Abstract
Over the past 20 years children have benefited from major improvements in both technology and clinical management of dialysis. Morbidity during dialysis sessions has decreased with seizures being exceptional and hypotensive episodes rare. Pain and discomfort have been reduced with the use of chronic internal jugular venous catheters and anesthetic creams for fistula puncture. Non-invasive technologies to assess patient target dry weight and access flow can significantly reduce patient morbidity and health care costs. The development of urea kinetic modeling enables calculation of the dialysis dose delivery, Kt/V, and an indirect assessment of the intake. Nutritional assessment and support are of major importance for the growing child. Even if the validity of these “urea only” data is questioned, their analysis provides information useful for follow-up. Newer machines provide more precise control of ultrafiltration by volumetric assessment and continuous blood volume monitoring during dialysis sessions. Buffered bicarbonate solutions are now standard and more biocompatible synthetic membranes and specific small size material dialyzers and tubing have been developed for young infants. More recently, the concept of “ultrapure” dialysate, i.e. free from microbiological contamination and endotoxins, has developed. This will enable the use of hemodiafiltration, especially with the on-line option, which has many theoretical advantages and should be considered in the case of maximum/optimum dialysis need. Although the optimum dialysis dose requirement for children remains uncertain, reports of longer duration and/or daily dialysis show they are more effective for phosphate control than conventional hemodialysis and should be considered at least for some high-risk patients with cardiovascular impairment. In children hemodialysis has to be individualized and viewed as an “integrated therapy” considering their long-term exposure to chronic renal failure treatment. Dialysis is seen only as a temporary measure for children compared with renal transplantation because this enables the best chance of rehabilitation in terms of educational and psychosocial functioning. In long term chronic dialysis, however, the highest standards should be applied to these children to preserve their future “cardiovascular life” which might include more dialysis time and on-line hemodiafiltration with synthetic high flux membranes if we are able to improve on the rather restricted concept of small-solute urea dialysis clearance.
Keywords: Hemodialysis, Children, Guidelines
Introduction
The European Paediatric Dialysis Working Group was established in 1999 by pediatric nephrologists from different European countries with a major interest in dialysis. The group has already published guidelines, mainly on peritoneal dialysis [1, 2]. Hemodialysis practices for children have improved over the ensuing 20 years, especially because of technological developments and the evolution from “minimum/adequate” to “optimum/maximum” dialysis prescription [3]. Therefore, new general recommendations seem necessary.
These guidelines were initiated and discussed at meetings of the group and refined by e-mail discussion to develop a consensus of opinion, on the basis of cumulative clinical experience and reported studies. This paper will discuss the main factors affecting hemodialysis prescription and management in children.
In some European countries hemodialysis (HD) is often preferred for children over the age of five years [3]. In contrast, peritoneal dialysis (PD) is offered to the younger children especially under the age of two years or weighing less than 10 kg. A multicenter European study has, however, found that 73% of 189 children were older than five years when peritoneal dialysis was started [4]. Factors ranked as first priority for choice of therapy [4] included age of the child (30%), parent choice (27%), distance from unit (14%), patient choice (11%), social condition (7%), and unable to do one mode (6%). Nevertheless important differences appear in the individual countries. Usually, however, HD is not offered to children less than 5 years old unless there are important contra-indications for PD [1]. For older children HD is applied for drop-outs from the PD program or if there are medical (rare) or psychosocial (more often) reasons for not performing PD.
Choosing a mode of dialysis, either HD or PD, for a child requires consideration, among other factors, of the probable impact of either mode of dialysis on the maintenance of residual renal function (RRF), because of its specific impact on patient outcome. Although there is no general consensus, peritoneal dialysis has been associated with less risk of RRF loss [5, 6]. Overall the choice of the mode of dialysis is just a part of the integrated care model, each child should be considered for a combined dialysis-transplantation program.
Provision of adequate vascular access remains the single greatest obstacle to successful HD, especially in infants. Unlike in the USA, where patients frequently use a central catheter for vascular access [7], in Europe an arteriovenous fistula is the most common vascular access for chronic/long term dialysis [8]. According to the K-DOQI guidelines, the percentage of catheters in a dialysis unit for adults should be less than 10%, although many pediatric centers do not meet this standard, because of the difficulty of creating fistulas in smaller children, especially in children less than 2 years of age.
During the past two decades there have been many improvements in the technology [3]: bicarbonate used as buffer in the dialysis solution, volumetrically controlled ultrafiltration, smaller dialysis lines and synthetic membranes useful even for babies, modeling of ultrafiltration rate and dialysate composition, on line hemodiafiltration and the concept of ultrapure dialysate, i.e. sterile and pyrogen free. Non invasive technologies to assess patient target dry weight and access flow offer a potential decrease in dialysis morbidity and costs [9]. Recently marketed medications to improve anemia, for example erythropoïetin even darbepoietin, and iron infusion, contribute to the clinical improvement of the hemodialysis session [10].
Dialysis adequacy quantification by urea kinetic modeling enables a more specific approach to dialysis dosing and indirect assessment of protein intake, despite the limited value of small-solute clearance [11]. Nevertheless, it has been widely accepted that clinical results depend at least in part on the dialysis dose delivered [12, 13, 14, 15]. In fact, a single center experience shows the beneficial impact of longer dialysis duration on clinical outcome in children [13]. However, it is now becoming more and more evident that increasing the dialysis dose when delivered only three times weekly is an unphysiological strategy, self limited by the potential increased risk of hemodynamic and electrolytic disturbances [16, 17, 18]. In adult care, there is a growing interest in the use of daily dialysis, because long term experience has shown good results [16, 17, 18]. In children only a pilot study in one center supported the positive impact of daily dialysis in very non-compliant adolescents [19].
In children the hemodialysis prescription should be individualized. Choice of the mode of hemodialysis should take into account the presumed waiting time before kidney transplantation as a “ justification” for the use of “ the best available” mode having the highest cost and, conversely, being supported by very limited/preliminary studies only [13, 19].
The importance of the choice of material used for dialysis and its application should not obviate the need for management of the entire child with ESRF, especially regarding optimum nutrition [20]. Because dialysis per se is not able to correct completely the numerous functions of the kidney lost during ESRF, medications and dietary recommendations are needed in children on hemodialysis [20]. Recombinant growth hormone is often needed considering the growth velocity rate of children on chronic dialysis [3].
Guideline 1: the dialysis unit
hemodialysis should be delivered in a “pediatric” dialysis center with a multidisciplinary support team which supports individualized and integrated therapy
nutrition, growth, and educational support are of major importance
Because of the specific needs of children, hemodialysis should be delivered at the best, and probably only, in a pediatric dialysis unit [3, 4, 7] This includes the treatment of adolescents up to the age of 18 years and beyond depending upon their physical and psychological development and transition arrangements to adult units [21]. Taking care of a child with ESRF necessitates an engaged team consisting of doctors, nurses, dietician, psychologist, school teacher, play therapist, and social worker [22]. This “second family or support team” should be multidisciplinary and immediately available to the chronically ill child, both close and distant enough to stimulate normal family life, supporting a proper (school) education, leaving all possibilities open for “full” integration into society in the future.
Hemodialysis, in contrast with peritoneal dialysis, is usually performed in an hospital setting, with a frequency of three times per week for most patients. This frequency may be increased to address the specific needs of babies and/or adolescents requiring “more dialysis” [3, 13, 19].
Guideline 2: water quality
adequate in terms of biochemical composition
free from microbiological contamination
The dialysis machine needs water for dialysate production adequate in terms of biochemical composition and free from microbiological contamination, i.e. germs and endotoxins (Table 1). Water purification depends on the disposable water quality. Usually filtration with charcoal and the small sieving coefficient associated with reverse osmosis produces water for dialysis in accordance with the recommendations [23] (Table 2). Currently, all new dialysis machines have the ability to filter the dialysate through a high flux membrane, which increases microbiological purity.
Table 1.
Water contaminants and associated complicationsa
| Dissolved organic material | Complications |
|---|---|
| Contaminants: | |
| - Pesticides, herbicides | No documentation during dialysis |
| - Chloramines, chlorine compounds | Severe hemolytic anemia |
| Bacteria and pyrogens: | |
| - Bacteria | Bacteremia or septicemia |
| Fever, chills, shaking | |
| Hypotension and death | |
| - Pyrogens | Pyrogenic reaction-fever |
| Chills, uncontrollable | |
| Shaking, vomiting, hypotension |
aNot exhaustive
Table 2.
