Abstract
Neurotransmitter regulation of bone metabolism has been a subject of increasing interest and investigation. We reported previously that osteoblastic cells express a functional serotonin (5-HT) signal transduction system, with mechanisms for responding to and regulating uptake of 5-HT. The clonal murine osteocytic cell line, MLO-Y4, demonstrates expression of the serotonin transporter (5-HTT), and the 5-HT1A, and 5-HT2A receptors by real-time RT-PCR and immunoblot analysis. Immunohistochemistry using antibodies for the 5-HTT, and the 5-HT1A and 5-HT2A receptors reveals expression of all three proteins in both osteoblasts and osteocytes in rat tibia. 5-HTT binding sites were demonstrated in the MLO-Y4 cells with nanomolar affinity for the stable cocaine analog [125I]RTI-55. Imipramine and fluoxetine, antagonists with specificity for 5-HTT, show the highest potency to antagonize [125I]RTI-55 binding in the MLO-Y4 cells. GBR-12935, a relatively selective dopamine transporter antagonist, had a much lower potency, as did desipramine, a selective norepinephrine transporter antagonist. The maximal [3H]5-HT uptake rate in MLO-Y4 cells was 2.85 pmol/15 min/well, with a Km value of 290 nM. Imipramine and fluoxetine inhibited specific [3H]5-HT uptake with IC50 values in the nanomolar range. 5-HT rapidly stimulated PGE2 release from MLO-Y4 cells; the EC50 for 5-HT was 0.1 μM, with a 3-fold increase seen at 60 min. The rate limiting enzyme for serotonin synthesis, tryptophan hydroxylase, is expressed in MLO-Y4 cells as well as osteoblastic MC3T3-E1 cells. Thus, osteocytes, as well as osteoblasts, are capable of 5-HT synthesis, and express functional receptor and transporter components of the 5-HT signal transduction system.
Keywords: bone, neurotransmitter, transporter, osteoblast, osteocyte
Introduction
Neurotransmitter regulation of bone metabolism has been a subject of increasing interest and investigation. Collectively, anatomical and in vitro studies suggest that bone metabolism may be influenced by the nervous system [1–10]. These immunohistochemical and biochemical studies of nervous system components in bone may reflect not only sensory and vascular regulatory functions for neurotransmitters, but potentially neurohormonal control of bone cell activities. Evidence for this hypothesis includes the demonstration that receptors for neuropeptides, catecholamines, and excitatory amino acids are present on bone cells, and some of these agonists (such as VIP, CGRP or glutamate) may influence bone resorption and formation ([11, 12]; reviewed in [13]). These observations have been extended recently with the work on leptin regulation of bone formation. These studies have demonstrated that leptin exerts an antiosteogenic effect through a central hypothalamic pathway [14]. Leptin appears to regulate both osteoblastic bone formation and osteoclastic bone resorption [46]. In addition, neuropeptide Y (NPY) and hypothalamic Y2 receptors, which are involved in appetite control, also regulate bone formation via a central mechanism [15]. Further work has demonstrated that the peripheral mediators of leptin antiosteogenic function appear to be neuronal, in that genetic or pharmacological ablation of adrenergic signaling leads to a leptin-resistant high bone mass [16]. Leptin may exert a direct stimulatory effect on bone growth as well [17].
Complementary to these findings are reports of the effects of neurotransmitter transporter expression/deletion on bone function. In osteoblast and osteocyte cells, expression and regulation of the excitatory amino acid glutamate/aspartate transporter (GLAST) by mechanical loading has been described [4]. We have demonstrated that disruption of the dopamine transporter (DAT) gene in mice [18] results in deficiencies in skeletal structure and integrity. More recently, we have analyzed skeletal structure in mice with disruption of the serotonin transporter gene (5-HTT−/− mice) [19]. 5-HTT−/− mice have reduced bone mass, size and strength compared with wild type littermates. Bone formation rates are reduced compared to wild type animals. No influence of null mutation of the 5-HTT gene was found on skeletal mechanosensitivity.. It is not known whether this skeletal phenotype reflects direct or indirect effects of the 5-HTT on bone.
5-HTT and DAT are members of a highly homologous family of neurotransmitter transporters for bioactive amines. These transporters cause intracellular accumulation of neurotransmitters by reuptake from the extracellular fluid through a sodium/chloride dependent cotransport process (for review see [20]). Presynaptic transporters that reduce neurotransmitter concentrations in the synapse are a major mechanism for terminating synaptic transmission [21]. Augmentation of synaptic activity by inhibition of sodium-dependent monoamine transport forms the basis for the mechanism of action of important antidepressant drugs.
Westbroek et al [22] demonstrated the expression of mRNA for the serotonin (5-HT) 2B receptor in chicken osteocytes, osteoblasts, and periosteal fibroblasts, a population containing osteoblast precursor cells. In addition, they found mRNA expression for the 5-HT2A, 5-HT2B, and 5-HT2C receptors in murine osteoblasts. They also demonstrated that occupancy of the 5-HT2B receptor stimulates proliferation of periosteal fibroblasts, and activation of 5-HT2 receptors decreases nitric oxide synthesis in mechanically stimulated osteoblasts. We confirmed expression of 5-HT2A and 5-HT2B receptor proteins, and demonstrated that the 5-HT1A and 5-HT1D receptors and the 5-HTT are expressed in osteoblastic cells [23]. 5-HT receptors are expressed in both cultured osteoblastic cell lines and normal differentiating rat osteoblasts, and the 5-HTT is expressed in all osteoblastic cell lines examined. 5-HTT activity is down-regulated by PMA treatment in osteoblastic cells. Finally, 5-HT potentiates PTH regulation of AP-1 activity in rat osteoblastic UMR 106-H5 cells. Gustafsson et al found that 5-HT enhances proliferation of mesenchymal stem cells and primary osteoblasts, as well as 5-HT2A receptor expression [24]. Thus osteoblasts possess a functional system for both responding to and regulating 5-HT activity.
In light of our demonstration of 5-HTT and 5-HT receptor expression in primary osteoblast cultures, including during the mineralization phase, we decided to explore the expression of these proteins in the next phase of osteoblast differentiation, i.e., osteocytes. We now demonstrate that 5-HTT and 5-HT receptors are expressed in osteocytic cell lines and in situ in bone. Furthermore, we show that the transporter functions with a pharmacologic profile consistent with 5-HTT expressed in other cell types. 5-HT also stimulates PGE2 release in the MLO-Y4 osteocytic cell line. Finally, osteocytic and osteoblastic cells express the rate-limiting enzyme for 5-HT synthesis. This data extends our previous observations in osteoblasts, and together with more recent data demonstrating osteopenia associated with reduced bone formation rates in 5-HTT knockout mice, suggests that the 5-HT signal transduction system may play a significant role in osteoblast recruitment and/or osteoblast/osteocyte differentiation.
