Abstract
Physical activity protects against cardiovascular disease, and physiological cardiac hypertrophy associated with regular exercise is usually beneficial, in marked contrast to pathological hypertrophy associated with disease. The p110α isoform of phosphoinositide 3-kinase (PI3K) plays a critical role in the induction of exercise-induced hypertrophy. Whether it or other genes activated in the athlete's heart might have an impact on cardiac function and survival in a setting of heart failure is unknown. To examine whether progressive exercise training and PI3K(p110α) activity affect survival and/or cardiac function in two models of heart disease, we subjected a transgenic mouse model of dilated cardiomyopathy (DCM) to swim training, genetically crossed cardiac-specific transgenic mice with increased or decreased PI3K(p110α) activity to the DCM model, and subjected PI3K(p110α) transgenics to acute pressure overload (ascending aortic constriction). Life-span, cardiac function, and molecular markers of pathological hypertrophy were examined. Exercise training and increased cardiac PI3K(p110α) activity prolonged survival in the DCM model by 15–20%. In contrast, reduced PI3K(p110α) activity drastically shortened lifespan by ≈50%. Increased PI3K(p110α) activity had a favorable effect on cardiac function and fibrosis in the pressure-overload model and attenuated pathological growth. PI3K(p110α) signaling negatively regulated G protein-coupled receptor stimulated extracellular responsive kinase and Akt (via PI3K, p110γ) activation in isolated cardiomyocytes. These findings suggest that exercise and enhanced PI3K(p110α) activity delay or prevent progression of heart disease, and that supraphysiologic activity can be beneficial. Identification of genes important for hypertrophy in the athlete's heart could offer new strategies for treating heart failure.
Keywords: heart failure, signal transduction, heart growth, athlete's heart
Regular physical activity plays an important role in the prevention and treatment of chronic diseases including obesity, diabetes, cardiovascular disease, cancer, depression, and osteoporosis through a broad range of actions and pathways (1, 2). Identification of genes that are critical for the beneficial effects of exercise may offer new opportunities for treating chronic disease. Here, we describe this approach in the failing heart. Heart failure is a debilitating disease with mortality rates, hospitalizations, and prevalence rates increasing (3). Cardiac dilatation and/or hypertrophy are characteristics of most forms of heart failure, and a large heart is a poor prognostic sign. A paradoxical exception is the cardiac hypertrophy that occurs in the athlete (physiological hypertrophy). In contrast to pathological hypertrophy in disease states, which is associated with fibrosis, dysfunction, altered cardiac gene expression, and increased morbidity and mortality (4–6), physiological hypertrophy shows a normal organization of sarcomeres and fibers, normal or enhanced cardiac function, and a relatively normal pattern of cardiac gene expression (6, 7). It is now clear that some signaling molecules play distinct roles in regulating cardiac growth in the athlete's heart as opposed to growth associated with the diseased heart (8–10). Exercise activates the insulin-like growth factor 1 (IGF1)–phosphoinositide 3-kinase (PI3K, p110α) pathway (11–13), and we have specifically shown that class IA PI3K plays a critical role in the induction of cardiac growth induced by exercise training (8, 14). Whether the genes and pathways activated in the athlete's heart have a therapeutic role for improving survival in a setting of heart failure is unknown. To examine whether activation of PI3K(p110α; subsequently referred to as PI3K) and exercise have an impact on lifespan or cardiac function in models of heart disease, PI3K activity was increased or decreased in two mouse models [a transgenic (Tg) model of dilated cardiomyopathy (DCM) and a surgical model of pathologic hypertrophy (pressure overload)], and the DCM model was subjected to progressive swim training.
Results
In this study, we used a mouse model of DCM (DCM-TG9) and cardiac-specific Tg mice with increased PI3K activity (constitutively active PI3K, caPI3K) or decreased PI3K activity (dominant negative PI3K, dnPI3K) (15, 16). DCM-TG9 mice display typical signs of DCM, including ventricular dilatation, impaired systolic function, congestive heart failure, and premature death. DCM-TG9 mice have a very reproducible time of death (maximum lifespans of males and females are 13 and 11 weeks, respectively) and develop heart failure symptoms (fatigue/sedentary behavior, labored respiration, skin cold to touch, peripheral edema) a few days before death (16). A further advantage of this model is that drugs that delay the progression of heart failure in humans (captopril or metoprolol) improve survival (16). caPI3K-Tg mice have elevated cardiac PI3K activity and hearts that are 20% larger and exhibit normal cardiac function (physiological hypertrophy, “athlete's heart”). By contrast, dnPI3K-Tg mice have depressed PI3K activity and hearts that are 20% smaller (15).
Three studies were performed. In the first, it was determined whether exercise training improved the lifespan of DCM-TG9 mice; in the second, DCM-TG9 mice were genetically crossed with PI3K-Tg mice (caPI3K and dnPI3K); and in the third, PI3K-Tg mice were subjected to a surgical model of pressure overload (ascending aortic constriction).
Exercise Training Prolonged Survival in a DCM Model.
