fetal loss can be induced by injection of “TH1-educated” CCR5+ proinflammatory effector TH cells, which also home into the uterus (35). In normal pregnancy, dominance of the effector TR cells leads to tolerance (6). If, however, this dominance of effector TR cells is broken, be it experimentally by interference with effector TR cell recruitment, by injection of proinflammatory effector TH cells (35), or naturally by a uterine infection (44, 49), the balance can quickly tip toward an aggressive immune response. In the case of pregnancy this rapidly inducible flexibility might be of advantage. Uterine infections are likely to spread to the fetus. In this scenario it is better for the maternal immune system to sacrifice a fetus that is likely to be damaged rather than risk both the mother's life and that of her unborn offspring (44).
CCR5 has multiple ligands, among them CCL4 (12), which is involved in the modulation of the recruitment/retention of TR cells to activated antigen-presenting cells (11). We build on this finding by demonstrating that in transwell migration assays CCL4 predominantly acts on CCR5+ effector TR cells rather than on the TR cell population as a whole. This makes CCL4 a likely candidate for the recruitment of effector T cells to the uterus. Indeed, we observed a strong correlation between the expression of CCL4 and the accumulation of TR cells, but not with that of the other CCR5 ligands CCL3 and CCL5. Yet, the lack of a detectable difference in uterine Foxp3 mRNA levels between wild-type and CCR5 gene deletion mutant mice shows that CCR5-deficient TR cells still find their way to the uterus. Although we cannot formally exclude compensatory mechanisms in the gene deletion mutants, we think it is more likely that there are CCR5/CCL4-independent mechanisms of TR cell recruitment. This, however, does not rule out a function for CCR5 in the accumulation of effector TR cells.
A hint comes from the fact that in the case of allogeneic pregnancies more CCR5+ cells accumulate in the uterus than in syngeneic pregnancies. The only difference between the two scenarios is the presence or absence of paternal alloantigen. We have previously demonstrated that the expansion of TR cells during pregnancy is independent of paternal alloantigen (6), yet we find this alloantigen-dependent difference in the number of CCR5+ cells in the uterus. Given that activation of TR cells can induce CCR5 expression, it is not too far-fetched to speculate that the CCR5+ cells found in the uterus are likely to be antigen-experienced or even alloantigen-induced effector TR cells. The fact that, in contrast to wild-type TR cells, CCR5-deficient TR cells are not able to completely prevent fetal loss highlights that the accumulation of CCR5+ effector TR cells is of biological importance. Pregnancy is clearly not the only scenario in which the antigen-specificity of the recruited TR cell appears to be essential. In a TetTNFα/CD80 inducible diabetes model those TR cells that can prevent the onset of disease preferentially accumulate in the pancreatic lymph nodes and islets (50). The accumulation of antigen-specific TR cells appears not to be restricted to self-antigens, because it also can be observed in chronic Leishmania major infections (51). Recently, Yurchenko et al. (52) examined the migration of TR cells to sites of L. major infection and demonstrated that CCR5 has an important role in the homing and presence of TR cells at their site of action, interference with which dramatically affects the outcome of the infection. In another study, tracking of CCR5+ TR cells revealed their long-term accumulation in graft-versus-host disease target organs, whereas CCR5-deficient TR cells were impaired in this accumulation, leading to decreased survival of the graft recipients (33). These findings are in agreement with our interpretation that CCR5+ TR cells are antigen-specific effector TR cells.
Furthermore, we were able to demonstrate that CCR5-deficient TR cells are handicapped in their accumulation in the gravid uterus when adoptively transferred into a wild-type animal. In contrast, TR cells constitutively expressing a CCR5 transgene appear to have a competitive advantage. In summary (Fig. 5e), we propose that CCR5 is responsible for an enhanced recruitment and/or retention of those TR cells that have already been activated by antigen in the periphery. In addition, TR cells that have migrated into the uterus in a CCR5-independent manner might become activated and possibly expand within the uterus itself. These cells are likely to be retained, in preference over those that have not been activated and thus do not express CCR5.
Materials and Methods
Experimental animals, FACS analysis, quantitative real-time RT-PCR, and adoptive transfers are described in detail in supporting information (SI) Materials and Methods.
Preparation of Uterine Tissue.
Uteri (implanted and nonimplanted segments) were separated from surrounding tissue (including fetuses/placenta), immersed in liquid nitrogen, pulverized, and resuspended in RNA lysis buffer. Total RNA was prepared by using the RNeasy kit (Qiagen, Crawley, U.K.) including on-column DNase digestion, as per the manufacturer's instructions. For FACS analysis uterine tissue was collagenase A (Roche Diagnostics, Burgess Hill, U.K.) digested for 15 min.