Definitions of water and dialysate quality (levels given as an upper limit for water-quality definition [70])
| Bacterial growth (cfu mL−1) | Endotoxin (EU mL−1) | Cytokine-induction | |
|---|---|---|---|
| AAMI, water | 200 | 5 | + |
| European pharmacopoeia | |||
| Regular water | 100 | 0.25 | + |
| Ultra-pure | 0.01 | 0.03 | – |
| Sterile | 10−6 | 0.03 | − |
In hemodiafiltration using an on-line technique [24, 25] with direct production from the dialysate of the hemofiltration substitution fluid, the dialysate benefits from double ultrafiltration, producing an ultrapure dialysate which is sterile and endotoxin free, at least at detectable levels. This ultrapure dialysate should limit the risks related to microbiological contamination, i.e. inflammatory process induction with both acute and chronic consequences [23]. This level of ultrapure dialysate is also required for synthetic high-flux membrane use even or especially when used in a conventional hemodialysis mode. Decontamination or sterilization by chemical agents or by heating should be performed in line with water, before final dialysate production by the dialysis machine, by filtration and osmosis installation and water distribution, without any break between sterilization and final dialysate. Quality control of the water for the dialysate should be performed regularly with regard to chemical composition (at least once per year), and final dialysate purity should be assessed with regard to bacteria and endotoxins (more regularly, depending in part on the mode of dialysis, weekly for high-flux membrane use) (Table 2) [23].
Guideline 3: the dialysis machine
volumetric ultrafiltration control
option for both single and double-needle dialysis
In the last decade numerous innovations in equipment have been developed by different manufacturers [3]. But the relevance to child outcome remains unknown, because of the absence of sufficient controlled study results. Nevertheless the following innovations seem essential: dialysate production by double dilution pumps using volumetric ultrafiltration control and blood pumps with double pumps available for single-needle dialysis.
Other “high-tech” innovations only deserve mention because of their limited application in “expert” centers: individual modeling of the dialysis session with monitoring of ultrafiltration and dialysate solute concentration (i.e. sodium, bicarbonate); polyvalency machine which enables not only conventional dialysis but also hemofiltration and hemodiafiltration providing the highest standard in terms of tolerance and efficiency [24, 25]. Newer dialysis machines provide monitoring of hematocrit variation as a major promising innovation [9, 26] and direct urea kinetic monitoring [27]. There is a restricted offer for blood thermal monitoring to avoid loss of calories to the dialysate or to prescribe cooled dialysate [28].
All these innovations enable individualized hemodialysis for the children, but their regular application should take into consideration the balance between the expected benefits and the costs.
Guideline 4: blood lines
available in infants/babies size
biocompatible material
A range of blood lines are available for dialysis of babies to dialysis of the largest adolescent. They should be considered for their biocompatibility, type of sterilization (ethylene oxide-free), and the blood volume required [3].
Guideline 5: principles of blood purification
small solute clearance and more, from diffusion process (urea) to convection (other uremic toxins “middle molecules”) mass transport
hemodiafiltration is an option to consider to obtain “maximum” dialysis efficiency
Uremic toxin extraction in dialysis [3, 24] is related to a combination of the diffusion process and convection mass transport (Table 3). In hemodialysis (HD), blood purification depends mostly on a diffusion process secondary to a concentration gradient, which ensures the best elimination of small molecules (urea). HD clearance (KHD) correlates directly with blood flow rate. In hemofiltration (HF), uremic toxin extraction is mostly dependent on convection mass transport secondary to a pressure gradient, which optimizes the elimination of both low and middle-molecular-weight compounds. HF clearance (KHF) directly correlates with ultrafiltration flow rate which is limited by the blood flow rate. In the post dilution mode, i.e. replacement fluid in the venous line chamber located after the dialyzer membrane, maximum filtrate flow rate is less than half the blood flow rate; it is usually one third, to limit the risks of excessive hemoconcentration. In the predilution mode, i.e. replacement fluid perfusion in the arterial line chamber, which is situated before the dialyzer membrane, maximum filtrate flow rate should be two thirds of or equal to the blood flow rate. Hemodiafiltration (HDF) combines HD and HF simultaneously, which enables blood purification by both a diffusive process and convective mass transport. HDF clearance (KHDF) in post-dilution mode is measured by use of the Granger formula [24]:
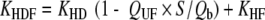 |
Table 3.
Dialyzer membrane permeability: diffusion and convection
| Diffusion process | Convection mass transport | |
|---|---|---|
| Membrane area | Ultrafiltrate flow (QUF) | |
| Mass-transport coefficient | Hydraulic permeability | |
| Concentration gradient | Transmembrane pressure (TMP; mmHg) | |
| Blood flow×extraction coefficient | Membrane area | |
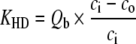 |
Sieving coefficient (S)* | |
| ci and co are inlet and outlet solute concentrations | ||
| Molecular permeability | ||
*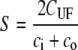
|
||
| CUF is the ultrafiltrate solute concentration | ||
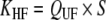 |
(postdilution) | |
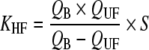 |
(predilution) | |
KHD is hemodialysis clearance and KHF is hemofiltration clearance
On replacement of QUF×S by KHF and Qb by Kmax (maximum achieved clearance) the formula for KHDF becomes:
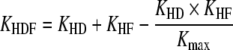 |
Thus it is clear that in terms of blood purification KHDF enhances the clearance of a uremic toxin if HF or HD clearances are lower than the Kmax (equal to the blood flow rate). HDF with a highly permeable membrane is as efficient as HD for low-molecular-weight compounds, but is more efficient than HF for low-molecular-weight compounds [29]. Moreover, HDF, besides its blood purification efficiency, is associated with a lower intradialytic morbidity rate [30, 31], as is HF [3]. On-line HDF [24, 25], in which filtered dialysate free of toxins and pyrogens is used as replacement fluid, enables use of an elevated convection fluid rate, especially in predilution mode, and facilitates a dialysis dose increase without a cost increase. The use of ultrapure dialysate, i.e. sterile and pyrogen free, as used for on-line HDF dialysate should reduce the diseases possibly associated with chronic inflammation related to contaminated dialysate [23], e.g. β2 microglobulin amyloidosis, hypercatabolism with loss of lean body mass, decreased growth rate, fibrosis and cardiovascular diseases. A high flux membrane [32], with an elevated ultrafiltration coefficient of permeability enabling backfiltration from the dialysate to the blood compartment, which is called retrofiltration, increases these risks, especially with contaminated dialysate [23].
Guideline 6: extracorporeal blood access and circulation
fistula vascular access is preferred for long-term chronic hemodialysis
in young children, less than 15 kg, the time needed to develop a fistula before it can be used could be some months
the double-needle technique is the standard, but single needle with double pump system is an alternative
a single lumen catheter with clamps offers for small children an acceptable compromise between a very low extracorporeal blood volume and valuable dialytic efficacy
total extracorporeal blood volume (needles, tubing and dialyzer) should, approximately, be less than 10% of patient total blood volume
anticoagulation in the extracorporeal circuit is achieved either with conventional heparin or with low-molecular-weight heparin
an extracorporeal blood flow rate (QB) of 150–200 mL min−1 m−2 or 5–7 mL min−1 kg−1 is often sufficient
The success of chronic hemodialysis depends on good vascular access: internal arteriovenous fistulae (AVF), shunt (AVS), graft (AVG) or central venous catheter. The type of access used is variable depending on factors in different units and countries, for example surgical experience, patient age and size, the time available before dialysis must be started, and the presumed waiting time before transplantation. Patient choice plays a major part, especially with adolescents.