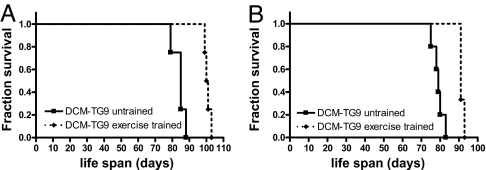
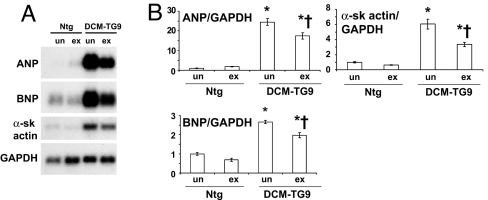
Although it is generally believed that strenuous exercise may overburden the diseased heart, and hence contraindicated to patients with DCM, no human trial has demonstrated that progressive exercise training has either a beneficial or deleterious effect on survival, possibly because of confounding factors including medications, diet, smoking, and exercise compliance. Consequently, it was important to demonstrate that exercise had a favorable impact in our model before examining whether PI3K activity had any effect. Swim training significantly improved lifespan in the model of DCM in both males and females [Fig. 1 and supporting information (SI) Fig. 9]. The mean age of males and females was increased by ≈20% and 16%, respectively. Ventricular gene expression of brain natriuretic peptide (BNP), atrial natriuretic peptide (ANP), and α-skeletal actin is up-regulated in DCM-TG9 mice compared with nontransgenic (Ntg) mice (16). Swim training significantly reduced the expression of these genes (Fig. 2). Of note, DCM-TG9 mice started swim training before displaying significant functional abnormalities [i.e., training commenced at 4 weeks of age (16)]; however, at this age hearts from these mice already show molecular markers of pathological hypertrophy, including ANP and BNP (SI Fig. 10).
Fig. 1.
Swim training prolongs the lifespan of mice with DCM. Kaplan–Meier survival curves of untrained and exercise-trained DCM-TG9 mice. (A) Males, n = 4 in each group; curve comparison, P < 0.007. (B) Females, untrained n = 5, exercise-trained n = 3, P < 0.02).
Fig. 2.
Swim training reduced the expression of fetal genes in mice with DCM. (A) Representative Northern blot showing total RNA from ventricles of untrained (un) and exercise trained (ex) Ntg and DCM-TG9 mice. (B) Quantitative analysis of fetal gene expression (fold change). GAPDH was used to normalize for loading of RNA. Mean values for Ntg untrained (un) mice were normalized to 1 (n = 3 or 4 in each group). ∗, P < 0.05 vs. Ntg untrained mice. †, P < 0.05 vs. DCM-TG9 untrained mice. α-sk actin, α-skeletal actin.
Enhanced PI3K Activity Prolonged Survival in a DCM Model.
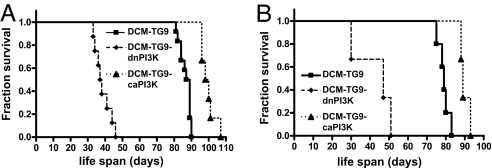
PI3K activity is an important regulator of exercise-induced cardiac growth (8). To examine whether PI3K can influence the progression of heart failure, we genetically crossed DCM-TG9 mice with caPI3K or dnPI3K-Tg mice. PI3K transgene expression had no effect on Tg protein levels in the DCM-TG9 model (data not shown). Tg expression of dnPI3K dramatically accelerated the progression of heart failure, shortening lifespan in both males and females (Fig. 3 and SI Fig. 11), whereas caPI3K transgene expression significantly delayed the onset of symptoms and prolonged survival. Furthermore, the increase in lifespan observed with caPI3K expression was similar to that observed in response to swim training (≈20%).
Fig. 3.
Enhanced PI3K activity improves the lifespan of mice with DCM. Survival curves of male (A) and female (B) DCM-TG9 mice (n = 12 and 5, respectively) with and without increased PI3K activity (DCM-TG9-caPI3K mice; n = 6 and 3, respectively) or decreased PI3K activity (DCM-TG9-dnPI3K mice; n = 8 and 3, respectively). Curve comparison shows for DCM-TG9 vs. DCM-TG9-caPI3K mice, male, P < 0.0002; female, P < 0.02 and for DCM-TG9 vs. DCM-TG9-dnPI3K mice, male, P < 0.0001; female, P < 0.005.
PI3K Activity Provides Protection Against Subsequent Pressure Overload.
To ascertain whether PI3K activity had an impact on another model of cardiac disease, adult PI3K-Tg mice were subjected to pressure overload (aortic banding). Sham-operated caPI3K and dnPI3K-Tg mice displayed no echocardiographic signs of hyperfunction or dysfunction as measured by fractional shortening (Table 1). Each banded group had a similar aortic gradient (Table 1, AomaxPg). In banded Ntg mice there were no changes in left ventricular (LV) end-systolic or –diastolic volumes and systolic function was maintained (Table 1, see fractional shortening). This finding is consistent with previous studies in which pressure overload for 1 week induced compensated hypertrophy (8, 17). There was a marked increase in LV end-systolic and –diastolic volumes in banded dnPI3K-Tg mice, and systolic function was significantly depressed compared with the other banded groups (Table 1). By contrast, there was a trend for a reduction in LV end-systolic volume in banded caPI3K-Tg mice, and systolic function was significantly greater than that of banded Ntg mice (Table 1). Consistent with these findings, the lung weight/body weight (BW) ratio (an index of pulmonary congestion and elevated LV end-diastolic pressure) was significantly elevated in banded dnPI3K-Tg mice compared with banded caPI3K-Tg mice (Table 2).
Table 1.