Methods
Cell Culture
Media, buffers, supplements and reagents for cell culture were obtained from GIBCO BRL-Life Technologies (Grand Island, NY) and Sigma Chemical Co. (St. Louis, MO). The immortalized mouse osteocytic cell line MLO-Y4 (generous gift of Dr. Lynda Bonewald) was cultured in α-MEM containing 5% bovine serum at 37°C in 5% CO2. The MC3T3-E1 cell line (generous gift of Dr. Peter Rotwein) was cultured in MEM, supplemented with l-glutamine and HEPES, containing 5% bovine serum at 37°C in 5% CO2. The A293 cell line (ATCC #CRL-1573) was cultured in Minimum Essential (Eagle) Media containing 1.0 mM sodium pyruvate and 10% heat inactivated horse serum at 37°C in 5% CO2. B14 cells (a generous gift of Dr. Mary Eaton) were grown in DMEM/F12 containing 10% fetal calf serum at 33°C in 5% CO2. For the immunoblot and real-time RT-PCR studies, B14 cells were differentiated in DMEM/F12/B16 media at 39°C in 5% CO2 prior to harvesting.
Immunoblot Protocol
For assessment of 5-HT receptor protein expression, whole cell extracts were prepared in RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1mM PMSF, 1 mM EDTA, 5ug/ml aprotinin, 5 ug/ml leupeptin, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS), incubated on ice for 20 min, cleared by centrifugation (13000 rpm x 5 min), and supernatant protein was subjected to SDS-PAGE on 10% gels. Western blot analysis was performed on equalized total protein concentrations (as determined with bicinchonic acid analysis (BCA; Pierce), using Immobilon-P PVDF membranes (Millipore, Billerica, Massachusetts) and subsequent immunoblotting. Following incubation with primary antibody (see below), blots were exposed to secondary antibody conjugated with HRP, and visualized with ECL reagent (Amersham). Immunoblots were performed using rabbit polyclonal antibodies against the 5-HTT (Chemicon, Temecula, CA; 5 μg/ml) and the 5-HT1A receptor (Santa Cruz Biotechnology, Santa Cruz, CA; 1:250) and a mouse monoclonal antibody against the 5-HT2A receptor (BD PharMingen, San Diego, CA). Secondary antibodies were either goat anti-rabbit (Promega) for the 5-HTT and the anti-5-HT1A receptor, or goat anti-mouse (Promega) for the 5-HT2A receptor. As a loading control for total protein, blots were stripped and reprobed with an anti-α-tubulin antibody at 1:1000 (Sigma). Molecular weight standards were Precision Plus Standards (Bio-Rad, Hercules, CA). Quantitative analysis of the proteins was performed by volume densitometry using Optiquant Software (PerkinElmer Life and Analytical Sciences, Inc, Boston, MA) after scanning of the film (ScanMaker 9800XL, Microtek, Carson, CA) in the linear range. Data is presented as the protein to α-tubulin ratio to correct for variations in protein loading and then normalized to control values for comparison between cell lines.
Real-time RT-PCR analysis
Expression of 5-HTT, 5-HT1A and 5-HT2A receptors and TPH1 mRNA was determined by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis with the iCycler IQ Real Time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA) using a one-step procedure. Total RNA was extracted from MLO-Y4, MC3T3-E1, B14, and A293 cell cultures using the RNA Stat-60 kit (Tel-Test, Inc. Friendswood, TX) followed by DNase treatment with RQ1-DNase (Promega, Madison WI) digestion and Zymo-spin column purification according to manufacturer’s instructions (Zymo Research, Orange, CA). Twenty ng RNA was reverse transcribed and amplified in a 25 μl reaction mix containing 1x QuantiTect SYBR Green RT-PCR Master Mix (Qiagen, Valencia CA) and 0.5 μM each primer. Gene-specific primers for mouse were from the Qiagen QuantiTect Primer Assay. For the 5-HTT, gene sequence number was QT00163345, accession number NM_010484; for the 5-HT1A receptor, gene sequence number was QT00250516, accession number NM_008308; for the 5HT2A receptor, gene sequence number was QT00282947, accession number NM_172812; for TPH1, gene sequence number was QT00152565, accession number NM_009414. Relative expression of the RT-PCR product was determined using the comparative ΔΔCt method [25], after normalizing expression to total RNA measured with RiboGreen (Molecular Probes, Eugene, OR, USA) [26]. Individual reaction kinetics were also analyzed using a five-fold dilution series of total RNA to ensure each qRT-PCR did not differ significantly from 100%. Following PCR, reaction products were melted over the temperature range 55°C to 95°C in 0.5°C increments, 10 sec per increment to ensure only the expected PCR product was amplified per reaction.
Immunocytochemistry
6 week old Wistar Rats were sacrificed by pentobarbital injection and infused with 1% glutaraldehyde in PBS by intracardiac injection. Tibiae were dissected, fixed in glutaraldehyde 1%, decalcified in EDTA 4%/glutaraldehyde 1%, and embedded in Epon. Semithin sections from decalcified samples were laid on silanized slides (Dako, Carpinteria, CA) and dried overnight at 37°C. Epon was removed with 13.3% (w/v) potassium hydroxide in a methanol/propylene oxyde (2/1) mixture and rehydrated. Sections were treated for 20 min with 100 mM glycine and 50 mM ammonium chloride in Tris buffer, pH 7.6 in order to saturate free aldehydic groups. Endogenase peroxidase activity was inhibited by incubation for 15 min with 1% sodium azide and 1.5% H202 in 50% methanol, and nonspecific immunoreactions were blocked for 30 min in 10% normal goat serum (NGS) in tris buffered saline (TBS) containing 0.01% bovine serum albumin. Sections were incubated overnight with specific antibodies diluted in TBS containing 1% NGS. Antibodies used for immunocytochemistry were the following: control sections for polyclonal antibodies (5-HTT and 5-HT1A; Santa Cruz Biotechnology) were stained with nonimmune rabbit serum, while control sections for mouse monoclonal antibody (5-HT2A; BD PharMingen) were stained with the monoclonal antibody MOPC21 which has no known hapten or antigen binding activity. All control antibodies were used at the same dilutions as primary antibodies.
The concentrations of antibodies used were 5-HTT: 2 μg/ml; 5-HT1A: 1 μg/ml; 5-HT2A: 2.5 μg/ml. Antigen-antibody complexes were detected with EnVisionTM/HRP System (Dako), and revealed with 3-3′ diaminobenzidine tetrahydrochloride (DAB) (Dako) in Tris buffer containing 0.01% H202. Sections were counterstained with Meyer’s hemalum, dehydrated and mounted in Xam (Gurr-BDH Laboratory, Poole, UK).
5-HTT Binding Assays/[3H]5-HT Uptake Assay
5-HTT binding and uptake assays were performed as previously described [23] with the following modifications: for [125I]RTI-55 binding, the pellet prepared from a 150 mm diameter plate of cells was resuspended in 2 ml of 0.32 mol/L sucrose. Assays contained approximately 45 μg of protein. For [3H]5-HT uptake, assays were conducted for 15 min at 30°C.