A catheter is more commonly used in the USA than in Europe [7, 8]. A catheter can be a primary access particularly in acute renal failure or chronic renal failure with acute presentation, in small children and in the case of a presumed short period on chronic hemodialysis. Internal jugular vein catheter access is superior to subclavian vein; it admittedly preserves the future arteriovenous fistula implantation on the arm. Femoral catheter access should be used only for “rescue and transient” access if intensive care is needed: it is easy to perform but with a higher risk of infection and thrombosis. A double lumen cuffed catheter, at least 8 French, is mostly preferred for children and has been reported to have a survival rate as high as 60 to 85% in one year [33], or as low as 30% [34]. Nevertheless in small infants a single lumen catheter used with the alternative clamps technique offers an acceptable compromise between recirculation and both the amount of extracorporeal blood volume and the achieved blood flow [35]. Thrombosis, a major cause of catheter failure, is reported to be between 9 and 46% [34]. Thrombosis causing poor flow can be corrected to salvage the catheter by different methods: catheter replacement over guidewire, systemic oral anticoagulation and local urokinase or tissue plasminogen activator instillation [36]. Loss of catheter access related to infection has decreased during the last decade; the aggressive use of antibiotics and perhaps antibiotic lock therapy, although not universally accepted, account for this lower rate of infection related catheter loss [34, 36, 37].
Microsurgery enables creation of a functional AVF at the wrist in most children, even small ones [8] but only a few surgeons are trained for vascular microsurgery, which therefore is rarely used. Creation of a fistula at the elbow is a second-choice vascular access. With a non functional cephalic vein, a basilic vein transposition, i.e. superficialization, is possible [38]. Synthetic grafts should be reserved for children who have exhausted autologous veins and should be used in children only very rarely. For all these reasons preoperative evaluation of the vessels to determine the correct choice of vein before the operation is mandatory. The non-dominant arm should be regarded as first choice of fistula implantation. The survival rate for a AVF is higher than the survival rate for a catheter, with more than two thirds of the children having a functioning AVF at four years [8]. With a basilic vein superficialization the fistula should not be used before full healing (2 to 6 weeks) to avoid a dissecting hematoma. Otherwise the time needed for venous development before use depends on the age of the patient and the place of the AVF (distal or proximal). In small children this period of time is often a delay of months. Before surgery it is essential to avoid venopuncture of the selected arm in the weeks before AVF creation. It is of interest to protect the dominant arm from the beginning of taking care of a child with “chronic dialysis risk” to enable, if necessary, implantation of a fistula. Such venoprotection should not be forgotten for peritoneal dialysis children, even babies/infants. For a period of time before surgery, especially for small children, [8] dilatation of the veins by immersion of the forearm in hot water is advantageous, a maneuver enhanced by placement of a tourniquet. A proximal AVF with a high blood flow, usually close to 1000 mL min−1 m−2 , is a risk factor for cardiac failure. Nevertheless, the major complication is thrombosis, consequent to local stenosis. Therefore, follow up of the access flow is essential, on the one hand clinically: auscultation (the sound of the AVF is maximum at the surgical site and decreases with distance from the fistulae), observation (elevation of the forearm should induce emptying of the previous dilated veins, and on the other hand by Doppler ultrasound or vascular access flow monitoring [9]. Application of regular access flow monitoring can be used to detect vascular stenosis before complete AVF thrombosis [9]. But it should be remembered that “Transonic” access flow monitoring can only be performed with double-pump dialysis and is not available for pediatric-sized blood lines.
The extracorporeal blood flow rate is achieved through venous puncture, most often via two needles, one for blood aspiration called the arterial needle, one for venous reinjection called the venous needle. The distance between the needles should be sufficient to limit recirculation, which is best prevented by opposite orientation of the needles: the arterial one toward the fistula, the venous one in the opposite direction. Usually the needle size is 17-gauge at initiation of dialysis; thereafter considering patient need and fistula development 16 or 14-gauge needles, particularly in adolescents, can be used to achieve a sufficiently high blood flow rate. Pain related to the puncture should be prevented by anesthetic cream (Emla or Amelop); this advance is important for both the children and nurses [39].
An extracorporeal blood flow rate (QB) of 150–200 mL min−1 m−2 , 5–7 mL min−1 kg−1, is often sufficient to achieve the targeted goals with double needle dialysis; in small children QB is determined using body weight (BW, kg): (BW+10)×2.5=QB (mL min−1). The arterial blood aspiration pressure should be monitored if possible and kept between 150–200 mmHg to limit endothelial trauma.
For single-needle dialysis in children the highest blood flow rate is obtained with a double pump system (venous flow higher than arterial flow) monitored by the pressure, system called time pressure regulation. The risk of recirculation is important with the latter; some machines limit this risk more than others, especially with the addition of clamps. Conversely for small infants a single lumen catheter used with the alternative clamps technique is an acceptable compromise between recirculation and both the extracorporeal blood volume and the achieved blood flow [35].
The total extracorporeal blood volume (needles, tubing, and dialyzer) should preferably be less than 10 % of patient total blood volume. This is essential for small children; however, the relative normal hemoglobin level obtained with erythropoïetin therapy enables this volume to be exceeded slightly without significant hypotension at the end of dialysis session when the patient reaches dry body weight. Nevertheless, it should be kept in mind that the higher the extracorporeal blood volume, the higher the volume of returned fluid, which will load the patient with fluid at the end of the dialysis session. (In very small children the substitution by air may be necessary to limit blood loss on one side and high substitution volume on the other side, but is very dangerous and should be strictly monitored.) System priming with saline, albumin, and sometimes blood should be applied in the first dialysis sessions with babies or small infants.
Anticoagulation of the extracorporeal blood volume is performed either by use of conventional, heparin with continuous infusion of 20 to 30 IU kg−1 h−1, or with low-molecular-weight heparin at 1 mg kg−1 as a bolus at the beginning of the dialysis session. If the hematocrit is over 35%, the risk of clotting is increased. Regional citrate anticoagulation is sometimes used especially when acute dialysis is needed [2]. Predilution treatment, feasible in either hemofiltration or hemodiafiltration, reduces the risk of clotting and even enables dialysis without anticoagulation in some circumstances. In the presence of thrombopenia heparin-toxicity is to be suspected.
The venous blood line has a pediatric size air-trap chamber to limit extracorporeal blood volume. The dialysis membrane is protected by an arterial chamber of expansion which in small children is often not incorporated in the line to reduce the extracorporeal blood volume. Prevention or treatment of ethylene oxide allergy is possible by using steam sterilization of needles, lines, and membranes; this is becoming the preferred option throughout Europe.
Guideline 7: which dialyzer membrane to “choose”
synthetic membrane, low flux, capillary configuration
high-flux membrane use requires use of ultrapure dialysate
removal of urea and other uremic toxins dialytic should be considered, especially in chronic, long-term dialysis
Three general types of membrane are available at present [32]: unmodified cellulose (low flux and so-called bioincompatible membranes), modified/regenerated cellulose (low flux or high flux; so-called relatively biocompatible), synthetic (low flux or high flux; so called relatively biocompatible).
The choice of a dialyzer membrane should take into account the following (Table 4):
the biocompatibility of the material towards leucocytes and complement activation
the blood volume priming requirement, which is membrane area-related
- the permeability, determined in the most simple way by two characteristics:
- hydraulic permeability (CUF) measured in mL per mmHg of transmembrane pressure achieved per hour, i.e. either low permeability, CUF under 5 mL mmHg−1 h−1 (low-flux membrane), and high permeability, CUF over 15 to 20 mL mmHg−1 h−1 (high-flux membrane)
- molecular permeability determined at least by the molecular weight of the molecule considered, usually between 0.8 and 0.9 for urea and lower for the other uremic toxins with a cut off of zero for albumin. In practice this cut off is often under a molecular weight of 20,000 Daltons. The profile of this molecular permeability [40, 41] is a specific characteristic of each manufactured dialysis membrane. Highly permeable membranes give the theoretical potential for middle-molecular-weight (Babb theory; 500 to 2,000 Daltons) uremic toxins being removed during dialysis. In adult dialysis patients the clinical benefits of improved removal of middle molecules by high flux, large pore, biocompatible membranes, more or less established, are [41]: reduction of uremia related amyloidosis, maintenance or residual renal function, and reduction of inflammation, malnutrition, anemia, dyslipidemia, and mortality.
The absorption capacity on to the membrane, (IL1, TNF, IL6, β2 microglobulin):a characteristic of synthetic membranes
Table 4.