Echocardiography data from mice subjected to pressure overload
| Data measure | Ntg mice |
dnPI3K mice |
caPI3K mice |
|||
|---|---|---|---|---|---|---|
| Sham (n = 4) | Band (n = 5) | Sham (n = 5) | Band (n = 6) | Sham (n = 5) | Band (n = 5) | |
| Body weight, g | 30.6 ± 1.4 | 26.9 ± 1.8 | 29.8 ± 1.2 | 27.6 ± 1.9 | 29.3 ± 0.6 | 30.8 ± 0.9 |
| Heart rate, bpm | 441 ± 41 | 315 ± 66 | 487 ± 29 | 353 ± 38 | 515 ± 25 | 320 ± 71* |
| IVS, mm | 0.73 ± 0.08 | 0.93 ± 0.10† | 0.84 ± 0.05 | 0.94 ± 0.06 | 0.95 ± 0.05 | 1.27 ± 0.07*‡ |
| LVPW, mm | 0.74 ± 0.06 | 0.82 ± 0.09 | 0.80 ± 0.04 | 0.83 ± 0.07 | 0.93 ± 0.03 | 1.16 ± 0.06*‡ |
| LVEDD, mm | 3.88 ± 0.25 | 3.68 ± 0.19 | 3.24 ± 0.16 | 3.96 ± 0.12* | 3.77 ± 0.19 | 3.58 ± 0.11 |
| LVESD, mm | 2.11 ± 0.24 | 2.18 ± 0.29 | 1.65 ± 0.13 | 2.81 ± 0.17*‡ | 1.94 ± 0.11 | 1.71 ± 0.10§¶ |
| LVESV, mm3 | 10.5 ± 3.1 | 13.2 ± 4.5 | 4.8 ± 1.0 | 23.4 ± 3.9*‡ | 7.6 ± 1.2 | 5.2 ± 1.0§¶ |
| LVEDV, mm3 | 60.6 ± 10.4 | 51.7 ± 7.9 | 35.1 ± 4.8 | 62.6 ± 5.3†† | 55.1 ± 7.9 | 46.4 ± 4.5 |
| Fractional shortening, % | 46 ± 3 | 42 ± 5 | 49 ± 3 | 29 ± 3‡ | 48 ± 1 | 52 ± 2¶‖ |
| AomaxPg, mmHg | 3 ± 1 | 29 ± 6* | 3 ± 0 | 21 ± 2* | nd | 26 ± 5** |
Pressure overload was induced by aortic banding (Band). IVS, interventricular septum thickness; LVPW, LV posterior wall thickness; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; LVESV, LV end-systolic volume; LVEDV, LV end-diastolic volume; AomaxPg, aortic pressure gradient; nd, not determined.
*, P < 0.05 vs. sham from the same genotype.
†, P < 0.1 vs. sham from the same genotype.
‡, P < 0.05 vs. all other groups.
§, P < 0.1 vs. Ntg Band.
¶, P < 0.05 vs. dnPI3K band.
‖, P < 0.05 vs. Ntg band.
**, P < 0.05 vs. Ntg sham.
††, P < 0.05 vs. dnPI3K sham (unpaired t test).
Table 2.
Postmortem analysis of mice subjected to pressure overload
| Data measure | Ntg mice |
dnPI3K mice |
caPI3K mice |
|||
|---|---|---|---|---|---|---|
| Sham (n = 7) | Band (n = 6) | Sham (n = 5) | Band (n = 6) | Sham (n = 5) | Band (n = 5) | |
| Age, days | 134 ± 23 | 122 ± 13 | 153 ± 16 | 140 ± 18 | 123 ± 23 | 141 ± 8 |
| BW, g | 29.5 ± 0.6 | 27.2 ± 1.5 | 29.4 ± 0.9 | 26.6 ± 1.2 | 28.4 ± 0.8 | 30.5 ± 1.0 |
| HW, mg | 127.1 ± 4.2 | 164.0 ± 7.6* | 101.1 ± 3.6† | 166.0 ± 10.2*† | 148.0 ± 3.7† | 185.1 ± 5.0†‡ |
| TL, mm | 16.8 ± 0.1 | 16.6 ± 0.1 | 17.1 ± 0.1 | 16.8 ± 0.1‡ | 16.8 ± 0.1 | 17.3 ± 0.1‡ |
| HW/BW, mg/g | 4.31 ± 0.12 | 6.09 ± 0.34* | 3.44 ± 0.04† | 6.27 ± 0.38*† | 5.21 ± 0.08† | 6.07 ± 0.10†‡ |
| HW/TL, mg/mm | 7.55 ± 0.25 | 9.85 ± 0.41* | 5.90 ± 0.19† | 9.90 ± 0.57* | 8.82 ± 0.20† | 10.67 ± 0.27†‡ |
| nHW/BW | 1.00 ± 0.03 | 1.41 ± 0.08* | 0.80 ± 0.01† | 1.46 ± 0.09*† | 1.21 ± 0.02† | 1.41 ± 0.02†‡ |
| nHW/TL | 1.00 ± 0.09 | 1.31 ± 0.05* | 0.78 ± 0.03† | 1.31 ± 0.08*† | 1.17 ± 0.03† | 1.41 ± 0.04†‡ |
| % increase in HW/BW (compared with sham from same genotype) | 41 ± 8 | 82 ± 11§ | 17 ± 2§¶ | |||
| % increase in HW/TL (compared with sham from same genotype) | 31 ± 5 | 68 ± 10§ | 21 ± 3¶ | |||
| LW/BW, mg/g | 5.63 ± 0.22 | 8.20 ± 1.15‡ | 6.00 ± 0.25 | 9.94 ± 1.40‡ | 5.44 ± 0.31 | 7.34 ± 0.45¶ |
nHW/BW, normalized HW/BW (values normalized to Ntg sham); nHW/TL, normalized HW/TL (values normalized to Ntg sham); LW, lung weight.
