Abstract
Purpose
To measure selected parameters of energy metabolism and adenosine triphosphate (ATP) production in passaged monolayer cultures of human retinal glial (Müller) cells to assess the effects of varying substrate and oxygen availability on the biochemistry and histologic integrity of these cells.
Methods
Confluent Müller cell cultures were incubated for up to 4 hours at 37°C in a modified minimal essential medium (no serum) under aerobic or mitochondrial-inhibited conditions in the presence and absence of 5 mM glucose or in the presence of lactate, pyruvate, glutamate, or glutamine. Cellular ATP levels, lactic acid production, and 14CO2 production from labeled glucose or glutamate were measured along with an examination of cellular morphology. Immunohistochemistry with antibodies to glial cell–specific proteins was also performed. Cells were positive for vimentin, but negative for glial fibrillary acidic protein and glutamine synthetase.
Results
Human Müller cells maintained ATP content aerobically at the same level for 4 hours in the presence and absence of glucose. ATP content was also maintained anaerobically at a value equal to that found aerobically, but only in the presence of glucose. ATP content in human Müller cells declined to a very low level when glycolysis was blocked by iodoacetate, and inclusion of lactate, pyruvate, glutamate, or glutamine did not restore the level of ATP. Aerobically, lactic acid production accounted for 99% of the total glucose used, whereas the oxidation of glucose by the mitochondria accounted for only 1%. When mitochondria were inhibited with antimycin A, there was only a modest (1.3-fold) increase in the rate of lactic acid production. No significant differences were found in the histologic appearance of the cells after mitochondrial blockade, but there was massive death of cells after inhibition of glycolysis with iodoacetate.
Conclusions
These results suggest that, in the presence of glucose and oxygen, cultured Müller cells obtain their ATP principally from glycolysis and have a low rate of oxygen consumption. This metabolic pattern may spare oxygen for retinal neurons, particularly in the inner nuclear and ganglion cell layers under normal physiological conditions. Furthermore, retinal Müller cells in culture are resistant to anoxia or absence of glucose, which provides a basis for understanding why Müller cells are less susceptible than neurons to ischemia or hypoglycemia.
The principal glial cell in the retina is the radially oriented Müller cell, which extends from the vitreal surface to 50% to 70% of retinal depth. Interest in the physiological properties of Müller cells began many years ago when Faber1 and Miller and Dowling2 first proposed that the b-wave of the electroretinogram (ERG) was generated by the Müller cells. This suggestion was based in part on findings in the central nervous system of the leech and the optic nerve of the frog and Necturus,3 that glial cell depolarizations arise in response to impulse activity in neurons. It is now known that Müller cells express many different types of voltage-gated ion channels4-6 and that their membrane conductance is dominated by potassium channels.7 Müller cells also express a number of receptors for retinal neurotransmitters and contain high-affinity uptake transporters for glutamate and γ-aminobutyric acid (GABA).8-10
It has also been thought that retinal Müller cells provide metabolic support to retinal neurons, because Müller cells are the principal storage site for glycogen.11 In times of metabolic stress (e.g., hypoglycemia) the breakdown of glycogen in the Müller cells could provide critical metabolites (e.g., lactic acid) for use by the deprived neurons. Glucose metabolism has been studied in isolated retinal Müller cells from the juvenile guinea pig, the major product of this metabolism being lactate.12,13 Because of the potential importance of glial–neuronal interactions in the retina and because our present understanding of Müller cell metabolism is limited, the present experiments were undertaken to provide additional information on the metabolic fate of glucose, aerobically and anaerobically, in cultured human retinal glial cells and on the response of Müller cells to hypoglycemia and hypoxia. The electrophysiological properties of ion channels in these passaged human Müller cells have been well documented.14,15
In the present study, measurements are reported of the pathways of glucose metabolism in cultured human Müller cells under aerobic and anaerobic conditions and the effects evaluated of alternative substrates and metabolic inhibitors on the energy status, morphologic integrity, and viability of these cells. Further, a summary model is provided of the relative contributions of lactic acid production and mitochondrial glucose oxidation to adenosine triphosphate (ATP) production in cultures of human Müller cells, and the present results and conclusions in retinal glia are compared with previously published measurements of these pathways of glucose metabolism in whole retina, cerebral astrocytes, peripheral axons, and several cell lines (e.g., HeLa and Madin–Darby canine kidney [MDCK]cells).
Materials and Methods
Cell Cultures
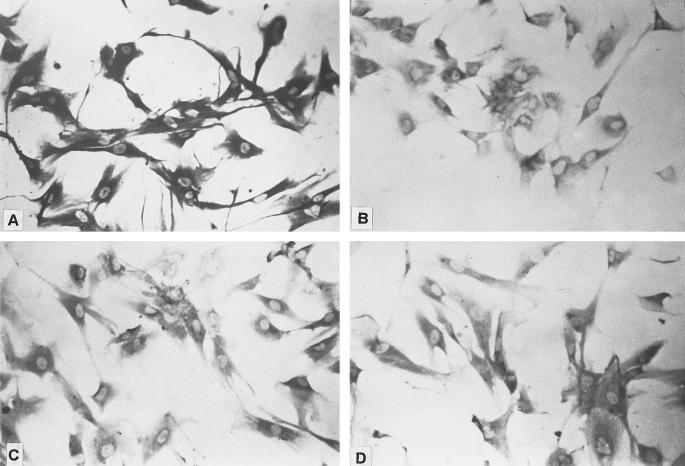
Cultures of human retinal glial cells from donor eyes were supplied within 8 to 16 hours after death by the Michigan Eye Bank and were prepared according to procedures published in detail previously.14-17 Dissociated cells were kept in a humidified environment of 95% O2-5% CO2 at 37°C and fed with a tissue culture medium (a 1:1 ratio of Dulbecco's modified Eagle's medium and Ham's F12 medium) and 20% bovine serum twice per week. When cultures reached 80% to 90% confluence they were split. Cells that had been in culture for three to five passages were used in this study. To help characterize the cultured cells, immunocytochemical staining was performed according to procedures outlined in the peroxidase staining kit (LSAB2; Dako, Carpinteria, CA) using antibodies to vimentin, glial fibrillary acidic protein (GFAP), and glutamine synthetase (GS). The cells were positive for vimentin, but were negative for GFAP and GS (Fig. 1. Immunoreactivity to vimentin is consistent with other studies showing that antibodies to this protein label Müller cells in mouse and rabbit adult retinas.18,19 In contrast, astrocytes in the adult rabbit retina are only weakly labeled with antibodies to vimentin,19 whereas retinal neurons and microglia20 are unreactive. In agreement with the low expression of GFAP-positive cells in rat retinal Müller cells in culture,21 the cultured human glial cells used in the present study did not seem to express GFAP to any appreciable extent, as monitored with a monoclonal antibody to this protein. It is well known that Müller cells in intact retinas also express GS.22,23 However, the absence of GS staining is consistent with the absence in these long-term cultures of neuron-glial interactions, which appear to be required for GS expression by Müller cells.24
Figure 1.