Cell Purifications and Proliferation Assays.
Lymphocytes were isolated from single-cell suspensions by using Lympholyte M or Mammal (Cedarlane, Ontario, Canada). CD4+ cells were isolated by depletion with anti-CD11b, anti-CD11c, anti-GR1, anti-CD19, and anti-CD8 antibodies (Becton Dickinson, Oxford, U.K.). CCR5 and CD25 subpopulations were isolated by positive selection by using an autoMACS (Miltenyi Biotec, Bisley, U.K.). For each step, the purity was >99.5% except for CCR5+, where experimental limitations allowed only 40% purity. To avoid cell density-dependent effects, the total cell number was kept constant. Cell counts were verified by FACS using CaliBRITE beads (Becton Dickinson). Effector cell suppression assays were performed by using CFSE-labeled target cells. A total of 2 × 104 CFSE+CD4+CD25− target cells were plated on U-bottom 96-well plates (Corning, Schipol-Rijk, The Netherlands) with 2 μg/ml plate-bound anti-CD3 (Becton Dickinson). To these either 2 × 104 freshly isolated CCR5-enriched cells or CCR5-depleted CD4+CD25+ cells or CD4+CD25+ CCR5-enriched cells isolated from CD4+CD25− cells that had been activated for 24 h with 2 μg/ml anti-CD3 and 10 ng/ml IL-12 (R & D Systems, Abingdon, U.K.) were added. After 72 h, the cells were analyzed by FACS by using CaliBRITE beads for volume-independent quantification. In some cases, [3H]thymidine incorporation was used (6).
Migration Assays.
Transwell migration assays were performed as described previously (11). CCL4 was used at 0–500 ng/ml. Migrated and input cells were analyzed by FACS.
Retroviral Transfection and Analysis of Transduced TR Cells.
The MLV-based retroviral plasmid m6pgfp carrying GFP and either CCR5 m6pgfp[CCR5] or blasticidine-S-deaminase m6pgfp[Blast] (control) were cotransfected with pCl-Eco packaging plasmid into 293TEBNA cell line by using CaPO4 precipitation. After 42 h, the supernatant was collected and used immediately. Freshly purified CD25+ T cells were activated by using plate-bound anti-CD3ε and murine IL-2 (20 ng/ml). After 36 h of activation, TR cells were transduced by using a 1:3 dilution of viral supernatant supplemented with 8 μg/ml polybrene, followed by centrifugation for 2 h at 450 × g and incubation at 37°C for 4 h. Transduced cells were cultured 36 h in medium containing murine IL-2 (10 ng/ml). Matching transduction efficiencies were confirmed by FACS. Female mice received 7.5 × 105 transduced cells i.v. and were allogeneically mated 48 h after transfer. Uterine and splenic lymphocytes were analyzed at E10.5 by FACS.
Supplementary Material
Acknowledgments
We thank F. Randow (Laboratory of Molecular Biology, Medical Research Council) for critical reading of the manuscript and provision of the m6p retroviral vector system and Theresa Langford and her team for expert animal handling. This work was supported by grants from the Arthritis Research Campaign (B0750) and the Carlsberg Foundation.
Abbreviations
- TR cell
regulatory T cell
- TH
T helper
- En
embryonic day n.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0604268104/DC1.