Dialyzer membranes: practical parameters of choice
| - Type of membrane: biocompatibility toward complement system |
| - Initial blood volume needed, i.e. area-related, quality of restitution |
| - Molecular permeability: maximum clearance for urea and the other uremic toxins, e.g. phosphate, related to potential patient osmotic risk |
| - Hydraulic permeability: possibility of use for HF or HDF procedure, but related to back filtration risk, high flux membranes need ultrapure dialysate |
| - Adsorption capacity on to the membrane (a characteristic of synthetic membranes) |
| - Cost |
For conventional dialysis low-flux membranes are suitable, but to achieve hemofiltration or hemodiafiltration high-flux membranes are necessary. The higher the hydraulic permeability, the higher is the backfiltration risk; this process could be limited both by permanent convective flow from the blood compartment to the dialysate compartment, as ultrafiltration (HF, HDF, or at least weight loss) and by use of ultrapure dialysate. Synthetic membranes seem the best theoretical choice but clinical justification of the relatively higher cost is uncertain [32]. Justification for use of high-flux synthetic membranes, as used in on-line HDF, remains a matter of debate for children on dialysis for short periods only while waiting for their kidney transplant.
Reuse of the membrane is not applied in practice for children.
Guideline 8: the dialysate
bicarbonate buffered,
low calcium level (1.25 mmol L−1) becomes the standard,
glucose concentration at physiological level,
dialysate quality control (germs and endotoxins) is required
The dialysate is prepared as a dilution of concentrate with water, ideally with ultrapure water. The composition of the dialysate has changed over the last two decades [42]. Acetate as buffer has been replaced by bicarbonate, with the development of machines with two separate dilution pumps, one for bicarbonate concentrate free from calcium, often as a powder, and one for the acid concentrate containing residual levels of acetate and the electrolytes (Na, K, Cl, Ca). The current use of oral calcium carbonate as a phosphate binder has mandated the need to decrease the calcium concentration of the dialysate, usually at a low rate, 1.25 mmol L−1 Ca2+, less often at a normal rate, 1.5 mmol L−1, avoiding the “historically” high level of 1.75 mmol L−1 Ca2+. In fact, the use of calcium carbonate combined with a high concentration of calcium in the dialysate, often led to an elevated Ca×P serum product, compared with the current recommendation of a product less than 5 mmol2 m−2 (60 mg2 dL−2) [43, 44]. This Ca×P serum product seems to be an important factor implicated in the vascular calcifications seen in the dialyzed patients [43], affecting even the dialyzed children [44, 45]. The need for glucose in the dialysate is of importance [46] and should be near the physiological concentration. Higher glucose concentrations or the introduction of parenteral feeding during dialysis will drive the potassium into the cells, leading to ineffective potassium-extraction [41].
Potassium-free dialysate is rarely used because of the theoretical risk of hypokalemia [42]. Therefore “low” (1–1.5 mmol L−1), “normal” (2–2.5 mmol L−1), and “high” (3–3.5 mmol L−1) potassium dialysate are available enabling individual adaptation and prevention of the arythmogenic potential of dialysis [42]. Nevertheless special attention should be devoted to avoiding any confusion among the “potassium charged” dialysates. Sodium concentrations have increased from the previous classical level of 132 mmol L−1 to a more physiological level of 138 to 144 mmol L−1. Newer machine capabilities enable dialysate profiles to change during a dialysis with respect to sodium and ultrafiltrate profiles [47, 48] to increase tolerated weight loss; and bicarbonate profiles [49], to enhance phosphate removal. Intermittent ultrafiltration rates, enabling better plasma refilling is the most common profile used. Similarly, the dialysate flow rate can be adapted to need, usually in the range 300 to 800 mL min−1. In general practice, 500 mL min−1 is used. The dialysate flow is usually in the opposite direction of the blood flow, separated by the membrane of the dialyzer. Dialytic thermal exchanges seem of importance especially for babies and/or high-flow dialysate use, leading to a risk of patient hypothermia. Control of thermal exchanges during a dialysis session is therefore available on a new machine [3, 28].
Guideline 9: post-dialytic dry weight assessment and adjustment
particularly difficult to define in growing children
no “unique” optimum method, importance of a clinical “pediatric” experience
need for regular assessment in a growing child
close collaboration with pediatric renal dietician
Patient dry weight is defined as the weight at the termination of a regular dialysis session, below which the patient will become symptomatically hypotensive. Incorrect estimation of dry weight will lead either to chronic fluid overload or chronic dehydration. Estimation of dry weight is particularly difficult in children for many reasons. First, the hypotensive tendency during a dialysis session is multifactorial and not only related to the ultrafiltration rate but also to the plasma refilling rate capacity [47, 48]. Second, body composition, i.e. total body water ratio to total body mass, is variable with age, especially during infancy and puberty. In infants and in adolescents dry weight must be assessed almost monthly to follow rapid body composition changes during a rapid growth period. This is also important under anabolic conditions such as with growth hormone treatment, and conversely under catabolic conditions such as the ill child with intercurrent infections or reduced food intake.
Clinical criteria used to assess hydration status are important but not always reliable. Therefore, different approaches have been proposed: assessment of total body water by bioelectrical impedance analysis [50], continuous measurement of hematocrit variations by non-invasive methods during dialysis [9, 26], plasma atrial natriuretic peptide or cyclic guanosine monophosphate determination [51], and, last, by echography of the inferior vena cava (IVC) [52, 53, 54, 55]. Measurement of the diameter of the IVC (IVCD) by ultrasound, expressed as an index to body surface-area in mm m−2, and the decrease on deep inspiration, called the collapse index, expressed as a percentage (%) seems to be an accurate non-invasive method easily performed serially. An IVCD between 8.0 and 11.5 mm m−2 and a collapse index between 40 and 75 % is considered as representing normovolemia [52, 53, 54, 55]. However, unlike body impedance, interstitial volume and sodium balance are not reflected by IVCD [55]. In fact all these approaches have to be balanced by clinical judgment and experience and combined with nutritional support.
Achievement of dry weight during ultrafiltration is associated with a drop of the hematocrite level. Ultrafiltration is well tolerated until a certain level of decrease of initial hematocrite, called “crash hematocrite” a patient individual characteristic, usually over 10% blood volume reduction over a 3-h session [56]. If the hematocrite curve is flat over time during a dialysis session, the patient could be considered as being over his optimum dry weight [9, 56]. In practice, monitoring of hematocrit (or blood volume) and guided ultrafiltration should avoid both fluid overload and hypotensive “crash hematocrit” and consequently approach more precisely the patient dry weight [9, 56].
Guideline 10: urea dialytic kinetic, dialysis dose, and protein intake assessment (nutrition)
Urea kinetic modeling (UKM) has been widely accepted as a method for dialysis dose assessment despite its limited value as a unique measure of dialysis adequacy. Does small solute clearance really matter? [11]. In adult patients the HEMO study suggested that increasing urea clearance above currently accepted target ranges does not lead to improved patient outcome [11]. Although urea per se is not toxic in concentrations normally encountered in dialysis patients, it may serve as a marker of unknown toxins of uremia, some of which are called “middle-molecular-weight” uremic toxins [11, 41].