Prostaglandin Assay
MLO-Y4 and MC3T3-E1 cells (80% confluent) were treated for 5, 10, 20, 30 and 60 min with 5-HT (100 μM) or vehicle for the time course studies, and 0.1–1000 μM for 30 min for the dose response studies. The amount of PGE2 in the medium following treatment was measured using a PGE2 EIA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Data Analysis
GraphPad Prism 3.0 (GraphPad Software, San Diego, CA) was used to analyze saturation and competition binding data and dose response curves, as well as for statistical analyses. IC50 values were converted to Ki values using the Cheng-Prusoff equation [27]. Significance was set at p<0.05.
Results
Western blot analysis demonstrated protein expression of the 5-HTT, as well as 5-HT1A and 5-HT2A receptors in the immortalized murine osteocytic MLO-Y4 cell line (Figure 1). We chose to evaluate expression of the 5-HT1A and 5-HT2A receptors because we previously demonstrated their expression in primary osteoblast cultures and osteoblastic cell lines [23]. 5-HTT expression was evaluated with a polyclonal antibody raised against a peptide mapping at the carboxy-terminus of the human serotonin transporter (Figure 1A). A dominant band of 62 kDa was identified in the immunoblots in MLO-Y4 (lane 2) and MC3T3-E1 (lane 3), and the differentiated B14 rat neuronal cell line, which has been shown to express the 5-HTT [28]. A doublet at 62 kDa was recognized in all three cell lines with the 5-HT1A antibody (Figure 1B); MLO-Y4 cells had an additional band at ~100 kDa. The 5-HT2A receptor antibody also recognized a doublet of 62 kDa in all three cell lines (Figure 1C).
Figure 1.
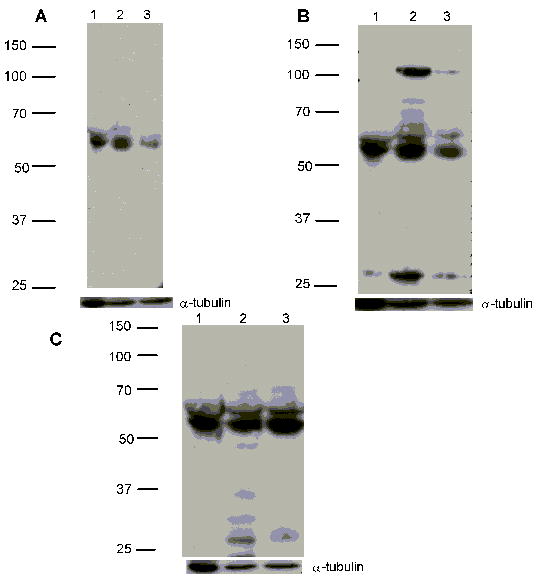
Immunoblot analysis of 5-HTT and 5-HT receptor expression in MLO-Y4 cells. Cell lysates were subjected to SDS-PAGE on 10% gels. Immunoblots were carried out as described in Methods, using rabbit polyclonal antibodies against the 5-HTT (Panel A) and the 5-HT1A receptor (Panel B), or mouse monoclonal antibodies against the 5-HT2A receptor (Panel C). Molecular weight markers are shown on the left of each blot. Lane 1: B14 (100 μg); lane 2: MLO-Y4 (100 μg); lane 3: MC3T3-E1 (100 μg). Bottom panel beneath each blot represents the same membranes reprobed with an anti-α-tubulin antibody as a loading control.
We performed quantitative densitometry of the immunoblots in order to assess the relative expression of the 5-HTT and 5-HT receptors in the different cell lines. Using the B14 cell line protein expression as the control, expression of the 5-HTT in the MLO-Y4 cells was 25 % greater, and in the MC3T3-E1 cells 32% reduced. For the 5-HT1A receptor, expression in MLO-Y4 cells was 23 % greater, and in MC3T3-E1 cells 5 % reduced compared to the B14 cells. Finally, for the 5-HT2A receptor, expression was increased by 24 % and 37 % in the MLO-Y4 and MC3T3-E1 cells, respectively, compared to B14 cells.
We confirmed mRNA expression for the 5-HTT, and 5-HT1A and 5-HT2A receptors in MLO-Y4, MC3T3-E1 and B14 cells by real-time RT-PCR analysis using primers directed against mouse sequences. Figure 2 shows the quantitative analysis of mRNA expression for the 5-HTT (panel A), 5-HT1A (panel B), and 5-HT2A (panel C) receptors in the cultured cells. Expression in the MLO-Y4 and MC3T3-E1 cells is normalized to the B14 cells. 5-HTT mRNA expression in the MLO-Y4 cells was >50-fold higher than in the B14 neuronal cell line, with the expression in MC3T3-E1 cells being slightly less than the B14 cells. This relationship was qualitatively similar to that seen with protein expression as determined by immunoblots. 5-HT receptor expression varied, with 5-HT1A receptor mRNA expression in MC3T3-E1>MLO-Y4=B14, differing from protein expression where MLO-Y4 cells showed the highest expression. 5-HT2A receptor mRNA expression demonstrated MLO-Y4>B14>MC3T3-E1, again differing from protein expression where MC3T3-E1 cells showed the highest level.
Figure 2.
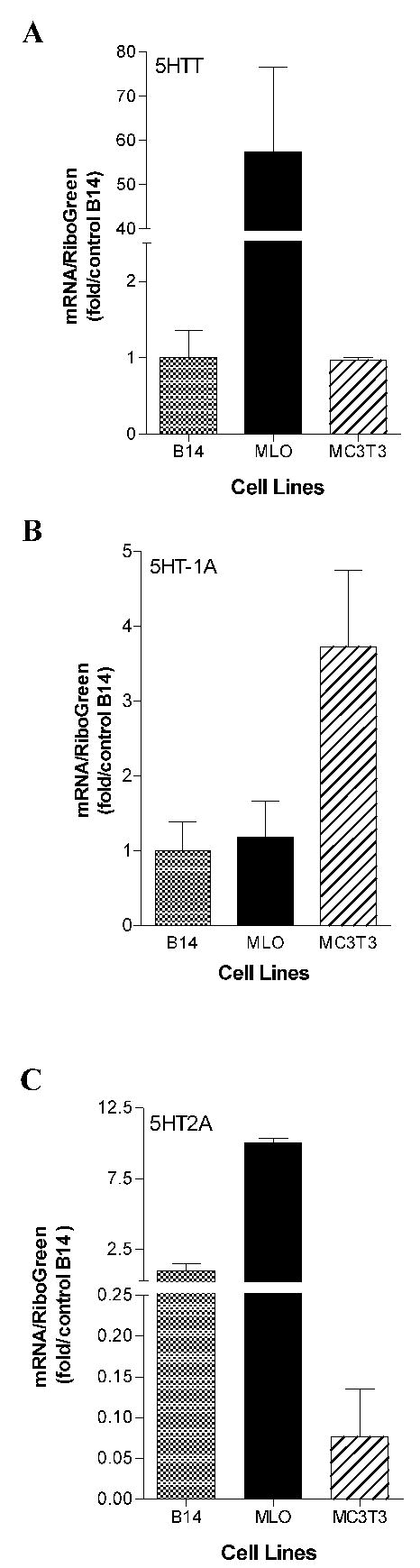
Real-time RT PCR analysis of 5-HTT, 5-HT1A and 5-HT2A receptor mRNA expression in MLO-Y4, MC3T3-E1 and B14 cells. Total RNA was isolated from the indicated cells (n=2). Expression of 5-HTT, 5-HT1A and 5-HT2A receptor mRNA was evaluated by real-time RT-PCR analysis after normalization to total RNA determined by RiboGreen assay. Data are expressed relative to the expression level in B14 cells as mean ± SEM. A: 5-HTT; B: 5-HT1A; C: 5-HT2A.