*P < 0.0001 vs. sham of the same genotype.
†, P < 0.05 vs. Ntg sham.
‡, P < 0.05 vs. sham of the same genotype.
§, P < 0.05 vs. Ntg band.
¶, P < 0.05 vs. dnPI3K band.
PI3K Activity Inhibits Hypertrophy in Response to a Pathological Stimulus.
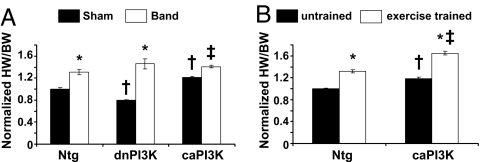
As reported, hearts from dnPI3K-Tg mice (under basal conditions or sham) were ≈20% smaller compared with Ntg mice, whereas hearts from caPI3K control/sham mice were ≈20% larger [Table 2, see normalized heart weight (HW)/BW sham values] (8, 15). In each of the three groups (Ntg, caPI3K, and dnPI3K) banding induced an increase in heart mass. When the HW/BW ratios were expressed as a percentage of sham from the same genotype, significant differences in the hypertrophic responses were apparent (Table 2, see percentage increase in HW/BW). These differences were not related to age. The HW/BW ratio increased by ≈40% in banded Ntg mice, whereas this response was exaggerated in banded dnPI3K-Tg mice (≈80%) and blunted in banded caPI3K-Tg mice (≈17%; Fig. 4A). To account for any changes in BW between groups, HWs were also normalized to tibial lengths (TLs). HW/TL ratios showed similar trends to HW/BW (Table 2). These data suggest that activation of PI3K can prevent excessive growth in response to a pathological stimulus. Consistent with these results, the gene expression changes of some ribosomal biogenesis- and protein biosynthesis-related genes (key features of cardiac hypertrophy) were significantly elevated in banded dnPI3K-Tg mice but not in other groups (SI Fig. 12).
Fig. 4.
Hypertrophic responses of PI3K-Tg mice to pressure overload and swim training. (A) Response of Ntg, dnPI3K, and caPI3K mice to aortic banding (band) for 1 week or the sham operation. HW/BW ratios are normalized to Ntg sham. Numbers for each group are shown in Table 2. *, P < 0.0001 vs. sham of the same genotype. †, P < 0.05 vs. Ntg sham. ‡, P < 0.05 vs. sham of the same genotype. (B) Response of Ntg and caPI3K mice to swim training for 4 weeks. HW/BW ratios are normalized to Ntg untrained mice. Numbers for each group are shown in SI Table 3. *, P < 0.05 vs. untrained mice of the same genotype. †, P < 0.05 vs. Ntg untrained mice. ‡, P < 0.05 vs. Ntg exercise-trained mice.
By echocardiography, there was a trend toward an increase in LV wall thickness in all banded groups (Table 1). However, the blunted hypertrophic response in banded caPI3K-Tg mice or the exaggerated response in banded dnPI3K-Tg mice cannot be accounted for by parallel changes in LV wall thicknesses. Rather, the changes seem to be related to changes in LV chamber dimensions and volumes. There was a significant increase in the chamber dimensions of dnPI3K-Tg mice in response to pressure overload and a trend for a reduction in caPI3K-Tg mice (Table 1).
Response of caPI3K-Tg Mice to Exercise Training.
To help exclude the possibility that hearts from aortic-banded caPI3K-Tg mice reached a critical mass at which no further hypertrophy was possible, a group of Ntg and caPI3K-Tg mice was subjected to a physiological stimulus (swim training for 4 weeks). In response to swim training both Ntg and caPI3K-Tg mice displayed a hypertrophic response (Fig. 4B and SI Table 3). This response was not blunted in caPI3K-Tg mice in comparison with Ntg mice.
Fetal Gene Expression in PI3K-Tg Mice Subjected to Pressure Overload.
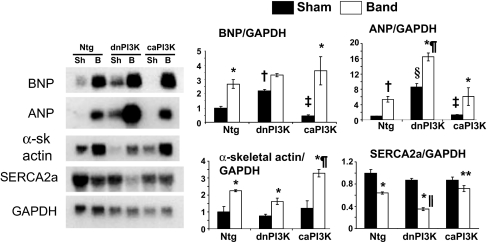
Aortic banding is commonly associated with up-regulation of the fetal gene program (18, 19). In banded Ntg mice, BNP, ANP, and α-skeletal actin were up-regulated compared with sham mice (Fig. 5). As described, ANP and BNP were up-regulated in dnPI3K-Tg mice without any intervention, i.e., control or the sham operation (15, 20). In response to banding, ANP expression rose in dnPI3K-Tg mice above that of banded Ntg or caPI3K-Tg mice. Interestingly, expression of α-skeletal actin increased more in banded caPI3K-Tg mice than the other banded groups. SERCA2a, a gene considered critical for maintaining cardiac contractility, fell in banded Ntg and dnPI3K-Tg mice. Notably the fall was greater in dnPI3K than Ntg mice, and there was no significant fall in banded caPI3K-Tg mice. This finding is consistent with the fall in systolic function in banded dnPI3K-Tg mice and preserved function in banded caPI3K-Tg mice (Table 1).
Fig. 5.