Immunocytochemical staining of human Müller cells with antibodies to vimentin (A), GFAP (B), GS (C), and a negative control (D). Cells show positive reactivity only to an antibody to vimentin.
An advantage of our culture system is the availability of relatively pure populations of Müller cells. As a result, cultures of human Müller cells have been useful in many studies.17,25 Nevertheless, the possibility of changes in these cells during the perimortem period and while in culture requires that in vitro findings concerning human Müller cells ultimately be confirmed in vivo.
Experimental Incubations
To prepare the culture wells for measurements of glucose metabolism, the serum-containing medium was decanted, and the cells were washed three times with serum-free, bicarbonate-buffered minimal essential medium (MEM). Confluent cultures were then incubated in 2 ml of serum-free medium in the presence of varying substrates for different times. The control medium contained 5 mM glucose and an MEM formulation of amino acids, in addition to typical extracellular concentrations of inorganic salts. Other substrates tested included glutamine, glutamate, lactate, and pyruvate. To selectively block the glycolytic pathway, cell cultures were incubated with iodoacetic acid (IAA), a potent inhibitor of triose phospate dehydrogenase.26 To block mitochondrial activity, cells were incubated in media containing 1 × 10−5 M antimycin A.27 In some cases, the medium was supplemented with 14C-glucose or 14C-glutamate to monitor the mitochondrial production of CO2.
Lactate Production and ATP Content of Cultured Glial Cells
These measurements were made using previously published methods, with some minor modifications appropriate for cell cultures versus whole, isolated retinas.27,28 Briefly, 0.05 to 0.1 ml of the media were removed at 30 minute intervals over several hours. Lactate was determined with a lactic acid dehydrogenase– based commercial kit (826-UV; Sigma, St. Louis, MO). Multiple sampling of the medium enabled the determination of the rate of appearance of lactic acid into the medium under the different incubation conditions. ATP content was measured both in fresh cell cultures and in cultures incubated under the experimental regimens. At the end of an incubation, the medium in the culture dish was decanted, and the dish was quickly rinsed three times with ice-cold saline. The saline was decanted, and the cells were scraped and collected into a total volume of 0.6 ml of 5% perchloric acid: two scrapes into successive additions of 0.3 ml of perchloric acid. The suspension of cells was sonicated in the cold for 90 seconds, then centrifuged at 10,000g for 10 minutes. An aliquot of the supernatant was diluted 200-fold, and the ATP content was measured using a firefly luciferase-based spectrofluorometric assay (Turner Systems, Mountain View, CA). Protein was determined with a BCA assay kit (Pierce, Rockford, IL).
Mitochondrial Glucose Oxidation
Cells were grown in special 75-mm2 flasks, each containing an extra side arm capped with a rubber septum. The incubation medium was the same (e.g., serum free) as during the other biochemical experiments except for the addition of 5 mM 14C-3,4 glucose or 1 mM 14C-1 glutamate (specific activity was approximately 50,000 counts per minute/mole for each substrate). Five milliliters of medium was present in each flask. The incubator was equilibrated with 20% O2-5% CO2-75% N2. At the end of the incubations, which lasted from 1 to 4 hours, the reaction was stopped and the 14CO2 released by addition of 1 ml of 2 N H2SO4 through the rubber septum and the 14CO2 collected in 0.5 ml hyamine contained in a vial inserted into the culture flask. Radioactivity was determined in a liquid scintillation spectrometer. Appropriate blanks and background measurements were performed in each experiment.
Enzyme Activities
Measurements were made of selected enzymes of glycolysis and the hexose monophosphate shunt (hexokinase, glyceraldehyde-3-phosphate dehydrogenase ([G3PDH], glucose-6-phosphate dehydrogenase [G6PDH], and lactic acid dehydrogenase [LDH]) and other metabolic enzymes (malate dehydrogenase, aspartate aminotransaminase, glutamate dehydrogenase, and GS). The standard straightforward procedures found in Bergmeyer29 were used for the measurements of all these enzymes except GS. Typically, culture dishes were rinsed three times with saline, 0.6 ml of an appropriate buffer (e.g., 0.1 M NaPO4 or 0.1 M triethanolamine) was added, and cells were scraped and collected in the buffer. The suspension was sonicated and centrifuged at 20,000g for 20 minutes. Aliquots of the supernatant were used for measurements of cytosolic enzyme activities using standard assay constituents and changes in OD340, reflecting an increase or decrease in the concentration of reduced nicotinamide adenine dinucleotide (NADH) or reduced nicotinamide adenine dinucleotide phosphate (NADPH), were monitored to obtain linear rates of reactions. Appropriate blanks (no substrate added) were monitored, and background rates were subtracted from the rates obtained with the substrate. The pellet was resuspended in buffer containing 0.2% Triton-X and was subsequently used for measurements of mitochondrial activities. GS activity was assayed by the method described by Thorndike and Reif–Lehrer30 after sonication and centrifugation of cells in 1 ml of a buffer mixture containing several protease inhibitors (phenylmethylsulfonyl fluoride, pepstatin A, and leupeptin). Inclusion of these inhibitors was necessary to prevent loss in activity of GS during the preparative stages.
Results
Figure 2 shows the production of lactic acid by cultured human retinal Müller cells in the presence and absence of glucose. Aerobic glycolysis was measured over the initial 2 hours. Subsequently, anaerobic conditions were established by adding antimycin A (Fig. 2, arrows), which blocks mitochondrial metabolism. During the next 2 hours, anaerobic lactic acid production was then monitored. In this way, a direct comparison between the rates of aerobic and anaerobic glycolysis could be made using the same cell population.