We next investigated the expression of the 5-HTT, the 5-HT1A and 5-HT2A receptors in sections of whole bone from rat tibia using immunohistochemistry. Figure 3 shows that the 5-HTT protein is expressed in osteoblasts and osteocytes, as are the 5-HT1A and 5-HT2A receptors. For each of the 6 rats, 12 sections were stained with each antibody, and in all sections osteoclasts were always negative. We have not found any correlation between the expression of serotonin receptors and transporter and osteocyte lacunae size and density. The lack of staining of some osteocytes may be associated with changes in osteocyte integrity as defined by Bentolila et al [29]. It is also possible that masking of the antigen by mineral or simply lack of expression of the transporter and receptor proteins accounts for negative staining. These observations confirm our findings in the immunoblot experiments (Figure 1).
Figure 3.
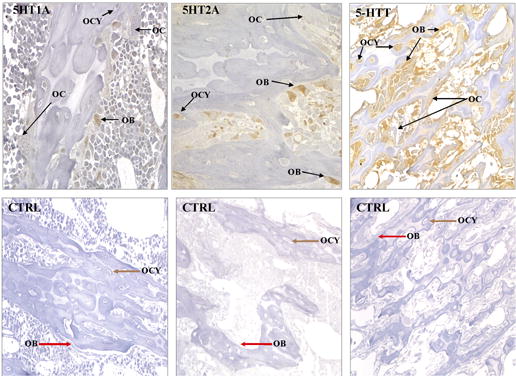
Immunostaining for 5-HTT, 5-HT1A and 5-HT2A receptors in rat tibia. Whole bone sections were prepared for immunohistochemistry as described in Methods. Antibody staining is shown in the upper panels; control staining with nonimmune rabbit serum (5-HTT and 5-HT1A) or irrelevant mouse monoclonal antibody (5-HT2A) is in the lower panels. The 5-HTT, 5-HT1A, and 5-HT2A receptors are expressed in both osteoblasts (OB) and osteocytes (OC), but not in osteoclasts (OC). Sections were counterstained with Meyer’s hemalum.
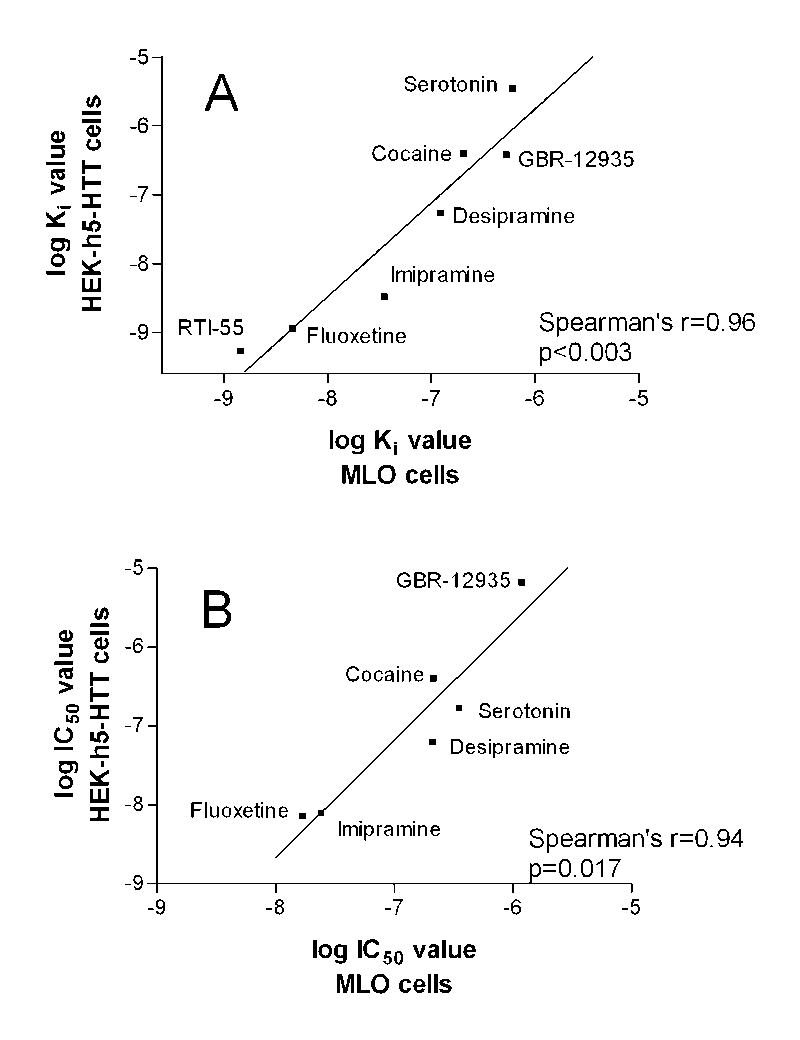
Binding and uptake studies to demonstrate functional expression of the 5-HTT were performed in the MLO-Y4 osteocytic cell line. Antagonist and substrate affinities for the 5-HTT were evaluated using [125I]RTI-55 binding to membrane preparations. [125I]RTI-55 is a stable cocaine analog that has high affinity for the 5-HT, dopamine and norepinephrine transporters. MLO-Y4 cells expressed high affinity [125I]RTI-55 binding sites, with a Kd value of 1.43 ± 0.43 nM and a density of 69 ± 23 fmol/mg protein. To pharmacologically characterize the binding site labeled by [125I]RTI-55, competition assays were conducted using six different compounds; the results are shown in Table 1. Two antagonists with specificity for 5-HTT, imipramine and fluoxetine, showed the highest potency in MLO-Y4 cells with affinities of 34.8 and 4.56 nM, respectively (Table 1, column 3). In contrast, GBR-12935, a relatively selective dopamine transporter antagonist, had a much lower potency (524 nM), as did desipramine, a selective norepinephrine transporter antagonist (122 nM). As shown in Figure 4A, the potencies for all drugs tested compared favorably with values measured in HEK 293 cells heterologously expressing the human 5-HTT (also known as hSERT, see [30]). Thus, the pharmacologic characterization of the 5-HT binding site in MLO-Y4 cells is highly consistent with the 5-HTT.