Fetal gene expression in PI3K-Tg mice subjected to pressure overload. (Left) Expression levels of BNP, ANP, α-skeletal actin (α-sk actin), and SERCA2a were examined in total RNA from ventricles of sham (Sh) and aortic-banded (B) mice by Northern blotting (representative blot). GAPDH was used to normalize for RNA loading. (Right) Quantitative analysis (fold change). Values were normalized to Ntg sham, n = 2–3 in the sham groups, n = 3 in each banded group. *, P < 0.05 vs. sham of the same genotype. †, P < 0.08 vs. Ntg sham. ‡, P < 0.05 vs. dnPI3K sham. §, P < 0.05 vs. Ntg sham. ¶, P < 0.05 vs. all other banded groups. ‖, P < 0.05 vs. Ntg band. **, P < 0.05 vs. dnPI3K band.
PI3K Activity Regulates Cardiac Fibrosis.
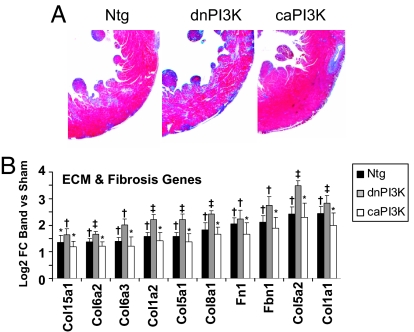
The LV wall of banded dnPI3K-Tg mice showed increased fibrosis compared with banded Ntg mice, whereas the LV wall of banded caPI3K-Tg mice showed less fibrosis (Fig. 6A). Consistent with this finding, the gene expression changes measured by microarray (as log 2-fold change of band vs. sham) of a large number of extracellular matrix- and fibrosis-related genes were greater in banded dnPI3K-Tg mice compared with banded Ntg mice, but lower in banded caPI3K-Tg mice (Fig. 6B). Two apoptosis-related genes showed a similar trend (SI Fig. 13).
Fig. 6.
Effect of PI3K activity on cardiac fibrosis. (A) Histological sections from the LV wall of Ntg and PI3K-Tg mice subjected to aortic banding. Fibrosis is blue on Masson's trichrome stain. (B) Gene expression changes measured by microarray [log 2 fold change (FC) of band vs. sham] of extracellular matrix (ECM)- and fibrosis-related genes. Col, procollagen types: 15a1, XV; 6a2 and 6a3, VI alpha 2 and 3; 1a1 and 1a2, I alpha 1 and 2; 5a1 and 5a2, V alpha 1 and 2; 8a1, VIII alpha 1; Fn1, fibronectin 1; Fbn1, fibrillin 1. n = 3 in each group. *, adjusted P < 0.05 for band vs. sham. †, adjusted P < 0.001 for band vs. sham. ‡, adjusted P < 0.0001 for band vs. sham.
PI3K Regulates G Protein-Coupled Receptor (GPCR)-Induced Extracellular Responsive Kinase (ERK)1/2 and Akt Activation.
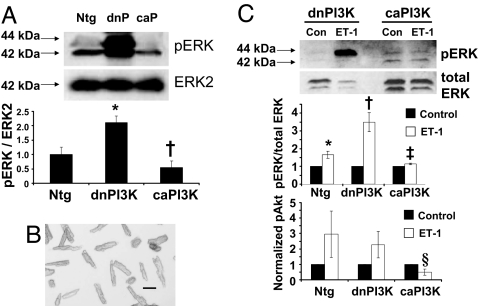
ERK1/2 activation is associated with the development of pressure overload-induced hypertrophy (21). To examine whether PI3K can inhibit pathological processes by regulating signaling molecules activated by pathological stimuli, we assessed ERK1/2 activation in heart lysates and isolated cardiomyocytes. pERK/ERK2 was elevated in unstressed hearts from dnPI3K-Tg compared with Ntg mice, whereas it tended to be lower in caPI3K-Tg mice (Fig. 7A). To examine whether these responses occur at the cellular level, studies were also performed on isolated adult cardiomyocytes from Ntg and PI3K-Tg mice (Fig. 7B). Ntg myocytes responded to endothelin 1 (ET-1; GPCR agonist) with an increased ERK1/2 phosphorylation compared with unstimulated myocytes (Fig. 7C Top and Middle). The ET-1-induced response was exaggerated in dnPI3K myocytes and tended to be blunted in caPI3K myocytes. Akt can be activated by receptor tyrosine kinases (RTKs), e.g., IGF1 receptor and GPCRs, e.g., ET receptors. RTK-induced Akt activation is elevated in hearts of caPI3K-Tg mice and depressed in dnPI3K-Tg mice (15). GPCR-induced activation of Akt by ET-1 tended to be increased in Ntg and dnPI3K-Tg myocytes but not caPI3K-Tg myocytes (Fig. 7C Bottom).
Fig. 7.