Figure 2.
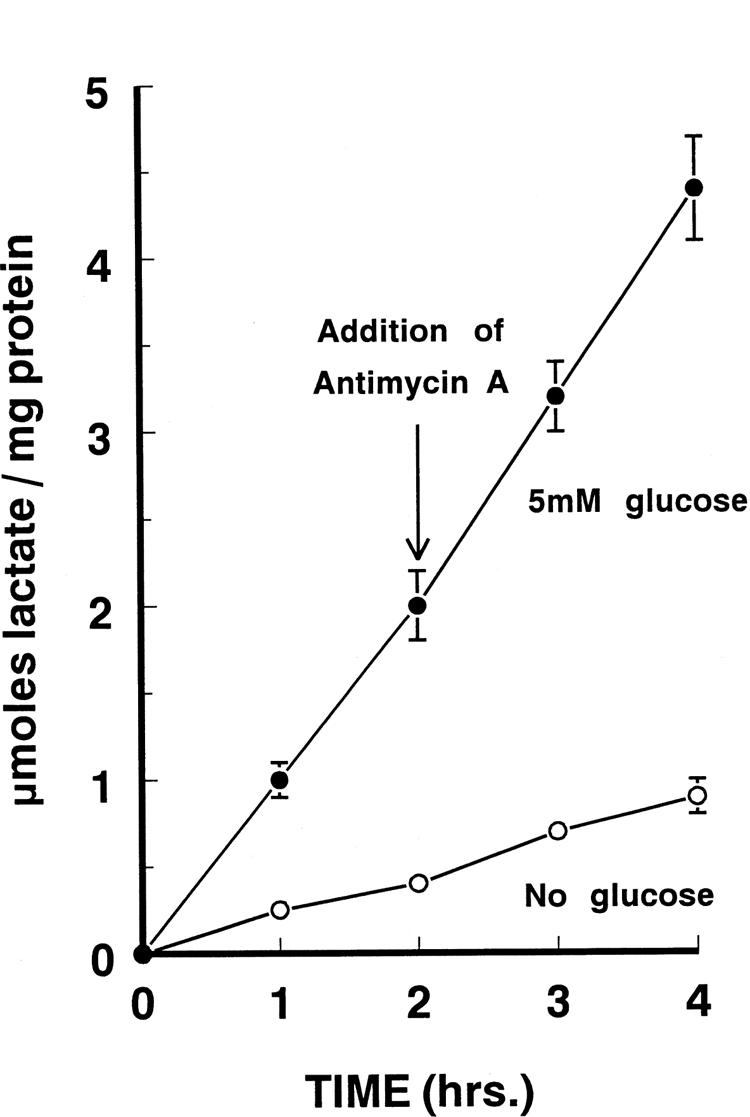
Rates of lactate production by cultured human retinal Müller cells as a function of time of incubation in the presence and absence of glucose. After 2 hours of culture in control media, 1 × 10−5 M antimycin A (final concentration) was added to the medium, and the rate of accumulation of lactate in the media was observed for an additional 2 hours. Each point represents the mean ± SD of 8 to 10 individual experiments (triplicate wells per experiment). When not shown, the SDs were smaller than the size of the symbol. (●), With glucose; (○), without glucose.
In the presence of glucose, the rate of lactic acid produced aerobically was 1.1 micromoles/mg protein per hour. After the addition of antimycin A, the rate of lactate production increased only modestly to 1.4 micromoles/mg protein per hour. Comparison of these rates of lactate production under aerobic and anaerobic conditions yielded a Pasteur effect of 1.3. When glucose was omitted from the incubation medium, lactate production was markedly reduced, both before and after the addition of antimycin A.
We compared the ATP content of cultured glial cells in the presence and absence of glucose. Table 1 shows that after a 4-hour incubation, the cells contained as much ATP aerobically in the presence of glucose as they did in the absence of glucose. With glucose in the bathing solution, the induction of anaerobic conditions did not significantly alter the glial cell content of ATP. However, the ATP content in these cells was depleted when glucose was absent and oxidative phosphorylation was inhibited with antimycin A. We also observed that inhibition of glycolysis by 0.1 mM iodoacetate (IAA) caused the ATP content to decline to a very low level in the absence or presence of glucose. Further evidence that oxidative metabolism plays only a small role in these glia is that the addition of substrates such as glutamine, glutamate, lactate, or pyruvate did not significantly increase the ATP content in the presence of IAA (Table 2).
Table 1.
ATP Content in Cultured Human Retinal Glia
| Condition | ATP (nanomoles/mg protein) |
|---|---|
| 5 mM glucose | 15.5 ± 2.0 |
| 5 mM glucose + 0.01 mM antimycin A | 14.8 ± 1.5 |
| 5 mM glucose + 0.1 mM IAA | 0.2 ± 0.1 |
| No glucose | 15.8 ± 1.9 |
| No glucose + 0.01 mM antimycin A | 0.1 ± 0.1 |
| No glucose + 0.1 mM IAA | 0.1 ± 0.1 |
Each value represents an average of at least six individual experiments with triplicate culture dishes per experiment. Duration of incubation, 4 hours. Data are means ± SD.
Table 2.
ATP Content in Cultured Human Retinal Glia as a Function of Exogenous Substrate
| ATP (nanomoles/mg protein) |
||
|---|---|---|
| Substrate | −IAA | +IAA* |
| None | 15.8 | 0.1 |
| 5 mM glucose | 15.5 | 0.2 |
| 1 mM glutamine | 15.4 | 0.2 |
| 1 mM glutamate | 15.0 | 0.2 |
| 5 mM lactate | 15.7 | 0.3 |
| 5 mM pyruvate | 15.2 | 0.2 |
Each value represents an average of at least six individual experiments with triplicate culture dishes per experiment. Duration of incubation, 4 hours.
0.1 mM.
In another set of experiments, we assessed the relative rates of lactate versus CO2 produced from labeled glucose or glutamate in cultured retinal glial cells. It was found that 14C-3,4 glucose was converted to 14CO2 at a very low rate (e.g., 0.006 ± 0.001 micromoles/mg protein per hour; n = 6), compared with the production of lactate (see Fig. 2). A similar low rate of mitochondrial oxidation (0.008 ± 0.002 micromoles/mg protein per hour; n = 6)) was found when 1 mM 14C-1 glutamate was the labeled substrate.