Table 1.
Drug potency for inhibition of [3H]5HT uptake by MLO-Y4 cells and [125I]RTI-55 binding to MLO-Y4 cell membranes.
| Drug | Inhibition of [3H]5HT uptake IC50 (nM) ± sem | Inhibition of [125I]RTI-55 binding Ki (nM) ± sem |
|---|---|---|
| RTI-55 | 1.43 ± 0.43 (Kd) | |
| Fluoxetine | 16.6 ± 4.1 | 4.56 ± 0.31 |
| Imipramine | 24.1 ± 2.5 | 34.8 ± 8.7 |
| Desipramine | 208 ± 15 | 122 ± 21 |
| Cocaine | 212.9 ± 3.3 | 202 ± 53 |
| Serotonin | 352 ± 37 | 600 ± 120 |
| GBR-12935 | 1180 ± 110 | 524 ± 28 |
For MLO-Y4 cells, typical binding experiments had 784 specific and 356 nonspecific cpm per assay containing ~30 μg protein, and typical uptake experiments had 7600 specific and 610 nonspecific cpm per well when uptake was conducted with 30 nM [3H]5-HT for 15 min at 30°C. A Kd value for [125I]RTI-55 of 1.43 nM was used in the Cheng-Prusoff correction for Ki values. All binding experiments were conducted in duplicate and all uptake experiments were conducted in triplicate (N=3–5). 5-HTT selective compounds have the following relative affinities for the DAT, NET and 5-HTT: fluoxetine 5-HTT>>NET>DAT; imipramine 5-HTT>>NET>>DAT. NET selective compound: DMI>5-HTT>>DAT. DAT selective compound: GBR 12935 DAT > 5-HTT =NET. The greater potency of 5-HT at inhibition of uptake as compared to inhibition of binding is typical of transporter substrates.
Figure 4.
Correlations of inhibitory potencies of antagonists and substrates in MLO and HEK-5HTT cells. (A) Ki values for [125I]RTI-55 binding. (B) IC50 values for [3H]5HT uptake. Ki and IC50 values for HEK-5HTT were reported in [30]). Spearman’s nonparametric correlational analysis was used. Conditions and analyses of the binding assays for the two cell lines were identical except for a larger amount of membrane protein used in the MLO-Y4 cell assays. The conditions for uptake were slightly different: uptake assays with MLO-Y4 cells were conducted with attached cells at 30°C for 15 min with 30 nM [3H]5-HT, whereas uptake assays with HEK-5-HTT cells were conducted with detached cells using a filtration assay at room temperature for 10 min with 20 nM [3H]5-HT. The conditions used with the MLO cells were optimal based on preliminary experiments (data not shown). The data are consistent with 5-HTT expression in MLO-Y4 cells.
In order to measure functional capacity of the 5-HTT in osteocytic cells, we performed uptake studies with [3H]5-HT in MLO-Y4 cultures. The specific uptake rate was linear with respect to time up to 20 min. At 15 min, non-specific uptake was less than 10% of specific uptake. The maximal [3H]5-HT uptake rate for MLO-Y4 cells was 2.85 ± 0.23 pmol/15 min/well, with a Km value of 290 ± 100 nM. Inhibition of specific [3H]5-HT uptake by imipramine and fluoxetine also demonstrated the high affinity of these antagonists for the 5-HTT in the MLO-Y4 cells, with IC50 values in the nanomolar range, again comparable to 5-HTT (hSERT) expressing HEK cells (Figure 4B). The rank order of potency of neurotransmitters for inhibition of [3H]5-HT uptake was 5-HT > dopamine > norepinephrine. Combined, the pharmacological profile of these binding and uptake studies indicates that the MLO-Y4 cells express functional 5-HTT.
MLO-Y4 cells, like primary osteocytes, have been shown to release prostaglandins in response to fluid flow treatment [31, 32]. 5-HT has been shown to stimulate PGE2 release from rat cultured mesangial cells [33]. To assess whether 5-HT might stimulate PGE2 release from MLO-Y4 cells, we treated the cells with 5-HT, and measured changes in PGE2 concentration in the culture medium. The time course of the 5-HT-induced PGE2 increase in conditioned medium is shown in Figure 5A. PGE2 increased by 5 min following 100 μM 5-HT stimulation, and at 60 min was 3-fold greater than vehicle control. The dose response curve for 5-HT is shown in Figure 5B. The EC50 for 5-HT-induced increase in PGE2 was 0.1 μM, with a peak effect at 1 μM.
Figure 5.
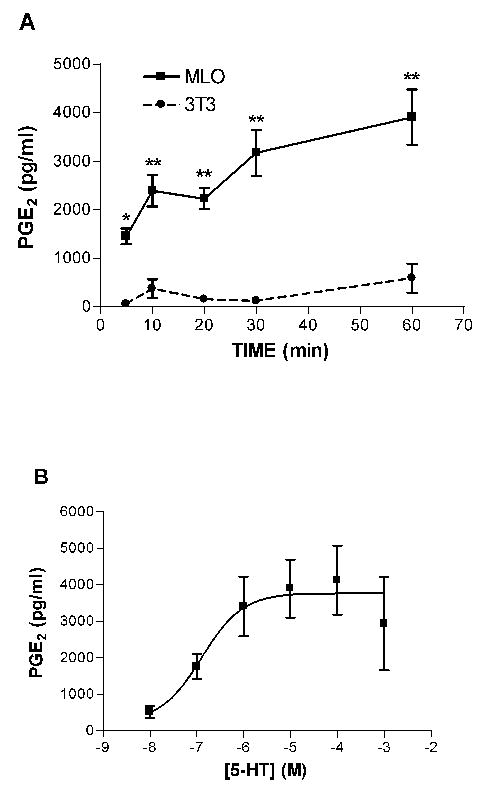
5-HT stimulates the release of PGE2 from MLO-Y4 cells. A: Time course. MLO-Y4 (80% confluent) or MC3T3-E1 cells (80% confluent) were treated for the indicated times with 5-HT (100 μM) or vehicle and the amount of PGE2 in the medium following treatment was measured using a PGE2 EIA kit (Cayman Chemical) according to the manufacturer’s instructions. Data shown were derived from three experiments performed in triplicate. Bars represent mean±SEM. Each data point represents treated minus vehicle control for that time point. Baseline (untreated) PGE2 concentrations were 643±196 pg/ml for MLO-Y4, and 554±224 for MC3T3-E1. *, p<0.01; **, p<0.001 compared to MC3T3-E1. B: Dose response. MLO-Y4 cells were treated for 30 min with the indicated doses of 5-HT and PGE2 in the medium was determined. Data shown were derived from two experiments performed in triplicate. Data points represent treated minus vehicle control. Bars represent mean±SEM.