ERK1/2 and Akt activation in heart lysates and/or isolated cardiomyocytes from PI3K-Tg mice. (A) (Upper) Western blot showing pERK1/2 in heart lysates (100 μg protein) from Ntg, dnPI3K (dnP), and caPI3K (caP) mice. pERK1/2 was normalized to ERK2. (Lower) Quantitative analysis. Mean values for Ntg mice were normalized to 1, n = 3 in each group. *, P < 0.05 vs. Ntg mice. †, P < 0.05 vs. dnPI3K mice. (B) Photograph of quiescent rod-shaped cardiomyocytes. (Scale bar: 0.1 mm.) (C) ERK1/2 and Akt activation in myocytes from Ntg, dnPI3K, and caPI3K mice to ET-1 stimulation vs. no stimulation (control, Con). (Top) Western blot shows pERK1/2 in myocytes (15 μg protein) from dnPI3K and caPI3K mice. (Middle) Quantitative analysis of pERK1/2 normalized to total ERK1/2. (Bottom) Quantitative analysis of pAkt normalized to Akt or GAPDH. Control values from each genotype were normalized to 1, n = 3 in each group. *, P < 0.08 vs. Ntg control myocytes. †, P < 0.05 vs. dnPI3K control myocytes. ‡, P < 0.05 vs. dnPI3K myocytes stimulated with ET-1. §, P < 0.05 vs. Ntg myocytes stimulated with ET-1 (unpaired t test).
Discussion
Regular aerobic exercise has many effects that are important in the prevention and treatment of cardiovascular disease (22–24). We have demonstrated that exercise training and enhanced PI3K activity have a positive impact on survival or cardiac function in a mouse model of DCM or pressure overload. Swim training and increased PI3K activity improved lifespan in the DCM model, whereas reduced PI3K activity dramatically reduced lifespan. Exercise training has favorable effects on cardiac function in heart failure patients and has been associated with a fall in plasma BNP levels (23, 25). The fast progression of disease in our model of DCM, particularly in double transgenics expressing both DCM-TG9 and dnPI3K, prevented us from routinely monitoring function by echocardiography (anesthesia was associated with premature death). However, swim training delayed or reversed the up-regulation of fetal genes including BNP, ANP, and α-skeletal actin in the DCM model, suggesting swim training had some beneficial cardiac effects. Voluntary cage wheel exercise was shown to reverse the up-regulation of fetal gene expression in a mouse model of hypertrophic cardiomyopathy (26). Although it may seem counterintuitive to subject mice with DCM to progressive and intense exercise training, this intervention was as effective or more effective than angiotensin-converting enzyme inhibition or β-blockade (16). Of note, DCM-TG9 mice commenced swim training at an age when molecular markers of pathological hypertrophy were present (i.e., ANP and BNP) but before they displayed significant systolic dysfunction.
We previously reported that dnPI3K-Tg mice develop signs of congestive heart failure in response to pressure overload after 1 week (8). The current study confirms these findings and extends them to animals with supranormal PI3K activity, i.e., caPI3K-Tg mice. Enhanced PI3K activity had a beneficial impact against subsequent pressure overload by inhibiting or preventing pathological processes, including dilatation, increases in LV volumes, excessive heart growth, fibrosis, and fall in SERCA2a gene expression. A number of clinical trials have found that reductions in LV volumes are a good indicator of favorable outcome with regard to morbidity and mortality (27).
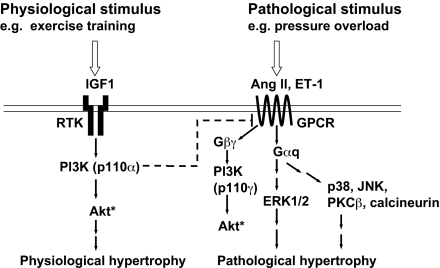
Class I PI3Ks play a critical role in the induction of physiological hypertrophy, whereas other signaling molecules induce pathological hypertrophy (Fig. 8) (6, 8, 14). The concept that the PI3K pathway can inhibit pathological growth in addition to promoting physiological growth is consistent with current and previous data obtained by using PI3K and IGF1 receptor Tgs. dnPI3K-Tg mice displayed an exaggerated response to a pathological stimulus (aortic banding) but a blunted response to a physiological stimulus (swim training) (8). By contrast, hearts with increased PI3K activity (caPI3K- or IGF1 receptor-Tgs) displayed a blunted response to aortic banding but not swim training (SI Fig. 14) (20). For this reason, we think it is unlikely that the blunted hypertrophic response in banded caPI3K-Tg mice and the exaggerated response in banded dnPI3K-Tg mice is simply caused by a larger heart requiring less hypertrophy and a smaller heart requiring more hypertrophy to meet the demands of pressure overload. Our data are also consistent with a recent study that reported Akt [downstream target of PI3K in the heart (28)] can promote physiological growth but antagonize pathological growth (10). Hearts of Akt1−/− mice were not different in size to WT, but showed an exaggerated hypertrophic response to aortic banding. These data also suggest that the hypertrophic response does not depend on heart size before the pathological stimulus. Akt promotes growth and survival in cardiac myocytes, and RTK-induced Akt activation was enhanced in caPI3K-Tg mice but depressed in dnPI3K-Tg mice (15). Akt is also activated in response to swim training (8), and Akt1−/− mice showed a blunted hypertrophic response to swim training (10). Thus, Akt is likely to play an important role in the protection provided by caPI3K transgene expression and exercise training. Consistent with this idea, systolic function was depressed in banded Akt1−/− mice but not WT controls (10).
Fig. 8.
Schematic illustrates physiological and pathological signaling cascades. A physiological stimulus acting via the IGF1-PI3K(p110α) pathway may inhibit signaling molecules downstream of GPCRs activated by pathological stimuli. Ang II, angiotensin II. Dotted line indicates it may not be a direct interaction. * indicates differential regulation of Akt by GPCRs vs. RTKs (31).