To further characterize the metabolic profile of these glia, we assayed the activities of selected enzymes of metabolism in cultured human retinal glial cells (Table 3). LDH and G3PDH had the highest specific activities in the cytosol. Relative to these high activities, malate dehydrogenase showed intermediate activity, and the activities of the other enzymes tested were extremely low. Of the enzymes found in the mitochondrial fraction, the order of activity was malate dehydrogenase, glutamate dehydrogenase, and aspartate aminotransaminase. GS activity was not detected in the cultures of human Müller cells used in this study.
Table 3.
Activities of Selected Enzymes of Glucose and Glutamate Metabolism in Cultured Human Glia
| Subcellular Fraction |
||
|---|---|---|
| Enzyme | Cytosol | Mitochondria |
| G3PDH | 1.60 ± 0.25 | — |
| Hexokinase | 0.04 ± 0.01 | — |
| LDH | 1.30 ± 0.20 | — |
| G6PDH | 0.03 ± 0.01 | — |
| Malate dehydrogenase | 0.44 ± 0.08 | 0.22 ± 0.07 |
| Aspartate aminotransaminase | 0.04 ± 0.01 | 0.03 ± 0.01 |
| Glutamate dehydrogenase | ND | 0.07 ± 0.02 |
| GS | ND | — |
Activities expressed as micromoles per minute per milligram protein; means ± SD. Each value is average of at least three experiments with triplicate culture dishes per experiment. ND, not detected.
Consistent with the finding that these glia were not dependent on oxidative metabolism, we found that the Müller cells were well maintained in the presence of antimycin A and glucose (Fig. 3). The morphologic appearance of the Müller cells was examined after 4-hour incubations with glucose. However, when glycolysis was inhibited with IAA, we observed pyknotic nuclei and other signs of cell death in virtually all the cultured glial cells. Consistent with the requirement for glycolysis, severe cellular disruption was also found when the cells were incubated for 4 hours with IAA and each of the various respiratory substrates tested in Table 2.
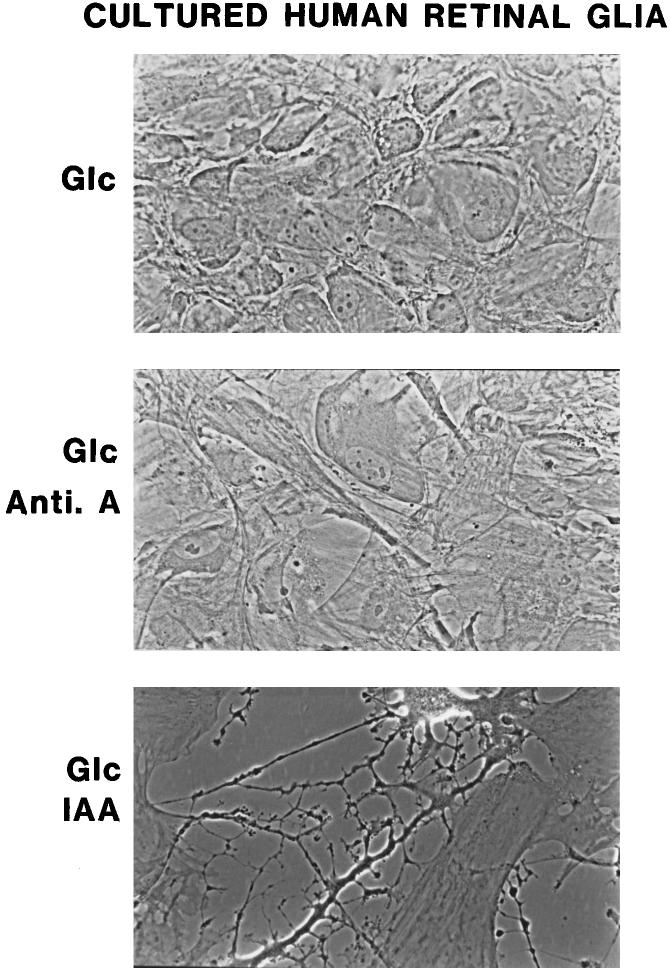
Figure 3.
Mophologic appearance of cultures of human Müller cells. Top: Cells were incubated with glucose (glc, control culture) and show typical well-preserved appearance of confluent cells used for all the metabolic studies. Middle: The appearance of Müller cells after a 4-hour incubation with glucose and 1 × 10−5 M antimycin A. Cells are clearly resistant to blockade of respiration. Bottom: Müller cells are significantly damaged ,and there is substantial loss of cells after incubation of the Müller cells for 4 hours with glucose and 0.1 mM IAA, an inhibitor of glycolysis.
Discussion
A characteristic feature of cerebral astrocytes in culture is their high rate of glucose utilization and lactate formation in the presence of oxygen.31-35 The predominant glial cell in the retina, the Müller cell, shares this metabolic feature. Thus, it has been shown that Müller cells in the guinea pig retina take up and phosphorylate glucose 36 and that acutely isolated Müller cells metabolize glucose aerobically primarily to lactate.13 The present results confirm and extend this work by showing that in cultured human Müller cells aerobic glycolysis is the major pathway of glucose metabolism. On the assumption that lactate, produced from glycolysis (1.1 micromoles/mg protein per hour), and CO2, produced from the mitochondrial oxidation of glucose (0.006 micromoles/mg protein per hour), are the only products of glucose metabolism in these cells, we calculated that under normal conditions of glucose and oxygen availability aerobic glycolysis accounts for 99% of total glucose utilization and mitochondrial glucose oxidation accounts for only 1%. It is recognized that this calculation represents an upper limit for the contribution of glycolysis to total glucose utilization, because guinea pig Müller cells have been shown to convert glucose also to amino acids.13 However, this latter activity is low in comparison with the total glycolytic activity in those cells. Therefore, aerobic glycolysis represents the primary pathway for glucose metabolism in Müller cells.
We propose that the significance of this metabolic pattern in human Müller cells is that it spares oxygen for consumption by retinal neurons, particularly in the inner nuclear and ganglion cell layers under normal physiological conditions. Further, as previously proposed,13,37 the lactate produced by the Müller cells may serve as a metabolic substrate used by surrounding retinal neurons.