A critical question in the analysis and extrapolation of our findings is the origin of the serotonin ligand that binds to cognate receptors and transporters in bone cells. It is possible that serotonergic innervation could be responsible, although serotonergic nerves have not been identified in bone. The other possibility is that bone cells themselves could produce serotonin, in which case the effects from the ligand would be autocrine/paracrine in nature. We investigated this hypothesis by assessing expression of the initial and rate limiting enzyme in serotonin synthesis, tryptophan hydroxylase (TPH) [34–36].
To assess TPH expression in bone cells, we performed real-time RT-PCR using primers for the TPH1 isoenzyme which is the form expressed in non-brain tissues such as gut, spleen and thymus (reviewed in [37]). The results are shown in Figure 6. The B14 cell line used as a positive control is a serotonergic cell line derived from embryonic day 13 rat medullary raphe cells which expresses the 5-HTT, 5-HT1A receptor, and TPH [28]. Both MLO-Y4 and MC3T3-E1 cell lines express mRNA for TPH1, and thus have the capability for serotonin synthesis. No amplified products were observed in A293 cells (Figure 6) or COS-7 cells (data not shown), which do not have detectable TPH enzyme activity [38].
Figure 6.
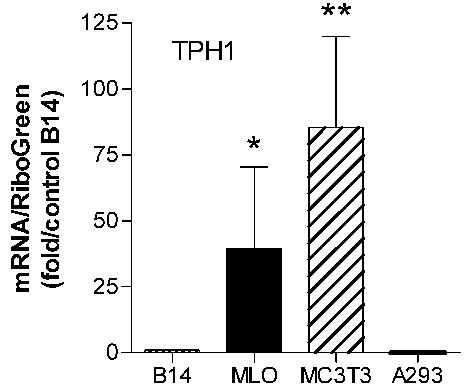
Real-time RT PCR analysis of TPH1 enzyme mRNA expression in MLO-Y4, MC3T3-E1, B14 and A293 cells. Total RNA was isolated from the indicated cells. Expression of TPH1 mRNA was evaluated by real-time RT-PCR analysis (n=6 for MLO-Y4 and MC3T3-E1, n=5 for B14, and n=2 for A293) after normalization to total RNA determined by RiboGreen assay. Data are expressed relative to the expression level in B14 cells as mean ±SEM. *, p<0.05 compared to B14; **, p<0.01 compared to B14 by Kruskal-Wallis test, with post-hoc analysis performed by Dunn’s multiple comparison test.
Discussion
We have demonstrated that the 5-HTT and 5-HT receptors are expressed in a clonal osteocytic cell line and in normal rat tibial osteocytes. This is the first report of 5-HTT expression in osteocytes, and extends our previous observations of 5-HTT and 5-HT receptor expression in osteoblasts [23]. Previously, 5-HT2B receptor mRNA expression was reported in osteocytes [22]. We have also demonstrated 5-HT stimulation of PGE2 levels in MLO-Y4 cells. Thus the 5-HT receptors expressed in osteocytes appear functional.
The functional role of 5-HTT expression in osteoblasts/osteocytes is unknown. However, we have reported that mice with disruption of the 5-HTT gene have reduced cortical and trabecular bone mass, with decreased breaking strength [19]. In addition, fluoxetine, a selective serotonin reuptake inhibitor, reduces bone accrual in growing mice. However, the 5-HTT KO mice do not appear to have any deficiency in responding to mechanical loading. Thus, the 5-HTT plays an important role in maintaining bone mass, but the mechanism is unknown.
The mouse 5-HTT cDNA sequence encodes a protein of 630 amino acids which contains twelve potential transmembrane domains [39]. Real-time RT-PCR analysis demonstrated a much higher level of expression of the 5-HTT in MLO-Y4 cells compared to MC3T3-E1 osteoblastic cells and the B14 rat neuronal cell line. Our polyclonal antibody raised against the carboxy-terminus of the human 5-HTT recognized a band of 62 kDa in MLO-Y4 and MC3T3-E1 cell extracts. The 5-HTT is differentially glycosylated in various rat tissues: 76 kDa in brain, 94 kDa in platelets and 60 kDa in both after deglycosylation [40]. The size of 5-HTT in bone suggests that it has less glycosylation than when expressed in other tissues. An anti-peptide antibody against a carboxy-terminal sequence of the human 5-HT1A receptor recognized a doublet of 62 kDa in extracts of MLO-Y4 and MC3T3-E1 cells (Figure 1B). Varied results have been reported for the molecular weight of the 5-HT1A receptor. A polyclonal antibody directed against residues 170–186 of the rat receptor recognized bands at 49.5, 42, and 39 kDa on immunoblots of crude hippocampal proteins [41], whereas another antibody against residues 258–274 recognized bands of 40 and 70 kDa in hippocampal membranes [42]. For the 5-HT2A receptor, we identifed a doublet of 62 kDa in MLO-Y4 and MC3T3-E1 cells, identical to that seen in MC3T3-E1 cells. For comparison, a monoclonal antibody raised against a fusion protein containing amino acids 1–72 of the human 5-HT2A receptor recognized bands of 53 and 58 kDa in rat brain [43].
In previous work, we demonstrated in differentiating primary cultures of fetal rat calvarial osteoblasts (rOB), that functional 5-HTT activity was detected late in culture (day 25), a time at which the cells are secreting osteocalcin and undergoing mineralization [44]. This result suggests that transporter uptake of 5-HT may influence events late in the differentiation paradigm of the osteoblast, i.e, during the transition to osteocytes. This observation correlates well with our current demonstration of 5-HTT uptake and binding activity in MLO-Y4 cells, an osteocytic cell line. The affinities of RTI-55 at the ligand binding site and of 5-HT for uptake in the MLO-Y4 cells (Kd=1.43 nM and Km=290 nM, respectively) are very similar to the values we observed in primary rat osteoblast cultures and osteoblastic cell lines.
Expression in bone cells of another neurotransmitter transporter has been described [4]. Messenger RNA and protein for the excitatory amino acid glutamate/aspartate transporter was found in osteoblasts and osteocytes. Regulation of the glutamate transporter protein was demonstrated by mechanical loading. Furthermore, mRNA expression has been demonstrated for a range of glutamate receptor subtypes in various osteoblast cell lines and primary cultures and in osteoclasts [1, 12]. In addition, functional glutamate receptors have been demonstrated in mammalian osteoclasts [45], and specific N-methyl-d-aspartate (NMDA) antagonists have been shown to prevent formation of the osteoclast sealing zone required for bone resorption [46]. It has also been shown that glutamate-containing fibers, among others, are present as a dense and intimate network in bone tissue [47]. Together, these studies suggest a functional system for glutamate in bone.