UKM facilitates identification of underdialyzed patients and recognition of dietary compliance. The measures most widely used to gauge dialyzer treatment are Kt/V, that is dialyzer urea clearance (K) multiplied by duration (t) of the dialysis session and divided by urea volume (V) of distribution, and the normalized protein catabolic rate (nPCR) [57, 58, 59]. Urea dialytic reduction rate (URR) is derived from the pre and post-dialysis serum urea values and quantitates urea removal by dialysis. URR expressed as the ratio post/pre should be at least equal to or lower than 0.35 and when expressed as the difference between pre and post-urea, divided by the predialysis value, should at least equal to or higher than 0.60 [60]. URR is proportional to dialysis efficiency, and thus to urea dialytic clearance. URR is inversely proportional to the urea refilling rate of the blood compartment and the extracellular space (EC) from the intracellular space (IC), called the transcellular urea mass transfer coefficient (Kie). URR is also correlated with the amount of urea dialytic removal (Kt) compared to the amount of urea body content (V) and thus to Kt/V. Usually urea dialytic clearance in children is low in comparison with the high Kie which is between 200 to 1000 mL min−1 (6 to 12 mL min−1 kg−1 BW) [58, 61]. Nevertheless, after dialysis the concentration of urea in plasma increases rapidly in an initial period, usually until 60 min postdialysis [62]. This postdialytic urea rebound (PDUR) is a multifactorial event [63, 64]. Vascular access and cardiopulmonary recirculation occurs within the first 2 to 3 min of discontinuing hemodialysis and account for 60 to 70% of total PDUR. Subsequently, tissue rebound occurs, because of intercompartmental, i.e. IC versus EC, urea dysequilibrium at the end of the dialysis session and tissue re-equilibration which is usually complete within one hour postdialysis, reaching the equilibrated postdialytic plasma urea concentration. For highly diffusible substances such as urea, distribution in total body water (TBW) seems to be limited by cardiovascular flow rather than diffusion [64]. The apparent IC–EC two-pool model should perhaps be the result of a regional blood flow distribution system in which approximately 80% of TBW (and thereby urea) is located in muscle, bone, and skin, with organs receiving only 20 to 30% of the cardiac output, i.e. low-flow system. The remaining organs contain only 20% of TBW (hence urea) but receive 70–80% of the cardiac output, high-flow system. One would expect the urea concentration in these organs to fall quite rapidly during dialysis. This flow system, and the vascular resistance model could explain the great variability of the PDUR among patients for which the IC–EC two-pool model is not accurate. Do some patients have thicker cell walls than others? By contrast the possible causes of vascular resistance variability, i.e. hypovolemia, hypertension, heart failure, hematocrit, alkalosis or acidosis, low-temperature dialysate, can explain PDUR variability. The URR variability could also be explained by vascular resistance changes over the dialysis session, at least for urea [64].
Kt/V calculation based on a single pool urea model neglects compartmental urea distribution in the body, hence PDUR, resulting in overestimation of actual Kt/V. Therefore, a two pool model should be applied, using instead of the urea plasma concentration at the end of the dialysis, the equilibrated urea, i.e. 60 min postdialysis [65, 66]. Other improvements from the initial formula are proposed to provide a more accurate Kt/V calculation: weight loss (UF/BW) and urea generation during the dialysis session (0.008td), leading to the Daugirdas and Schwartz formula proposed in 1994 [60]:
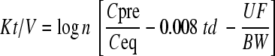 |
where td is the dialysis time (h), Cpre and Ceq are, respectively, the predialysis and equilibrated postdialysis urea concentrations, and UF/BW is the ultrafiltrate-to-body weight ratio (L kg−1)
The predialysis blood sample should be taken from the arterial line, before any rinsing. Because of the practical difficulty in obtaining the postdialysis equilibrated urea sample 60 min after the end of the dialysis, different indices have been proposed to estimate Ceq, for example using a 6 min [67] or a 15 min [68, 69] post treatment sample. The most important rule of the urea end dialysis sample should be the use of the “stop dialysate flow method” [67], measuring urea 6 min after angio access was removed and cardiopulmonary recirculation completed.
The other major cause of error for the Kt/V calculation is determination of V. The V, hence the TBW, could be calculated from a formula (Table 5) [71] or determined by bioimpedance measurements [50].
Table 5.
Formulas enabling calculation of the volume of distribution of urea in liters (total body water) using height, weight, sex and age (from Ref. [67])
| Boys: | Ht<132.7 cm | V=1.927+0.465/BW (kg)+0.0045/ht (cm) |
| Ht>132.7 cm | V=−21.1933+0.406/BW (kg)+0.209/ht (cm) | |
| Girls: | Ht<110.8 cm | V=0.076+0.507/BW (kg)+0.013/ht (cm) |
| Ht>110.8 cm | V=-10.313+0.252/BW (kg)+0.154/ht (cm) |
Guideline 11: dialysis dose and outcome
only “small solute urea clearance” prescription?
a minimum Kt/V urea level of 1.2–1.4 is thought to be desirable; adequacy tests should be performed monthly
dialysis and residual renal small-solute clearance are not equivalent
dialysis prescription should be adequate before being optimum, not only a “urea dialysis dose”
Although the optimum level of Kt/V required is matter of debate, a minimum Kt/V level of 1.2–1.4 is now thought to be desirable [11]. Overall, this Kt/V as an index of dialysis dose should only be analyzed in comparison with the nPCR, hence the diet, protein and caloric intake (Fig. 1). Because of the mathematical relationship between Kt/V and nPCR [72, 73] the real impact of these variables for a given patient would determine the therapy necessary for a patient to achieve an “urea dialysis dose”. Nevertheless increasing dialysis dose seems to have a direct impact on nutrition [74] and the combination of increased dialysis dose and adequate nutrition can promote normal growth in children treated with long-term hemodialysis [75]. Therefore malnutrition should be avoided [76] by using a diet survey, anthropometric measurements, and perhaps IGF1 determination [77].
Fig. 1.
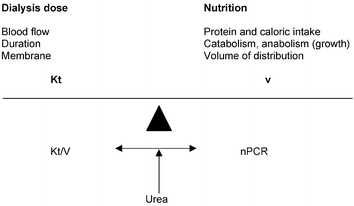
Dialysis prescription balance
Does small solute clearance, i.e. urea, really matter? [11]. Because of the limited number of children on chronic dialysis the relationship between optimum urea dialysis dose and patient outcome will be “difficult” to establish. It is, however, known that blood purification, dialysis and residual renal small solute clearance, are not equivalent [11, 78] giving more importance to other uremic toxins, whose removal is enhanced using high-flux dialyzer membranes with on-line hemodiafiltration [3, 24, 32, 41].
Even if the pediatric data only seem to be unique center experiences, the case for a greater urea dialysis dose [12] could be correlated with both growth rate enhancement [12, 13, 19, 78] and improved cardiac function [14, 19]. The duration of each hemodialysis session is also matter of debate, long duration being able to induce regression of left ventricular hypertrophy in adult patients [16] and being able in children [13] to promote growth and well being. In the same way daily dialysis seems related to better clinical results both in adults [17, 18, 77, 79] and in adolescents [19].
Dialysis prescription should be adequate before being optimum (Table 6) [80]. In long term chronic dialyzed children the individualized prescription should consider all the available new strategies to fully preserve at the best “the life chances” [13, 14, 19].
Table 6.
Hemodialysis prescription for children: adequate, before optimum
| - Dialysis modality should enable achievement of blood pressure control (without antihypertensive medications for most children), normal myocardial morphology and function |
| - Dialysis dose prescription should not only be an urea dialysis dose. Removal of the other uremic toxins should be considered, not only middle molecules but overall phosphate |
| - Dialysis frequency and duration must be adjusted to the tolerance of ultrafiltration to reach the dry weight. Ultrafiltration rate should not exceed 1.5±0.5% of body weight per hour (in theory no more than 5% BW loss per whole session ). Blood volume (hematocrite) guided ultrafiltration secure |
| - A regular diet survey is essential to maintain adequate protein and calorie intakes. Urea kinetic assessment enables not only urea dialysis dose calculation, i.e. Kt/V, but also estimation of protein intake by use of the PCRn calculation (protein catabolic rate). Fasting to enable a short duration three times a week dialysis schedule is inadequate care management |
| - Too fast ultrafiltration can induce hypotension and cramps during dialysis, usually during the second half time session, and fatigue and/or hang over after dialysis |
| - A small solute, e.g. urea, clearance which is too high is a factor of disequilibrium syndrome occurring during dialysis, usually after the first half/or one hour session time with headache, even seizures, nausea, vomiting, sleepiness or a hypertensive tendency with a narrow range between systolic and diastolic pressure values. Symptoms usually disappear a few hours after the end of the dialysis |
Guideline 12: the dialysis session, prescription, and monitoring
individual prescription is required: babies/infants/children specificities
assessment and adjustment is needed regularly in small/growing children
psychological preparation of the child and his family is needed, pain prevention is essential
The first dialysis session is of importance to induce child and parent confidence, therefore appropriate preparation is needed. The site of the puncture of the fistula, most often with a double needle, size gauge 17, is carefully chosen and determined so that the needles are sufficiently separated to limit recirculation. Pain prevention is essential by application of a xylocaine ointment (Emla) one hour before needle insertion [39]. Psychological preparation of the child and family is also needed to limit “anxious stress” [22]. An aseptic procedure is essential. The extracorporeal circulation is adapted to the level of arterial aspiration pressure if measurable by the machine to prevent endothelial vascular trauma (not less than −150 mmHg). The venous return pressure should not be more than +200 mmHg to prevent endothelial vascular trauma.