To determine whether PI3K has an impact on signaling molecules activated in response to pathological stimuli e.g., pressure overload (8, 21), we assessed ERK1/2 activation in heart lysates and isolated myocytes. pERK1/2 was elevated in unstressed hearts from dnPI3K-Tg mice but tended to be depressed in hearts from caPI3K-Tg mice. Differences in ERK1/2 activation were not found in the original characterization of the PI3K-Tg mice (15). However, pERK1/2 was increased in hearts of mice with muscle-specific deletion of class IA PI3Ks (14). The discrepancy between the studies is unclear but could be caused by variation between animals in the initial study. To examine whether the changes we observed in ERK1/2 activation in unstressed hearts represented changes in cardiomyocytes we measured pERK1/2 in response to ET-1 stimulation. Myocytes from dnPI3K-Tg mice showed an augmented pERK1/2 response to ET-1, whereas myocytes from caPI3K-Tg mice tended to show a blunted response. Together, these data suggest that PI3K signaling can inhibit ERK1/2 activation. Of note, even though ERK1/2 activation increases in a setting of pressure overload, the exact role of ERK1/2 remains uncertain (21). ERK1/2 activation has been associated with compensatory hypertrophy and cardiac protection (21). Thus, in hearts of dnPI3K-Tg mice in which RTK-induced Akt activation is depressed, ERK1/2 may be activated as a protective mechanism. However, assessment of pAkt in ET-1-stimulated myocytes suggests that PI3K acts upstream of ERK1/2 and has the potential to regulate other signaling molecules downstream of GPCR. Akt is activated by PI3K(p110α) to induce physiological hypertrophy but is also activated in response to GPCR agonists, e.g., ET-1 via another PI3K isoform (p110γ) that induces pathological hypertrophy (Fig. 8). P110γ negatively controls cardiac contractility in a setting of pressure overload (29). The biological significance of Akt activation in response to RTKs versus GPCRs is not completely understood but seems to be differentially regulated in the heart (30, 31). The blunted Akt response in ET-1-stimulated caPI3K myocytes compared with Ntg and dnPI3K myocytes suggests PI3K(p110α) may inhibit signaling molecules at the level of GPCR or G proteins (Fig. 8). GPCR attenuation could serve to both limit hypertrophy and restore contractility (through the attenuation of p110γ-dependent phosphodiesterase activity) (29). Generation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) in response to GPCR stimulation produced a negative feedback loop that caused internalization of GPCRs (32). One could speculate that generation of PIP3 in response to PI3K(p110α) could also internalize GPCRs.
A challenge in targeting the PI3K family with drugs is to understand how individual PI3K isoforms control normal physiology in different cell types. The use of isoform-specific inhibitors of PI3K has generated great interest in oncology (33, 34). Aberrant activation of Akt through PI3K permits cancer cells to bypass normal growth-limiting controls. Although PI3K inhibitors do not appear to lead to unmanageable metabolic disturbances, e.g., diabetes (34), our current findings raise concerns for patients who have heart disease or are at risk. Even though mice with reduced PI3K activity have normal cardiac function under basal conditions (15), they display accelerated heart failure in response to DCM or hypertension. Thus, the clinical use of PI3K inhibitors may require regimens that minimize cardiotoxicity or be contraindicated in patients with preexistent cardiomyopathy.
In summary, our findings suggest that exercise training and increased PI3K activity have a favorable impact on survival and cardiac function in models of cardiac disease. The cardioprotective role of PI3K may be caused, at least in part, by inhibition of pathological signaling cascades. In general, heart failure research and therapy has concentrated on identifying and inhibiting pathological processes. Our results demonstrate the potential of proactive therapeutic interventions based on stimuli and genes leading to growth in the athlete's heart.
Materials and Methods
Experimental Animals.
Animal ethics committees of Beth Israel Deaconess Medical Center, Washington University School of Medicine, and Alfred Medical Research and Education Precinct approved animal care and experimentation. The mouse model of DCM (DCM-TG9; caused by high-level Cre-recombinase), caPI3K and dnPI3K-Tg mice were generated as described (15, 16).
Echocardiography.
Mice were anesthetized with an i.p. injection of 2,2,2-tribromoethanol (0.4–0.6 mg/kg). Transthoracic echocardiography was performed by using a Sonos 5500 echocardiography system (Hewlett–Packard, Andover, MA) with a 15-MHz linear array transducer. LV wall thicknesses, LV chamber dimensions, LV volumes, and fractional shortening were determined from M-mode tracings (further details are presented in SI Materials and Methods). In aortic-banded mice, the degree of aortic stenosis was assessed as described (19).
Exercise Training.
DCM-TG9 mice were subjected to chronic swim training from 4 weeks of age for 10–11 weeks as described (8). This protocol causes athlete's heart (physiological hypertrophy) in Ntg mice (8). Mice were swum daily until they displayed prominent signs of congestive heart failure, i.e., labored breathing, fatigue, and sedentary behavior. A group of 3-month-old male caPI3K-Tg and Ntg mice were subjected to the same swim protocol for 4 weeks.
Tg Cross-Studies.
Male heterozygous DCM-TG9 mice were genetically crossed with female heterozygous caPI3K or dnPI3K-Tg mice.
Pressure Overload Studies.
Adult male Ntg, caPI3K, and dnPI3K-Tg mice were subjected to ascending aortic constriction or the sham operation by the same surgeon, without knowledge of the animals' genotype (17, 35). This model causes LV hypertension and hypertrophy. Cardiac function was assessed by echocardiography 1 week after surgery, and mice were killed for assessment of HW/BW and HW/TL ratios.
Gene Expression.