We have also begun to explore the functional correlates of 5-HT receptor and 5-HTT expression in osteoblastic cells. MLO-Y4 cells have extensive dendritic processes. Dye-transfer experiments show that they are functionally coupled, and this coupling is mediated by gap junction channels [48]. MLO-Y4 cells, like primary osteocytes, have been shown to release prostaglandins in response to fluid flow treatment [31, 32]. This prostaglandin release is thought to be involved in the stimulatory effects of fluid flow-induced shear stress on intercellular communication [49]. 5-HT has been shown to stimulate PGE2 release from rat cultured mesangial cells [33]. Our results demonstrate that 5-HT rapidly and in a dose-dependent manner stimulates release of PGE2 from MLO-Y4 cells. MC3T3-E1 osteoblastic cells showed very little response to 5-HT. We do not yet know if this increase in PGE2 release alters intercellular communication as observed for fluid flow treatment, for example by increasing connexin 43 levels. Our findings are in contrast to those of Locker et al, who investigated 5-HT regulation of PGE2 production in the C1 mesoblastic cell line which has osteogenic potential [50]. Their data demonstrates that 5-HT stimulates PGE2 production in the C1 cells via arachidonic acid-dependent cyclooxygenase (COX) activation at the beginning of mineral deposition; when C1 osteoblasts undergo conversion to osteocyte-like cells, COX activity is quenched. The reason for this apparent discrepancy is unclear; perhaps it is related to the difference in derivation of the two cell lines (C1 versus MLO-Y4).
A critical question in the analysis and extrapolation of our findings is the origin of the serotonin ligand that binds to cognate receptors and transporters in bone cells. It is possible that serotonergic innervation could be responsible, although serotonergic nerves have not been identified in bone. The other possibility is that bone cells themselves could produce serotonin, in which case the effects from the ligand would be autocrine/paracrine in nature. We investigated this hypothesis by assessing expression of the initial and rate limiting enzyme in serotonin synthesis, tryptophan hydroxylase (TPH) [34–36] in osteoblastic and osteocytic cells. Using real-time RT-PCR, we observed that TPH mRNA is expressed in MLO-Y4 and MC3T3-E1 cells at levels that exceed the rat neuronal B14 cell line. Gustaffson et al also found expression of TPH1 mRNA in MC3T3-E1 cells by one-step RT-PCR [24]. These findings indicate that osteocytes and osteoblasts are capable of synthesizing serotonin, and thus can produce paracrine/autocrine effects through this neurotransmitter on bone cells.
The nature of the serotonin-mediated interaction, if any, between osteoblasts and osteocytes is unknown. It may be that the much higher levels of TPH1 mRNA in the osteoblastic MC3T3-E1 cells is indicative of a higher level of serotonin-synthesizing capacity, and thus osteoblast-derived serotonin may be responsible for inducing a PGE2 response in osteocytic cells. Since osteocytes are mechanosensitive cells, it is tempting to hypothesize that serotonin might influence the threshold for PGE2 release in mechanically loaded cells. Our data in vivo in 5-HTT null mice did not find any evidence for altered mechanosensitivity, but it may be that the serotonin levels, which are increased in these mice, exceed the threshold for osteocyte activation. Alternative approaches to evaluate this hypothesis would include measuring the PGE2 response to serotonin activation in mechanically loaded osteocytic cells in vitro in the presence of serotonin receptor antagonists, or assessing the osteogenic response to loading in vivo in serotonin receptor null mice.
We have previously demonstrated that disruption of the dopamine transporter (DAT) gene in mice results in deficiencies in skeletal structure and integrity by mechanisms yet to be defined. [18]. These studies, combined with the results presented here, suggest that neurotransmitters, through their respective transporters and receptors, may play a significant but underappreciated role as signaling molecules that modulate skeletal health. Further, they suggest that one level of neurotransmitter action may be through direct or indirect effects on differentiating osteoblasts/osteocytes. Our findings regarding reduced bone mass and strength in 5-HTT knockout mice suggests that the 5-HT signal transduction system plays an important role in skeletal development or maintenance. Future work in our laboratory will investigate the mechanisms involved.
Acknowledgments
The authors gratefully acknowledge the excellent technical support provided by Les Alberque throughout the course of these studies.
Footnotes
Portions of this investigation were presented at the Twenty-fourth Annual Meeting of the American Society for Bone and Mineral Research, San Antonio, TX, USA, 2002 (Abstract SU270). This work was supported in part by the Medical Research Service of the Department of Veterans Affairs, and NIH grant DK54415 to MB.
References
- 1.Patton A, Genever P, Birch M, Suva L, Skerry T. Expression of an N-methyl-D-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone. 1998;22:645–649. doi: 10.1016/s8756-3282(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 2.Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25:119–125. doi: 10.1016/0165-1838(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 3.Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–171. doi: 10.1016/0196-9781(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Mason DJ, Suva LJ, Genever PG, Patton AJ, Steuckle S, Hillam RA, Skerry TM. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20:199–205. doi: 10.1016/s8756-3282(96)00386-9. [DOI] [PubMed] [Google Scholar]
- 5.Kruger L, Silverman J, Mantyh P, Sternini C, Brecha N. Peripheral patterns of calcitonin gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280:291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- 6.Hohmann E, Elde R, Rysavy J, Einzig S, Gebhard R. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:869–887. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 7.Duncan C, Shim S-S. The autonomic nerve supply of bone. J Bone Joint Surg. 1977;58-B:323–330. doi: 10.1302/0301-620X.59B3.19482. [DOI] [PubMed] [Google Scholar]
- 8.Miller M, Kashara M. Observations on the innervation of human long bones. Anat Rec. 1963;145:13–23. [Google Scholar]
- 9.Milgram J, Robinson R. An electron microscopic demonstration of unmyelinated nerves in the haversian canals of the adult dog. Bull Johns Hopkins Hosp. 1965;117:163–173. [Google Scholar]
- 10.Reimann I, Christensen S. A histological demonstration of nerves in subchondral bone. Acta Orthop Scand. 1977;48:345–352. doi: 10.3109/17453677708992006. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg P, Bostrom I, Mukohyama H. Neuro-hormonal control of bone metabolism: vasoactive intestinal peptide stimulates alkaline phosphatase activity and mRNA expression in mouse calvarial osteoblasts as well as calcium accumulation in mineralized bone nodules. Regul Pept. 1999;85:47–58. doi: 10.1016/s0167-0115(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 12.Chenu C, Serre C, Raynal C, Burt-Pichat B, Delmas P. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–299. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- 13.Lerner U. The role of skeletal nerve fibers in bone metabolism. The Endocrinologist. 2000;10:377–382. [Google Scholar]