During the first dialysis session, the blood flow rate is maintained at a low level to prevent the dysequilibrium syndrome secondary to too efficient solute removal during this first session. Therefore, the blood flow rate should be approximately 3 mL kg−1 BW (or 90 mL m−2), or even less, so that urea clearance will be less than 3 mL min−1 kg−1 BW, which is usually well tolerated even in small children and limits the development of the dysequilibrium syndrome. The duration of the first dialysis session should be short, no more than 3 h, or adapted to the ultrafiltration need. The dysequilibrium syndrome is most often only symptomatic after one to two hours of dialysis, with variable symptoms such as headache or seizure, vomiting, fatigue, sleepiness, or a hypertensive tendency with a narrow range between systolic and diastolic pressure values. If needed, mannitol infusion (1 g kg−1 BW over 1 to 2 h during dialysis) is effective in preventing the syndrome. Symptoms usually disappear a few hours after the end of the dialysis.
The extracorporeal blood flow rate, the duration of the session, and the number of sessions a week is progressively increased to individual patient need. Usually a blood flow rate of 150 to 200 mL min−1 m−2 and three sessions per week for 3 to 4 h per session achieve the minimum target prescription of 1.2 to 1.4 Kt/V [11].
The duration of a dialysis session is often prescribed to reach the anticipated dry weight at the end of the session. The total amount and the rate of ultrafiltration needed must be tolerable. A weight loss per hour of 1.5 to 2% of the BW is standard [3, 80] and most often well tolerated. Intermittent ultrafiltration with bicarbonate buffered dialysate which is not too warm (so called “cooled dialysate”), a normal “high” level of sodium (140 to 144 mmol L−1), which is not more than the normal concentration of sodium per liter plasma water, a normal hematocrit over 30% and optimally near 35% but not higher [78], and a mode of dialysis based on hemofiltration, i.e. (optimally HDF) are some of the major “tricks” used to enhance ultrafiltration tolerance [3, 48]. Intolerance of weight loss usually becomes symptomatic at the end of the dialysis session, when the patient is near his dry weight. Continuous blood volume monitoring during the session should become a real clinical support to enable optimum ultrafiltration tolerance (notion of crash hematocrit) [26, 56]. This information is limited to blood compartment changes. The interstitial space, which is mostly sodium-dependent, is better estimated by clinical assessment of edema or body weight [54, 55]. Rarely bed scales are used to assess more precisely the weight changes over a dialysis session.
For most infants and children weighing less than 10 kg the need for more than three sessions a week may become evident to enable adapted nutrition, i.e. milk that is ”water”, hence 4 to 5 sessions a week are frequently prescribed [3, 11]. The adequate number and duration of each session should avoid partial fasting to achieve the weight needed to facilitate a short dialysis duration [7, 21, 80]. The volume of fluid used for extracorporeal blood replacement at the end of the session should be limited, and preferably a glucose solution instead of saline solution be used, especially in infants without residual renal function [3].
At the beginning of the dialysis session clinical manifestations of bioincompatibility may occur. This first use reaction is related to the biocompatibility of the material in the extracorporeal circuit, i.e. membrane, lines or even the needle either during the first session, first contact with the “extracorporeal” material or thereafter for example, in a new dialysis center for holidays. The major positive diagnostic criteria is the onset within 20 min of starting dialysis, of the major symptoms of dyspnea, burning heat throughout the body or access site, angioedema, flushing or vascular collapse, or with minor symptoms such as itching, rhinorrhea, lacrymation, urticaria, or abdominal cramping. Even if its occurrence is rare, or underestimated in the event of intermittent minor symptoms only during the first hour of session, the risk could be substantial. Biocompatible membranes, steam-sterilized material, adequate flushing of the circuit before blood connection, are some of the most important prevention factors [32].
The dialysis per se should be regarded as part of an overall strategy for care including dietary adequacy and interdialytic therapy [1, 21]. A weight gain over 10% dry BW during the interval of two sessions is often correlated with global non-compliance [3, 80]. In these cases, major outcomes could even occur: first acute, i.e. hyperkalemia or pulmonary edema, second chronic, i.e. hyperparathyroidism, and third long term, i.e. cardiovascular and coronary involvement [44, 45].
Conclusions
Hemodialysis in children has benefited from major progress over the last 20 years. The morbidity of the sessions has decreased, even disappeared, seizures being exceptional, hypotensive episodes or headaches rare, and pain related to the fistula puncture effectively prevented by xylocaine ointment. The development of urea kinetic modeling enables calculation of the dialysis dose and indirect assessment of protein intake, nPCR. Even if the validity of these values is questioned their combined analysis provides an assessment and therefore is a “good thing”. The patient also benefits from the technological revolution. The newer machines enable precise control of ultrafiltration volumetric assessment and continuous blood volume monitoring during the session, buffered bicarbonate has become a standard technique, synthetic more biocompatible membranes and specific material available for babies/infants have been developed. Non invasive intervention, for example blood volume guided ultrafiltration have provided more adequate dialysis sessions and better dry weight assessment [81]. Last, the availability of erythropoietin [82] and of growth hormone and the promising results from enhanced dialysis dose on both growth and cardiac function [13, 20], all give the dialyzed child a real increased quality of life. In theory, reduction of dialysis prescription to only a urea dialysis dose achieved by three short (3-h) dialysis sessions, should be abandoned for long term dialyzed children and replaced by optimum dialysis obtained with longer (4 and more hours) and/or more frequent (daily: 5 to 6) sessions [13, 20, 79, 80]. But for such a daily dialysis strategy all the costs must be considered. On the one hand the financial cost cannot be neglected. For the patient bearing the burden, on the other hand, such an intensive dialysis prescription is acceptable only as an integrated therapy life project, a dialysis–transplantation program (HD, PD) with special regard for prevention of the vascular calcification [83]. Daily hemodialysis is one approach, perhaps the only one, to achieve phosphate purification [16, 17, 18, 19] and thereby maintain the calcium×phosphorus product in the optimum range of 3.3 to 4.4 mmol2 mL−2 [43].