The present results have also shown that human Müller cells are resistant to mitochondrial blockade. Thus, there is no significant difference between averaged ATP content in cells incubated under the control condition in comparison to the level of ATP found in cells incubated for 4 hours with antimycin A, a potent inhibitor of mitochondrial electron transport. Consistent with the maintenance of normal ATP levels in cells exposed to antimycin A is the finding that these cells are also indistinguishable morphologically from control cells. It is important to note that the preservation of ATP content and morphology in mitochondria-inhibited human Müller cells depends on the continued high production of lactic acid under the anaerobic condition, because inhibition by IAA of triose phosphate dehydrogenase, a glycolytic enzyme of particularly high specific activity (see Table 3), leads to the loss of ATP and death of human Müller cells within several hours.
It is of additional interest that cultured human Müller cells maintained their ATP content aerobically for many hours in the absence of any exogenous substrate (Tables 1 and 2). Clearly, one or more endogenous substrates are available to support ATP synthesis. That aerobic ATP content declined to a low level after inhibition of glycolysis with IAA suggests that ATP production is supported by endogenous hexose stores (i.e., glycogen). This appears to be the case even when noncarbohydrate substrates (amino acids) are included in the incubation media. In the absence of glucose, the rate of aerobic lactic acid production declined to a very low level. Accordingly, it is reasonable to conclude that under this condition the endogenous substrate is oxidized by the Müller cell mitochondria at a rate sufficient to maintain ATP content over many hours. This conclusion is consistent with the finding that only in the presence of oxygen are human Müller cells capable of maintaining their ATP content in the absence of an exogenous substrate. In this regard, human Müller cells appear to be a classic example of the Crabtree effect38: In the presence of glucose, respiration is inhibited, but if glycolysis is depressed by omitting glucose from the medium, respiration is stimulated. Indeed, the Crabtree effect has been observed only in tissues that have a high glycolytic capacity (e.g., mammalian retina and cancer cells).
It should be pointed out that although lactic acid is the major end product of glucose metabolism in cultured cerebral astrocytes,31-35 these cells39-41 also oxidize glucose and glutamate to CO2 under normal conditions at rates considerably higher than their rates of oxidation in human Müller cells in the present experiments. This suggests that the contribution of respiration to normal energy metabolism is quantitatively more important in cerebral glial cells than in retinal glial (Müller) cells.
Relevance to Retinal Ischemia
Our findings showing that cultured human Müller cells oxidize glucose at a low rate, generate most of their ATP from glycolysis, and are resistant to mitochondrial inhibition have relevance to certain manifestations of retinal ischemia. From a clinical standpoint, the insult most often associated with retinal ischemia is the absence of oxygen, in large part because of the high oxygen consumption of retinal neurons. Ischemic episodes of variable duration have been produced in vivo by elevation of intraocular pressure to a level higher than systolic pressure, thereby occluding choroidal and retinal blood flows. For example, Reinecke et al.42(p474) induced retinal ischemia in cats and reported that “retinas studied 48 hours after prolonged (90 minutes) ischemia showed progressive lysis and disappearance of the ganglion cells, followed by the rods and cones, the rod and cone nuclei, and bipolar cells, in that order.” Widespread damage to all populations of retinal neurons in vivo has also been reported after 60 to 90 minutes of ischemia in rats43 and rabbits.44 In contrast to the substantial neuronal degeneration observed in these retinas after an increase in intraocular pressure, Hughes43(p577) noted that “there appeared to be sparing of Müller cells in the inner nuclear layer,” and Johnson and Foulds44(p 52) stated that “the nuclei of the Müller cells appeared highly resistant to ischemia, being unaffected by even the longest period (120 minutes) of ischemia used.”
A higher resistance of Müller cells (and astrocytes) relative to retinal neurons was also found in owl monkeys after ischemia.45 These results are consistent with other findings showing that glial cells in the central nervous system are more resistant than neurons to anoxia or hypoglycemia.46 A simple explanation for the ability of retinal Müller cells to survive ischemia in vivo is that glycolysis generates enough ATP to maintain cellular viability, an activity that depends on adequate amounts of utilizable, endogenous carbohydrate (glycogen) stores in these cells.47 This is not likely to be the entire explanation, however, given the complexity of events associated with ischemia and reperfusion (e.g., local pH shifts, excitotoxic reactions and the generation of oxygen free radicals, to cite just a few examples). Indeed, Müller cells respond to ischemia in vivo by expressing GFAP,48 an effect that is typically viewed as a response to neuronal injury.49-52 Moreover, it has been suggested53 that stress to Müller cells caused by exposure to excitatory amino acids requires extensive interactions between neurons and Müller cells. Accordingly, Müller cells appear to possess mechanisms that enable them to withstand reasonably well both the direct effects of ischemia and the indirect effects that involve degenerative changes in neighboring retinal neurons.
Concluding Remarks on Glucose Metabolism in Retinal Müller Cells, Retinal Neurons, and Other Cells and Tissues
Just how much does aerobic glycolysis contribute to overall ATP production in cultured human Müller cells under the normal condition of glucose and oxygen availability? And what is its contribution in other cells and tissues, for comparison? To address these questions, we start with the fact that glycolysis yields 2 moles ATP per mole of glucose metabolized, and respiration yields 36 moles ATP per mole of glucose oxidized completely. This 18-fold difference in the efficiencies of these two pathways is at the core of the generally accepted belief that cells obtain virtually all their ATP from respiration and that in most species, tissues and organs have an absolute dependence on oxygen. This ratio of ATP production from respiration and glycolysis is true for the particular case in which the two molecules of pyruvate produced from one molecule of glucose are fully oxidized by the mitochondria (i.e., no lactate is produced in the respiring cell or tissue). This is the standard example most often cited in biochemistry texts, and leads to the view that in the grand scheme of energy production the glycolytic pathway is little more than the prelude to the main act of mitochondrial glucose oxidation. However, this is clearly not the case for Müller cells, nor is it the case for other cells and tissues that also produce lactic acid aerobically.