- 14.Ducy P, Amling M, Takeda S, Priemel M, Schilling A, Beil F, Shen J, Vinson C, Rueger J, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 15.Baldock P, Sainsbury A, Couzens M, Enriquez R, Thomas G, Gardiner E, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Inv. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker K, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 17.Gordeladze J, Drevon C, Syversen U, Reseland J. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 18.Bliziotes M, McLoughlin S, Gunness M, Fumagalli F, Jones S, Caron M. Bone histomorphometric and biomechanical abnormalities in mice homozygous for deletion of the dopamine transporter. Bone. 2000;26:15–19. doi: 10.1016/s8756-3282(99)00232-x. [DOI] [PubMed] [Google Scholar]
- 19.Warden S, Robling A, Sanders M, Bliziotes M, Turner C. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinol. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 20.Nelson N. The family of Na+/Cl− neurotransmitter transporters. J Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 21.Bohm S, Grady E, Bunnett N. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J. 1997;322 ( Pt 1):1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westbroek I, van der Plas A, de Rooij K, Klein-Nulend J, Nijweide P. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 23.Bliziotes M, Eshleman A, Zhang X-W, Wiren K. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29:477–486. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson B, Thommesen L, Stunes A, Tommeras K, Westbroek I, Waldum H, Slordahl K, Tamburstuen M, Reseland J, Syversen U. Serotonin and fluoxetine modulate bone cell function in vitro. J Cell Biochem. 2006 Jan 11; doi: 10.1002/jcb.20734. 10.1002/JCB.20734. [DOI] [PubMed] [Google Scholar]
- 25.Winer J, Jung C, Shackel I, Williams P. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto J, Beadles-Bohling A, Wiren K. Comparison of RiboGreen and 18S rRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques. 2004;36:54–60. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y, Prusoff W. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharamacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 28.Eaton M, Whittemore S. Autocrine BDNF secretion enhances the survival and serotonergic differentiation of raphe neruonal precursor cells grafted into the adult rat CNS. Exp Neurol. 1996;140:105–114. doi: 10.1006/exnr.1996.0121. [DOI] [PubMed] [Google Scholar]
- 29.Bentolila V, Boyce T, Fyhrie D, Drumb R, Skerry T, Schaffler M. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. doi: 10.1016/s8756-3282(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 30.Eshleman A, Carmolli M, Cumbay M, Martens C, Neve K, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Therap. 1999;289:877–885. [PubMed] [Google Scholar]
- 31.Klein-Nulend J, van der Plas A, Semeins C, Ajubi N, Frangos J, Nijweide P, Burger E. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995;9:441–445. doi: 10.1096/fasebj.9.5.7896017. [DOI] [PubMed] [Google Scholar]
- 32.Klein-Nulend J, Burger E, Semeins C, Raisz L, Pilbeam C. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Min Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Knauss T, Abboud H. Effect of serotonin on prostaglandin synthesis in rat cultured mesangial cells. Am J Phys (Renal Fluid Electrolyte Physiol) 1986;251:F844–F850. doi: 10.1152/ajprenal.1986.251.5.F844. [DOI] [PubMed] [Google Scholar]
- 34.Jequier E, Robinson D, Lovenberg W, Sjoerdsma A. Further studies on tryptophan hydroxylase in rat brainstem and beef pineal. Biochem Pharmacol. 1969;18:1071–1081. doi: 10.1016/0006-2952(69)90111-7. [DOI] [PubMed] [Google Scholar]
- 35.Lovenberg W, Jequier E, Sjoerdsma D. Tryptophan hydroxylation: measurement in pineal gland, brainstem and carcinoid tumor. Science. 1967;155:217–210. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- 36.Grahame-Smith DG. Tryptophan hydroxylation in brain. Biochem Biophys Res Comm. 1964;16:586–592. doi: 10.1016/0006-291x(64)90197-4. [DOI] [PubMed] [Google Scholar]
- 37.Walther D, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 38.D’Sa C, Arthur RJ, States J, Kuhn D. Tryptophan hydroxylase: cloning and expression of the rat brain enzyme in mammalian cells. Neurochem. 1996;67:900–906. doi: 10.1046/j.1471-4159.1996.67030900.x. [DOI] [PubMed] [Google Scholar]
- 39.Chang A, Chang S, Starnes D, Schroeter S, Bauman A, Blakely R. Cloning and expression of the mouse serotonin transporter. Mol Brain Res. 1996;43:185–192. doi: 10.1016/s0169-328x(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y, Melikian H, Rye D, Levey A, Blakely R. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azmitia E, Yu I, Akbari H, Kheck N, Whitaker-Azmitia P, Marshak D. Antipeptide antibodies against the 5-HT1A receptor. J. Chem. Neuroanat. 1992;5:289–298. doi: 10.1016/0891-0618(92)90016-j. [DOI] [PubMed] [Google Scholar]
- 42.Anthony T, Azmitia E. Molecular characterization of antipeptide antibodies against the 5-HT1A receptor: evidence for state-dependent antibody binding. Mol. Brain Res. 1997;50:277–284. doi: 10.1016/s0169-328x(97)00201-5. [DOI] [PubMed] [Google Scholar]
- 43.Guillet-Deniau I, Burnol A-F, Girard J. Identification and localization of a skeletal muscle serotonin 5-HT2A receptor coupled to the Jak/STAT pathway. J. Biol. Chem. 1997;272:14825–14829. doi: 10.1074/jbc.272.23.14825. [DOI] [PubMed] [Google Scholar]
- 44.Owen T, Aronow M, Shalhoub V, Barone L, Wilming L, Tassinari M, Kennedy M, Pockwinse S, Lian J, Stein G. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:213–221. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 45.Espinosa L, Itzstein C, Cheynel H, Delmas P, Chenu C. Active NMDA glutamate receptors are expressed by mammalian osteoclasts. J Physiol (Lond) 1999;518:47–53. doi: 10.1111/j.1469-7793.1999.0047r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itzstein C, Espinosa L, Delmas P, Chenu C. Specific antagonists of NMDA receptors prevent osteoclast sealing zone formation required for bone resorption. Biochem Biophys Res Commun. 2000;268:201–209. doi: 10.1006/bbrc.2000.2097. [DOI] [PubMed] [Google Scholar]
- 47.Serre C, Farlay D, Delmas P, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 48.Cheng B, Zhao S, Luo J, Sprague E, Bonewald L, Jiang J. Expression of functional gap junctions and regulation by fluid flow shear stress in osteocyte-like MLO-Y4 cells. J Bone Min Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 49.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald L, Jiang J. PGE2 is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142:3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 50.Locker M, Bitard J, Collet C, Poliard A, Mutel V, Launay J, Kellermann O. Stepwise control of osteogenic differentiation by 5-HT(2B) receptor signaling: nitric oxide production and phospholipase A2 activation. Cell Signal. 2006;18:628–639. doi: 10.1016/j.cellsig.2005.06.006. [DOI] [PubMed] [Google Scholar]