Footnotes
Published on behalf of the European Pediatric Dialysis Working Group. Contributing Members of the European Pediatric Dialysis Working Group EPDWG are: M. Ekim, University of Ankara, Ankara, Turkey; A. Edefonti, I Clinici di Perfezionamento, Milan, Italy; M. Fischbach, Hôpital de Hautepierre, Strasbourg, France; G. Klaus, University of Marburg, Marburg, Germany; K. Rönnholm, University of Helsinki, Helsinki, Finland; F. Schaefer, University of Heidelberg, Heidelberg; C. Schröder, Wilhelmina Kinderziekenhuis, Univ. of Utrecht, The Netherlands; E. Simkova, v Motol, Prague, Czech Republic; C. J. Stefanidis, A&P Kyriakou Children’s Hospital, Athens, Greece; V. Straszdins, University Hospital for Children, Riga, Latvia; J. Vande Walle, University of Ghent, Ghent, Belgium; A. Watson, Nottingham City Hospital, Nottingham, United Kingdom; A. Zurowska, University of Gdansk, Gdansk, Poland
References
- 1.Fischbach M, Stefanidis CJ, Watson AR (2002) Guidelines by an ad hoc European committee on adequacy of the pediatric peritoneal dialysis prescription. Nephrol Dial Transplant 17:380–385 [DOI] [PubMed]
- 2.Strazdins V, Stefanidis V, Watson AR, Harvey B (2004) Renal replacement therapy for acute renal failure in children: European guidelines. Pediatr Nephrol 19:199–207 [DOI] [PMC free article] [PubMed]
- 3.Fischbach M, Terzic J, Menouer S, Provot E, Bergere V (2001) Hemodialysis in children: principles and practice. Semin Nephrol 21:470–479 [DOI] [PubMed]
- 4.Watson AR, Thurlby D, Schröder C, Fischbach M, Schaefer F, Edefonti A, Stefanidis CJ, Rönnholm K, Zurowska A (2000) Choice of end stage renal failure therapy in eight European centres. Pediatr Nephrol 6(5):C38 [DOI] [PMC free article] [PubMed]
- 5.Feber J, Scharer K, Schaefer F, Mikova M, Janda J (1994) Residual renal function in children on haemodialysis and peritoneal dialysis therapy. Pediatr Nephrol 8:579–583 [DOI] [PubMed]
- 6.Fischbach M, Terzic J, Menouer S, Soulami K, Dangelser C, Helmstetter A, Gehant F (2001) Effects of automated peritoneal dialysis (APD) on residual urinary volume in children. Adv Perit Dial 17:269–273 [PubMed]
- 7.Bunchman TE (1996) Pediatric hemodialysis: lessons from the past, ideas for the future. Kidney Int 53 (Suppl):S64–S67 [PubMed]
- 8.Bourquelot P, Cussenot O, Corbi P, Pillion G, Gagnadoux MF, Bensman A, Loirat C, Broyer M (1990) Microsurgical creation and follow up of arteriovenous fistulae for chronic hemodialysis in children. Pediatr Nephrol 4:156–159 [DOI] [PubMed]
- 9.Goldstein SL, Smith CM, Currier H (2003) Non invasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol 14:2127–2131 [DOI] [PubMed]
- 10.Muller Wiefel DE, Amon O (1994) Use of recombinant human erythropoietin in children undergoing dialysis. Semin Dial 7(6):413–420
- 11.Goldstein SL (2004) Adequacy of dialysis in children: does small solute clearance really matter? Pediatr Nephrol 19:1–5 [DOI] [PubMed]
- 12.Sharma A (2001) Reassessing haemodialysis adequacy in children: the case for more. Pediatr Nephrol 16:283–290 [DOI] [PubMed]
- 13.Bell L, Espinosa P (2003) Intensive in center hemodialysis for children: a case for longer dialysis duration. Hemodial Int 7(4):290–295 [DOI] [PubMed]
- 14.Bakkaloglu SA, Ekim M, Kovak G, Attalay S, Tumer N (2001) Impact of dialysis adequacy on cardiac function in pediatric CAPD patients. Perit Dial Int 21:395–400 [PubMed]
- 15.Fischbach M, Terzic J, Menouer S, Provot E, Laugel V (2001) Normal statural growth in two infants on peritoneal dialysis: anecdotical or related to the whole management. Clin Nephrol 56:17–20 [PubMed]
- 16.Can CT, Floras JS, Miller JA, Richardson RMA, Pierratos A (2002) Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61:2235–2239 [DOI] [PubMed]
- 17.Maduell F, Navarro V, Torregrosa E, Rius A, Dicenta F, Cruz MC, Ferrero JA (2003) Change from three times a week on line hemodiafiltration to short daily on line hemodiafiltration. Kidney Int 64:305–313 [DOI] [PubMed]
- 18.Traeger S, Galland R, Arkouche W, Delawari E, Fouque D (2001) Short daily hemodialysis: a four year experience. Dial Transplant 30:76–86
- 19.Fischbach M, Terzic J, Laugel V, Dheu C, Menouer S, Helms P, Livolsi A (2004) Daily on line hemodiafiltration: a pilot experience in children. Nephrol Dial Transplant 19:2360–2367 [DOI] [PubMed]
- 20.Coleman JE, Edefonti A, Watson AR on behalf of the European Paediatric Peritoneal Working Group (2001) Guidelines by and ad hoc European committee on the assessment of growth and nutritional status in children on chronic peritoneal dialysis. Perit Dial Int 21:323
- 21.Watson AR, Shooter M (1996) Transitioning adolescents from paediatric to adult dialysis units. In: Khanna R (ed) Adv Perit Dial Publications 12:176–178 [PubMed]
- 22.Watson AR (1995) Strategies to support families of children with end stage renal failure. Pediatr Nephrol 9:628–631 [DOI] [PubMed]
- 23.Lonnemann G (2000) The quality of dialysate: an integrated approach. Kidney Int 58, S112–S119 [DOI] [PubMed]
- 24.Fischbach M (1994) Use of hemodiafiltration in children. Semin Dial 7(6):409–412
- 25.Canaud B (1998) On line hemodiafiltration: state of the art. Nephrol Dial Transplant 13 (Suppl 5):5–11 [DOI] [PubMed]
- 26.Jain SR, Smith L, Brewer ED, Goldstein SL (2001) Non-invasive intravascular monitoring in the pediatric hemodialysis population. Pediatr Nephrol 16:15–18 [DOI] [PubMed]
- 27.Van Hoeck KJM, Lilien MR, Brinkman DC, Schroeder CH (2000) Comparing a urea kinetic monitor with Daugirdas formula and dietary records in children. Pediatr Nephrol 14:280–283 [DOI] [PubMed]
- 28.Santoro A, Mancini E, Canova C, Mambelli E (2003) Thermal balance in convective therapies. Nephrol Dial Transplant 18 (Suppl 7):vii41–vii44 [DOI] [PubMed]
- 29.Fischbach M, Hamel G, Geisert J (1985) Efficiency of high permeable membranes in hemodiafiltration in children: an optimal method of purification. Int J Pediatr Nephrol 6:251–256 [PubMed]
- 30.Muller Wiefel DE, Rauch H, Wingen AM (1982) Hemofiltration in children. Contr Nephrol 32:128–131 [DOI] [PubMed]
- 31.Edefonti A, Galato R, Savage A (1982) Clinical impact of hemofiltration on dialysis discomfort in children. Int J Pediatr Nephrol 3:115–117
- 32.Bouré T, Vanholder R (2004) Which dialyzer membrane to choose? Nephrol Dial Transplant 19:293–296 [DOI] [PubMed]
- 33.Scharma A, Zilleruedo G, Abitbol C, Montane B, Strauss J (1999) Survival in children on chronic hemodialysis. Pediatr Nephrol 13:245–248 [DOI] [PubMed]
- 34.Goldstein SL, Macierowski CT, Jabs K (1997). Hemodialysis catheter survival and complication in children and adolescents. Pediatr Nephrol 1:74–77 [DOI] [PubMed]
- 35.Coulthard MG, Sharp J (2001) Hemodialysis in infants: theoretical limitations, and singles versus double lumen lines. Pediatr Nephrol 16:332–334 [DOI] [PubMed]
- 36.McDowell DE, Moss AH, Vasilakis C, Bell R, Pillai L (1993) Percutaneously placed dual lumen silicone catheters for long term hemodialysis. Am Surg 59:569–573 [PubMed]
- 37.Brittinger WD, Walker G, Twittenhoff WD, Konrad D (1997) Vascular access for hemodialysis in children. Pediatr Nephrol 11:87–95 [DOI] [PubMed]
- 38.Revers SP, Scher LA, Sheekan E, Lynn R, Veith FJ (1993) Basilic vein transposition: an underused autologous alternative to prosthetic dialysis angioaccess. J Vasc Surg 18:391–396 [PubMed]
- 39.Choy L, Collier J, Watson AR (1999) Lignocaine—prilocaine cream or amethocaine gel for venepunture. Acta Paediatr 88:961–964 [DOI] [PubMed]
- 40.Fischbach M, Hamel G, Koehl C, Geisert J (1989) β2 Microglobulin in hemodiafiltered children—long term efficiency follow up. Nephron 53:110–114 [DOI] [PubMed]