What is the meaning of these quantitative differences in the glycolytic and oxidative breakdown of glucose and how do these differences help us to understand why certain types of cells and tissues absolutely depend on oxygen for survival, whereas others do not? Figure 4 is a simple, straightforward attempt to provide a functional perspective on the contribution of glycolysis (and, by inference, respiration) to total cellular ATP production. Our assumptions are that the total amount of glucose used per unit of time is constant as the percentage of metabolized glucose converted to lactate varies from 0% to 100% and that the pyruvate not so used enters the mitochondria and is completely oxidized. Thus, because more lactate is formed, less pyruvate is available for oxidation by the mitochondria. The anaerobic case is defined for the condition when 100% of the pyruvate molecules are converted to lactate, and the ATP produced comes only from glycolysis. This definition is applied universally to all cells, tissues, and organisms, and it is easy to understand. However, the aerobic case is not as easy to define, because it represents the continuum of varying percentages (0%–99.9%) of glucose metabolized to lactate, and cells and tissues behave quite differently in terms of the relative rates of glycolysis and mitochondrial glucose oxidation.
Figure 4.
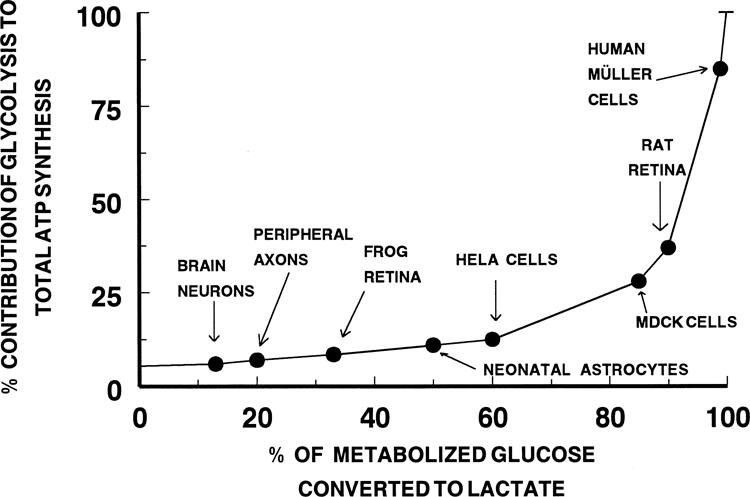
Glycolytic versus respiratory source of ATP. Theoretically derived curve of the fractional contribution of glycolysis to ATP generation based on the ratio of 2 glycolytic to 36 respiratory moles of ATP per mole of glucose metabolized. The percentage contribution of respiration to total ATP synthesis is the inverse of the ordinate. The data points (●) for brain neurons54 and peripheral axons,58 frog55 and rat27 retinas, and the four cell types (e.g., neonatal astrocytes,41 HeLa cells,56 MDCK,57 and human Müller cells [present study]) are placed on the curve according to their experimentally determined rates of glucose metabolism by the glycolytic and respiratory pathways.
Thus, the aerobic definition is tissue specific. It depends on the rates of the specific pathways of glucose metabolism, which are not always easy to establish. In the situation in which all the pyruvate molecules are oxidized (i.e., 0% converted to lactate) the glycolytic contribution to total ATP production is 5.5%, or one eighteenth, of the ATP coming from oxidation. As can be seen, the contribution of glycolysis to total ATP production increases very slowly as the amount of lactate formed aerobically increases to 80% of the total glucose used. Only when the amount of lactate produced from glucose aerobically equals 95% of the total glucose used (i.e., an 18-to-1 ratio, does the contribution of glycolysis to total ATP production exceed 50%.
Figure 4 includes data points for brain tissue54 and whole frog55 and rat retinas27 which are in very different regions of the continuum, as well as for several different types of cells maintained as monolayer cultures.41,56-58 Brain tissue under normal oxygenated conditions produces a small amount of lactate aerobically (13% of total glucose used), obtaining 6% of its ATP from aerobic glycolysis and 94% from respiration. On this basis, it is perhaps easy to understand why brain tissue is so sensitive to impairment of respiration. To make up for the loss in respiratory ATP, brain neurons must increase the rate of glucose consumption by 16.6-fold to produce the same amount of ATP as in the aerobic condition. The rapid vulnerability of brain neurons to absence of oxygen suggests that brain tissue fails to upregulate glycolysis sufficiently to compensate for the loss of respiratory ATP. In contrast, the rat retina sits at a point on the curve where 90% of the glucose used aerobically is converted to lactate and 36% of the ATP produced comes from glycolysis.27 When a rat retina is deprived of oxygen, anaerobic glycolysis increases approximately twofold (Pasteur effect), and ATP levels are maintained at 50% to 70% of the aerobic level.27,59 The resistance of this tissue to anoxia is linked to the relatively modest upregulation of glycolysis that is necessary to generate compensatory amounts of ATP. A somewhat similar situation is seen with human Müller cells in which a modest increase in glycolysis by 30% is all that is necessary to maintain ATP content after inhibition of the mitochondria. When glycolysis is the only ATP-generating pathway in MDCK cells,57 net ATP production is maintained at 80% of the control, aerobic rate. The frog retina represents an extreme case, because this tissue maintains its ATP content anaerobically equal to that found aerobically by upregulating its glycolytic activity by 8.5-fold when the mitochondria are inhibited.60
The normalized graph in Figure 4 is both useful and informative, although its application is limited to those cells and tissues that use glucose as the primary substrate for mitochondrial metabolism or produce a high amount of lactate aerobically. It is also important to point out that under the assumption of a fixed rate of total glucose used the absolute amount of total ATP produced decreases as the percentage of metabolized glucose converted to lactate increases. This decrease in ATP does not affect the implications and conclusions of the perspective offered in Figure 4, because this change in ATP produced is a straightforward result of the inefficiency of glycolysis in producing ATP. What can be inferred is that if the mammalian retina and brain tissue were producing ATP at the same rate per unit of time and per unit of tissue (e.g., wet weight or protein basis), the rate of glucose utilization by the retina would be nearly six times faster than glucose utilization by the brain. Further, for stimulated brain tissue to increase ATP synthesis from glycolysis by 25%, a 5.2-fold increase in glucose consumption would be needed, whereas if this 25% increase in ATP synthesis came only from mitochondrial glucose oxidation then only a 23% increase in glucose consumption would be needed. Finally, the extent to which pyruvate is incompletely oxidized to CO2 by the Krebs cycle (loss of carbon atoms to amino acids, for example) influences the validity of using the standard 36 moles of ATP/2 moles pyruvate oxidized in the calculation in Figure 1. Any reduction in this number results in an apparent increase in the relative efficiency of glycolysis vis-a-vis oxidation in producing ATP. At present, our understanding of this latter issue is incomplete.