References
- 1.Maloy KJ, Powrie F. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 2.Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, et al. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aluvihare VR, Kallikourdis M, Betz AG. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 7.Wood GW, Hausmann EH, Kanakaraj K. Cytokine. 1999;11:1038–1045. doi: 10.1006/cyto.1999.0513. [DOI] [PubMed] [Google Scholar]
- 8.Kitaya K, Nakayama T, Okubo T, Kuroboshi H, Fushiki S, Honjo H. J Clin Endocrinol Metab. 2003;88:1809–1814. doi: 10.1210/jc.2002-020980. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama T, Kitaya K, Okubo T, Kuroboshi H, Daikoku N, Fushiki S, Honjo H. Fertil Steril. 2003;80:1461–1465. doi: 10.1016/j.fertnstert.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Lathbury LJ, Salamonsen LA. Biol Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 11.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 12.Meyer A, Coyle AJ, Proudfoot AE, Wells TN, Power CA. J Biol Chem. 1996;271:14445–14451. doi: 10.1074/jbc.271.24.14445. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature. 1999;401:708–712. [PubMed] [Google Scholar]
- 14.Geginat J, Sallusto F, Lanzavecchia A. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. J Immunol. 2002;168:2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 16.Wang CR, Liu MF. Clin Exp Immunol. 2003;132:371–378. doi: 10.1046/j.1365-2249.2003.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 18.Bagaeva LV, Williams LP, Segal BM. J Neuroimmunol. 2003;137:109–116. doi: 10.1016/s0165-5728(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 19.Cameron MJ, Arreaza GA, Grattan M, Meagher C, Sharif S, Burdick MD, Strieter RM, Cook DN, Delovitch TL. J Immunol. 2000;165:1102–1110. doi: 10.4049/jimmunol.165.2.1102. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho-Pinto C, Garcia MI, Gomez L, Ballesteros A, Zaballos A, Flores JM, Mellado M, Rodriguez-Frade JM, Balomenos D, Martinez AC. Eur J Immunol. 2004;34:548–557. doi: 10.1002/eji.200324285. [DOI] [PubMed] [Google Scholar]
- 21.Machado FS, Koyama NS, Carregaro V, Ferreira BR, Milanezi CM, Teixeira MM, Rossi MA, Silva JS. J Infect Dis. 2005;191:627–636. doi: 10.1086/427515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luangsay S, Kasper LH, Rachinel N, Minns LA, Mennechet FJ, Vandewalle A, Buzoni-Gatel D. Gastroenterology. 2003;125:491–500. doi: 10.1016/s0016-5085(03)00903-x. [DOI] [PubMed] [Google Scholar]
- 23.Glass WG, Lane TE. J Virol. 2003;77:191–198. doi: 10.1128/JVI.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W, Faia KL, Csizmadia V, Smiley ST, Soler D, King JA, Danoff TM, Hancock WW. Transplantation. 2001;72:1199–1205. doi: 10.1097/00007890-200110150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Abdi R, Smith RN, Makhlouf L, Najafian N, Luster AD, Auchincloss H, Jr, Sayegh MH. Diabetes. 2002;51:2489–2495. doi: 10.2337/diabetes.51.8.2489. [DOI] [PubMed] [Google Scholar]
- 27.Hancock WW, Wang L, Ye Q, Han R, Lee I. Curr Opin Immunol. 2003;15:479–486. doi: 10.1016/s0952-7915(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 28.Glass WG, Liu MT, Kuziel WA, Lane TE. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffnagle GB, McNeil LK, McDonald RA, Murphy JW, Toews GB, Maeda N, Kuziel WA. J Immunol. 1999;163:4642–4646. [PubMed] [Google Scholar]
- 30.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. J Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 31.Welniak LA, Wang Z, Sun K, Kuziel W, Anver MR, Blazar BR, Murphy WJ. Exp Hematol. 2004;32:318–324. doi: 10.1016/j.exphem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Kurihara T, Ryseck RP, Yang Y, Ryan C, Loy J, Warr G, Bravo R. J Immunol. 1998;160:4018–4025. [PubMed] [Google Scholar]
- 33.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC. Eur J Immunol. 2004;34:377–387. doi: 10.1002/eji.200324469. [DOI] [PubMed] [Google Scholar]
- 36.Thornton AM, Shevach EM. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 37.Drake PM, Red-Horse K, Fisher SJ. Dev Dyn. 2004;229:877–885. doi: 10.1002/dvdy.10477. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama M, Okabe H, Takakura K, Fujiyama Y, Noda Y. Br J Obstet Gynaecol. 1999;106:725–730. doi: 10.1111/j.1471-0528.1999.tb08374.x. [DOI] [PubMed] [Google Scholar]
- 39.Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Am J Pathol. 2001;159:2199–2213. doi: 10.1016/S0002-9440(10)63071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caballero-Campo P, Dominguez F, Coloma J, Meseguer M, Remohi J, Pellicer A, Simon C. Mol Hum Reprod. 2002;8:375–384. doi: 10.1093/molehr/8.4.375. [DOI] [PubMed] [Google Scholar]
- 41.Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Nat Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 42.Barthlott T, Kassiotis G, Stockinger B. J Exp Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 44.Trowsdale J, Betz AG. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 45.Thornton AM, Piccirillo CA, Shevach EM. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 46.Horiguchi K, Kitagawa-Sakakida S, Sawa Y, Li ZZ, Fukushima N, Shirakura R, Matsuda H. J Heart Lung Transplant. 2002;21:1090–1100. doi: 10.1016/s1053-2498(02)00443-6. [DOI] [PubMed] [Google Scholar]
- 47.Balashov KE, Rottman JB, Weiner HL, Hancock WW. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I. Int J Cancer. 2005;116:949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 49.Abrahams VM, Mor G. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Green EA, Choi Y, Flavell RA. Immunity. 2002;16:183–191. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 51.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.