- 41.Vanholder RC, Glorieux GL, De Smet RV (2003) Back to the future: middle molecules, high flux membranes and optimal dialysis. Hemodial Int 7(1):52–57 [DOI] [PubMed]
- 42.Locatelli F, Covic A, Chazot C, Leunissen K, Luno J, Yaqoob M (2004) Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant 19:758–796 [DOI] [PubMed]
- 43.Block GA, Port FK (2000) Reevaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysed patients. Recommendations for a change in management. Am J Kidney Dis 35(6):1226–1237 [DOI] [PubMed]
- 44.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood onset chronic renal failure. Circulation 106:100–105 [DOI] [PubMed]
- 45.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. New Engl J Med 342:1478–1483 [DOI] [PubMed]
- 46.Fischbach M, Terzic J, Cousandier E, Hamel G, Geisert J (1998) Glucose charged dialysate for children on hemodialysis: acute dialytic changes. Pediatr Nephrol 12:60–62 [DOI] [PubMed]
- 47.Fischbach M, Zita N, Birmele B, Geisert J (1998) Sequential hypertonic dialysis (SHD) in children. Pediatr Nephrol 2(4):442–446 [DOI] [PubMed]
- 48.Fischbach M, Mengus L, Simeoni U, Durringer R, Mark J, De Geeter B, Hamel G, Geisert J (1991) Dialyse à double profil: ultrafiltration et sodium variables. Description et validation clinique chez l’enfant. Néphrologie 12:179–183 [PubMed]
- 49.Fischbach M, Hamel G, Simeoni U, Geisert J (1992) Phosphate dialytic removal: enhancement of phosphate cellular clearance by biofiltration (with acetate free buffer dialysate). Nephron 62:155–160 [DOI] [PubMed]
- 50.Wuhl E, Fush Ch, Scharer K, Mehls O, Schaefer F (1996) Assessment of total body water in paediatric patients on dialysis. Nephrol Dial Transplant 11:75–80 [PubMed]
- 51.Sitter T, Holzgartner V, Wolfram G, Toepfer M, Klare B, Gerzer R, Schiff H (1999) Assessment of hypervolemia by cyclic 3′,5′-guanonsine monophosphate in pediatric patient on hemodialysis. Nephron 83:287–288 [DOI] [PubMed]
- 52.Cheriex EC, Leunissen KML, Janssen JHA, Mooy JMV, Hooff JP (1989) Echography of the inferior vena cava is a simple and reliable tool for estimation of dry body weight in haemodialysis patients. Nephrol Dial Transplant 4:563–568 [PubMed]
- 53.Sommez F, Mir S, Ozyurek AR, Cura A (1996) The adjustment of postdialysis dry weight based on non-invasive measurements in children. Nephrol Dial Transplant 11:1564–1567 [PubMed]
- 54.Katzarski KS, Charra B, Laurent G, Lopot F, Divino Filho JC, Nisell J, Bergstrom J (1997) A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive hemodialysis patients. Am J Kidney Dis 30:459–465 [DOI] [PubMed]
- 55.Dietel T, Filler G, Ryszard G, Wolfish N (2000) Bioimpedance and inferior vena cava diameter for assessment of dialysis dry weight. Pediatr Nephrol 14:903–907 [DOI] [PubMed]
- 56.Schroeder KL, Sallusto JE, Ross EA (2004) Continuous haematocrit monitoring during intradialytic hypotension: precipitous decline in plasma refill rates. Nephrol Dial Transplant 19:652–656 [DOI] [PubMed]
- 57.Harmon WE (1994) Kinetic modeling of hemodialysis in children. Semin Dial 7(6):392–397
- 58.Marsenic O, Pavlicic D, Peco-Antic A, Bigovic G, Jovanovic O (2000) Prediction of equilibrated urea in children on chronic hemodialysis (HD). Perit Dial Int 20 (Suppl 1):S95 [DOI] [PubMed]
- 59.Evans JHC, Smye SW, Brocklebank JT (1992) Mathematical modeling of haemodialysis in children. Pediatr Nephrol 6:349–353 [DOI] [PubMed]
- 60.Daugirdas JT, Schneditz D (1994) Postdialysis urea rebound: measurement prediction and effects of regional blood flow. Dial Transplant 23:166–173
- 61.Maasrani M, Jaffrin MY, Fischbach M, Boudailliez B (1995) Urea creatinine and phosphate kinetic modeling during dialysis: application to pediatric hemodialysis. Int J Artif Organs 18:122–129 [PubMed]
- 62.Fischbach M, Boudailliez B, Foulard M (1997) Phosphate end dialysis value: a misleading parameter of hemodialysis efficiency. Pediatr Nephrol 11:193–195 [DOI] [PubMed]
- 63.Pedrini LA, Zereck S, Rasmy S (1988) Causes, kinetics and clinical implications of postdialysis urea rebound. Kidney Int 34:817–825 [DOI] [PubMed]
- 64.Schneditz D, Van Stone JC, Daugirdas JT (1993) A regional blood circulation alternative to in series two compartment urea kinetic modeling. ASAIO J 39:M573–M577 [PubMed]
- 65.Buur T, Bradbury MG, Smye SW, Brocklebank JT (1994) Reliability of hemodialysis urea kinetic modeling in children. Pediatr Nephrol 8:574–578 [DOI] [PubMed]
- 66.Marsenic O, Peco Antic A, Jovanovic O (1999) Comparison of two methods for predicting equilibrated Kt/V (eKt/V) using true eKt/V value. Pediatr Nephrol 13:418–422 [DOI] [PubMed]
- 67.Geddes CC, Traynor J, Walbaum D, Fox JG, Mactier RA (2000) A new method of post dialysis blood urea sampling: the stop dialysate flow method. Nephrol Dial Transplant 15:517–523 [DOI] [PubMed]
- 68.Goldstein SL, Sorof JM, Bremer ED (1999) Evaluation and prediction of urea rebound and equilibrated Kt/V in the pediatric hemodialysis population. Am J Kidney Dis 33:518–522 [DOI] [PubMed]
- 69.Goldstein SL, Bremer ED (2000) Logarithmic extrapolation of a 15 minute postdialysis BUN to predict equilibrated BUN and calculate double pool Kt/v in the pediatric hemodialysis population. Am J Kidney Dis 36(1):98–104 [DOI] [PubMed]
- 70.Lonneman G (2000) Should ultrapure dialysate be mandatory. Nephrol Dial Transplant 15 (Suppl 1):55–59 [DOI] [PubMed]
- 71.Cheek DB, Mellits D, Elliott D (1966) Bodywater, height and weight during growth in normal children. Am J Dis child 112:312–317 [DOI] [PubMed]
- 72.Movilli E (1996) Simplified approaches to calculate Kt/V. It’s time for agreement. Nephrol Dial Transplant 11:24–27 [PubMed]
- 73.Stein A, Walls J (1994) The correlation between Kt/V and PCR: a self fulfilling prophecy. Nephrol Dial Transplant 9:743–745 [PubMed]
- 74.Fischbach M, Boudailliez B, Foulard M (1996) Adequacy of dialysis estimated by urea kinetics in children: is there a benefit of a larger dialysis dosis. Nephron 72:104–105 [DOI] [PubMed]
- 75.Tom A, McCauley L, Bell L, Rodd C, Espinosa P, Yu G, Yu J, Girardin C, Sharma A (1999) Growth during maintenance hemodialysis: impact of enhanced nutrition and clearance. J Pediatr 134:464–471 [DOI] [PubMed]
- 76.Riella MC (2000) Malnutrition in dialysis: malnourishment or uremic inflammatory response. Kidney Int 57:1211–1232 [DOI] [PubMed]
- 77.Besbas N, Ozdemir S, Saatci U, Coskem T, Ozen S, Topaloglu, Bekkaloglu A (1998) Nutritional assessment of children on hemodialysis: value of IGF1, TNF alpha and IL beta. Nephrol Dial Transplant 13:1484–1488 [DOI] [PubMed]
- 78.Chadha V, Blowley DL, Warady BA (2001) Is growth a valid outcome measure of dialysis clearance in children undergoing peritoneal dialysis? Perit Dial Int 21(Suppl 3):S179–S184 [PubMed]
- 79.Pierratos A (2001) Introduction: entering the era of daily hemodialysis. Adv Ren Replace Ther 8:223–226 [DOI] [PubMed]
- 80.Twardowski Z J (2003) We should strive for optimal hemodialysis: a criticism of the hemodialysis adequacy concept. Hemodial Int 7(1):5–6 [DOI] [PubMed]
- 81.Michael M, Brewer FP, Goldstein SC (2004) Blood volume monitoring to achieve target dry in pediatric hemodialysis patients. Pediatr Nephrol 19:432–437 [DOI] [PubMed]
- 82.Schroeder CH (2004) The European Pediatric Peritoneal Dialysis Working group. The management of anemia in pediatric peritoneal dialysis patients. Guidelines by an ad hoc European committee. Pediatr Nephrol 18:805–809 [DOI] [PMC free article] [PubMed]
- 83.Querfeld U (2004) The clinical significance of vascular calcification in young patients with end-stage renal disease. Pediatr Nephrol 19:478–484 [DOI] [PubMed]