Footnotes
Supported in part by National Eye Institute Grants EY 10015 (BSW), EY 06931 (DGP), EY05230 (Core, Eye Research Institute) and EY 00785 (Core, Kellogg Eye Institute). DGP is a Research to Prevent Blindness Senior Scientific Investigator.
Commercial relationships policy: N.
References
- 1.Faber DF. Analysis of slow transretinal potentials in response to light. State University of New York at Buffalo; 1969. PhD thesis. [Google Scholar]
- 2.Miller RF, Dowling JE. Intracellular responses in the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electro-retinogram. J Neurophysiol. 1970;33:323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- 3.Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond. B Biol Sci. 1967;168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 4.Newman EA. Membrane physiology of retinal glial (Müller) cells. J Neurosci. 1985;5:2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman EA, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 6.Puro DG, Stuenkel EL. Thrombin-induced inhibition of potassium currents in human retinal glial (Müller) cells. J Physiol. 1995;485:337–348. doi: 10.1113/jphysiol.1995.sp020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman EA. Distribution of potassium conductance in mammalian Müller (glial) cells: a comparative study. J Neurosci. 1987;7:2423–2432. [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz EA. L-glutamate conditionally modulates the K current of Müller glial cells. Neuron. 1993;10:1141–1149. doi: 10.1016/0896-6273(93)90062-v. [DOI] [PubMed] [Google Scholar]
- 9.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;325:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz EA, Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the Salamander retina. J Physiol. 1990;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwabara T, Cogan D. Retinal glycogen. Arch Ophthalmol. 1961;66:96–104. doi: 10.1001/archopht.1961.00960010682013. [DOI] [PubMed] [Google Scholar]
- 12.Poitry–Yamate CL, Tsacopolous M. Glucose metabolism in freshly isolated Müller glial cells from a mammalian retina. J Comp Neurol. 1992;320:257–266. doi: 10.1002/cne.903200209. [DOI] [PubMed] [Google Scholar]
- 13.Poitry–Yamate CL, Poitry S, Tsacopolous M. Lactate released by Müller cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puro DG, Mano T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. J Neurosci. 1991;11:1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puro DG, Yuan JP, Sucher NJ. Activation of NMDA receptor-channels in human retinal Müller cells inhibits inward-rectifying potassium currents. Vis Neurosci. 1996;13:319–326. doi: 10.1017/s0952523800007562. [DOI] [PubMed] [Google Scholar]
- 16.Puro DG, Mano T, Chan C-C, Fukuda M, Shimada H. Thrombin stimulates the proliferation of human retinal glial cells. Graefes Arch Clin Exp. Ophthalmol. 1990;19:169–173. doi: 10.1007/BF00935728. [DOI] [PubMed] [Google Scholar]
- 17.Puro DG. Calcium channels of human retinal glial cells. Methods Neurosci. 1994;19:68–81. [Google Scholar]
- 18.Drager UC, Edwards DL, Barnstable CJ. Antibodies against filamentous components in discrete cell types of the mouse retina. J Neurosci. 1984;4:2025–2042. doi: 10.1523/JNEUROSCI.04-08-02025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnitzer J. Immunocytochemical studies on the development of astrocytes, Müller (glial) cells and oligodendrocytes in the rabbit retina. Dev Brain Res. 1988;44:59–72. doi: 10.1016/0165-3806(88)90118-6. [DOI] [PubMed] [Google Scholar]
- 20.Gariano RF, Sage EH, Kaplan HJ, Hendrickson AE. Development of astrocytes and their relation to blood vessels in fetal monkey retina. Invest Ophthalmol Vis Sci. 1996;37:2367–2375. [PubMed] [Google Scholar]
- 21.Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro, I: an improved method for isolation and culture. Exp Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 22.Riepe RE, Norenberg MD. Müller cell localization of glutamine synthetase in rat retina. Nature. 1977;268:654–655. doi: 10.1038/268654a0. [DOI] [PubMed] [Google Scholar]
- 23.Lewis GP, Erickson PA, Kaska DD, Fisher SK. An immunocytochemical comparison of Müller cells and astrocytes in the cat retina. Exp. Eye Res. 1988;47:839–853. doi: 10.1016/0014-4835(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 24.Linser PJ, Moscona AA. Induction of glutamine synthetase in embryonic neural retina: Its suppression by the gliatoxic agent α-aminoadipic acid. Dev Brain Res. 1981;1:103–119. doi: 10.1016/0165-3806(81)90097-3. [DOI] [PubMed] [Google Scholar]
- 25.Puro DG. Müller cells: Dynamic components of the retina. In: Toyota J, Murakami M, Kaneko A, Sito T, editors. The Retinal Basis of Vision. Elsevier; Amsterdam: 1999. pp. 233–248. [Google Scholar]
- 26.Cohen LH, Noell WK. Glucose catabolism of rabbit retina, before and after development of visual function. J Neurochem. 1960;5:253–276. doi: 10.1111/j.1471-4159.1960.tb13363.x. [DOI] [PubMed] [Google Scholar]
- 27.Winkler BS. A quantitative assessment of glucose metabolism in the isolated rat retina. In: Christen Y, Doly M, Droy–Lefaix M-T, editors. Les Seminaires ophtalmologiques dIPSEN: Vision et Adaptation. Elsevier; Amsterdam: 1995. pp. 78–96. [Google Scholar]
- 28.Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmeyer HU. Methods of Enzymatic Analysis. 2nd ed. 1–4. Academic Press; New York: 1974. [Google Scholar]
- 30.Thorndike J, Reif–Lehrer L. A sensitive assay for glutamyl transferase. Enzyme. 1971;12:235–241. doi: 10.1159/000459536. [DOI] [PubMed] [Google Scholar]