Gene expression in LV samples was determined by Northern blotting or microarray analysis (detailed methods are in SI Materials and Methods).
Isolated Adult Cardiomyocytes.
Adult cardiomyocytes from 4- to 5-month-old male mice (Ntg, caPI3K, or dnPI3K-Tg) were isolated as described with some modifications (36, 37). Details are presented in SI Materials and Methods. Plated myocytes were stimulated with or without ET-1 (10−7 M) for 10 min.
Protein Analysis.
Protein from hearts and myocytes were prepared as described (28, 36). Phosphorylation of ERK1/2 and Akt were assessed by Western blotting. Details are presented in SI Materials and Methods.
Statistical Analysis.
Results are presented as mean ± SE. Differences between groups were compared by using one-way ANOVA followed by the Fisher's protected least-significant difference or Tukey post hoc test. Kaplan–Meier survival curves were constructed by using Prism (GraphPad, San Diego, CA) and compared by using a log rank test. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank M. Li and O. Rozhitskaya for technical assistance and P. Kang (Beth Israel Deaconess Medical Center) for PI3K-Tg mice. This work was supported by National Institutes of Health Grant RO1 HL65742 (to S.I.); National Health and Medical Research Council Program Grant 225108 (to G.L.J.); and National Health and Medical Research Council Project Grants 367600 (to J.R.M.), 317801 (to E.A.W.), and 317802 (to E.A.W.). J.R.M. was supported by Career Development Award 317835/CR 04M1716 cofunded by the National Health and Medical Research Council and National Heart Foundation of Australia. E.A.W. is a National Health and Medical Research Council Research Fellow. A.B. was supported by the Deutsche Akademie der Naturforscher Leopoldina. P.Y.J. is a Scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine (Grant K12-HD001487).
Abbreviations
- PI3K
phosphoinositide 3-kinase
- caPI3K
constitutively active PI3K
- dnPI3K
dominant negative PI3K
- HW
heart weight
- BW
body weight
- Ntg
nontransgenic
- Tg
transgenic
- DCM
dilated cardiomyopathy
- LV
left ventricular
- GPCR
G protein-coupled receptor
- ERK
extracellular responsive kinase
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- ET-1
endothelin 1
- IGF1
insulin-like growth factor 1
- TL
tibial length
- RTK
receptor tyrosine kinase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606663104/DC1.
References
- 1.Briazgounov IP. World Health Stat Q. 1988;41:242–250. [PubMed] [Google Scholar]
- 2.Warburton DE, Nicol CW, Bredin SS. Can Med Assoc J. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker WH, Mullooly JP, Getchell W. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, et al. Circulation. 1997;95:766–770. doi: 10.1161/01.cir.95.4.766. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 6.McMullen JR, Sadoshima J, Izumo S. In: Molecular Mechanisms of Cardiac Hypertrophy and Failure. Walsh RA, editor. London: Taylor & Francis; 2005. pp. 117–136. [Google Scholar]
- 7.Fagard RH. Cardiol Clin. 1997;15:397–412. doi: 10.1016/s0733-8651(05)70348-9. [DOI] [PubMed] [Google Scholar]
- 8.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Proc Natl Acad Sci USA. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 10.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 11.Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, Michelucci A, Colella A, Galanti G. Circ Res. 2001;89:977–982. doi: 10.1161/hh2301.100982. [DOI] [PubMed] [Google Scholar]
- 12.Koziris LP, Hickson RC, Chatterton RT, Jr, Groseth RT, Christie JM, Goldflies DG, Unterman TG. J Appl Physiol. 1999;86:1436–1442. doi: 10.1152/jappl.1999.86.4.1436. [DOI] [PubMed] [Google Scholar]
- 13.Perrino C, Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, Depinho RA, Izumo S, Cantley LC. Mol Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. J Cardio Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 18.Izumo S, Nadal-Ginard B, Mahdavi V. Proc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 20.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, et al. J Biol Chem. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 21.Bueno OF, Molkentin JD. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 22.Jennings GL. Clin Exp Pharmacol Physiol. 1995;22:209–211. doi: 10.1111/j.1440-1681.1995.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 23.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 24.Giannuzzi P, Mezzani A, Saner H, Bjornstad H, Fioretti P, Mendes M, Cohen-Solal A, Dugmore L, Hambrecht R, Hellemans I, et al. Eur J Cardiovasc Prev Rehabil. 2003;10:319–327. doi: 10.1097/01.hjr.0000086303.28200.50. [DOI] [PubMed] [Google Scholar]
- 25.Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. J Am Coll Cardiol. 2006;47:1835–1839. doi: 10.1016/j.jacc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 26.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Circ Res. 2006;98:540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 27.Konstam MA, Udelson JE, Anand IS, Cohn JN. J Cardio Fail. 2003;9:350–353. doi: 10.1054/j.cardfail.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, et al. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Sadoshima J, Trimarco B. Circ Res. 2005;96:180–188. doi: 10.1161/01.RES.0000152968.71868.c3. [DOI] [PubMed] [Google Scholar]
- 31.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Circ Res. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhaesebroeck B, Rohn JL, Waterfield MD. Cell. 2004;118:274–276. doi: 10.1016/j.cell.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 35.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 36.Woodcock EA, Lambert KA. J Mol Cell Cardiol. 1997;29:3275–3283. doi: 10.1006/jmcc.1997.0553. [DOI] [PubMed] [Google Scholar]
- 37.Sambrano GR, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull JT. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.