- 31.Pauwels PJ, Opperdoes FR, Trouet A. Effects of antimycin, glucose deprivation and serum on cultures of neurons, astrocytes and neuroblastoma cells. J Neurochem. 1985;44:143–148. doi: 10.1111/j.1471-4159.1985.tb07123.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu A, Hertz L. Metabolic sources of energy in astrocytes. In: Hertz L, Kvamme E, McGeer EG, editors. Glutamine, Glutamate and GABA in the Central Nervous System. Alan R. Liss; New York: 1983. pp. 431–438. [Google Scholar]
- 33.Walz W, Murkerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1:366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- 34.Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- 35.Swanson RA, Benington JH. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18:515–521. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- 36.Poitry–Yamate C, Tsacopolous M. Glial (Müller) cells take up and phosphorylate 3H-2-deoxy-D-glucose in a mammalian retina. Neurosci Lett. 1991;122:241–244. doi: 10.1016/0304-3940(91)90868-t. [DOI] [PubMed] [Google Scholar]
- 37.Tsacopolous M, Poitry–Yamate CL, MacLeish PR, Poitry S. Trafficking of molecules and metabolic signals in the retina. Prog Retinal Eye Res. 1998;17:429–442. doi: 10.1016/s1350-9462(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 38.Sussman I, Erecinska M, Wilson DF. Regulation of cellular energy metabolism: the Crabtree effect. Biochim Biophys Acta. 1980;591:209–223. doi: 10.1016/0005-2728(80)90153-x. [DOI] [PubMed] [Google Scholar]
- 39.Yu A, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982;39:954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- 40.McKenna MC, Tildon JT, Stevenson JH, Huang X. New insights into the compartmentation of glutamate and glutamine in cultured rat brain astrocytes. Dev. Neurosci. 1996;18:380–390. doi: 10.1159/000111431. [DOI] [PubMed] [Google Scholar]
- 41.Lopes-Cardozo M, Larsson OM, Schousboe A. Acetoacetate and glucose as lipid precursors and energy substrates in primary cultures of astrocytes and neurons from mouse cerebral cortex. J Neurochem. 1986;46:773–778. doi: 10.1111/j.1471-4159.1986.tb13039.x. [DOI] [PubMed] [Google Scholar]
- 42.Reinecke RD, Kuwabara T, Cogan DG, Weis DR. Retinal vascular patterns, Part V: Experimental ischemia of the cat eye. Arch Ophthalmol. 1962;67:470–475. doi: 10.1001/archopht.1962.00960020470015. [DOI] [PubMed] [Google Scholar]
- 43.Hughes WF. Quantitation of ischemic damage in the rat retina. Exp Eye Res. 1991;53:573–582. doi: 10.1016/0014-4835(91)90215-z. [DOI] [PubMed] [Google Scholar]
- 44.Johnson NF, Foulds WS. The effects of total acute ischaemia on the structure of the rabbit retina. Exp Eye Res. 1978;27:45–59. doi: 10.1016/0014-4835(78)90052-0. [DOI] [PubMed] [Google Scholar]
- 45.Anderson DR, Davis EB. Sensitivities of ocular tissues to acute pressure-induced ischemia. Arch Ophthalmol. 1975;93:267–274. doi: 10.1001/archopht.1975.01010020277006. [DOI] [PubMed] [Google Scholar]
- 46.Silver IA, Deaa J, Erecinska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience. 1997;78:589–601. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno K, Sato K. Reassessment of histochemistry of retinal glycogen. Exp Eye Res. 1975;21:489–497. doi: 10.1016/0014-4835(75)90130-x. [DOI] [PubMed] [Google Scholar]
- 48.Kim IB, Kim KY, Joo CK, et al. Reaction of Müller cell after increased intraocular pressure in the rat retina. Exp. Brain Res. 1998;121:419–424. doi: 10.1007/s002210050476. [DOI] [PubMed] [Google Scholar]
- 49.Bignami A, Dahl D. The radial glial of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28:62–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 50.Eisenfeld AJ, Bunt-Milan AH, Sarthy PV. Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:1321–1328. [PubMed] [Google Scholar]
- 51.Erickson PA, Fisher SK, Guerin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res. 1987;44:37–48. doi: 10.1016/s0014-4835(87)80023-4. [DOI] [PubMed] [Google Scholar]
- 52.Burns MS, Robles M Müller cell GFAP expression exhibits gradient from focus of photoreceptor light damage. Curr Eye Res. 1990;9:479–486. doi: 10.3109/02713689008999613. [DOI] [PubMed] [Google Scholar]
- 53.Heidinger V, Dreyfus H, Sahel J, Christen Y, Hicks D. Excitotoxic damage of retinal glial cells depends upon normal neuron-glial interactions. Glia. 1998;23:150–159. doi: 10.1002/(sici)1098-1136(199806)23:2<146::aid-glia6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Clarke DD, Lajtha AL, Maker HS. Intermediary metabolism. In: Siegel G, Agranoff B, Albers RW, Molinoff P, editors. Basic Neurochemistry. Vol. 541. Raven Press; New York: 1989. p. 564. [Google Scholar]
- 55.Sickel W. Retinal metabolism in dark and light. In: Fuortes MGF, editor. Handbook of Sensory Physiology: Physiology of Photoreceptor Organs. VII/2. Springer–Verlag; Berlin: 1972. pp. 667–727. [Google Scholar]
- 56.Stanisz J, Wice BM, Kennell DE. Comparative energy metabolism in cultured heart muscle and HeLa cells. J Cell Physiol. 1983;115:320–330. doi: 10.1002/jcp.1041150316. [DOI] [PubMed] [Google Scholar]
- 57.Lynch RM, Balaban RS. Energy metabolism of renal cell lines, A6 and MDCK: regulation by Na-K ATPase. Am J Physiol. 1987;252:C225–C231. doi: 10.1152/ajpcell.1987.252.2.C225. [DOI] [PubMed] [Google Scholar]
- 58.Greene DA, Winegrad AI. Effects of acute experimental diabetes on composite energy production metabolism in peripheral axons and Schwann cells. Diabetes. 1981;30:967–974. doi: 10.2337/diab.30.11.967. [DOI] [PubMed] [Google Scholar]
- 59.Winkler BS, Arnold MJ, Brassell M, Sliter D. Glucose dependence of glycolysis, hexosemonophosphate shunt activity, energy status, and the polyol pathway in retinas isolated from normal (non-diabetic) rats. Invest Ophthalmol Vis Sci. 1997;38:62–71. [PubMed] [Google Scholar]
- 60.Fliesler SJ, Richards, McKay S, Winkler BS. In vitro metabolic competence of the frog retina: effects of glucose and oxygen deprivation. Exp Eye Res. 1997;64:683–692. doi: 10.1006/exer.1996.0281. [DOI] [PubMed] [Google Scholar]