Abstract
Tuberculosis (TB) continues to ravage humanity, causing 2 million deaths per year. A vaccine against TB more potent than the current live vaccine, bacillus Calmette–Guérin (BCG), is desperately needed. Using two commercially available strains of BCG as host strains, BCG Connaught and Tice, we have constructed two recombinant BCG vaccines stably expressing and secreting the 30-kDa major secretory protein of Mycobacterium tuberculosis (M. tb.), the primary causative agent of TB. We have tested the efficacy of the two strains in the highly susceptible guinea pig model of pulmonary TB, a model noteworthy for its close resemblance to human TB. Animals immunized with the recombinant BCG vaccines and challenged by aerosol with a highly virulent strain of M. tb. had 0.5 logs fewer M. tb. bacilli in their lungs and 1 log fewer bacilli in their spleens on average than animals immunized with their parental conventional BCG vaccine counterparts. Statistically, these differences were highly significant. Paralleling these results, at necropsy, animals immunized with the recombinant BCG vaccines had fewer and smaller lesions in the lung, spleen, and liver and significantly less lung pathology than animals immunized with the parental BCG vaccines. The recombinant vaccines are the first vaccines against TB more potent than the current commercially available BCG vaccines, which were developed nearly a century ago.
One of the highest priorities of tuberculosis (TB) research is a vaccine more potent in humans than the current vaccine, bacillus Calmette–Guérin (BCG). Despite widespread use of BCG, TB continues to ravage humanity. Each year, Mycobacterium tuberculosis (M. tb.), the primary causative agent of TB, causes approximately 8 million new cases of TB and 2 million deaths, making it the world's most lethal infectious agent (1). Adding to the problem, strains of M. tb. resistant to the major antibiotics used to treat TB are emerging rapidly worldwide (2).
BCG is a live attenuated vaccine derived from Mycobacterium bovis by Albert Calmette and Camille Guérin between 1906 and 1919. More then 3 billion doses of the vaccine have been administered worldwide (3). Although BCG is relatively safe and inexpensive, its efficacy is highly variable, ranging from −35% (recipients more likely to develop TB) to +80% in clinical trials (3). One reappraisal of BCG trials concluded that the most carefully conducted trials showed relatively high efficacy (4), and a comprehensive metaanalysis estimated BCG efficacy to be ≈50% (5). BCG is considered clearly efficacious in protecting against tuberculous death, meningitis, and miliary TB (5, 6).
Experimental approaches to developing an improved vaccine against TB have included the use of attenuated mycobacteria, subunit vaccines, and DNA vaccines (7). Until now, none of these has proved to be more potent than BCG in any animal model or even closely comparable in the highly susceptible and clinically relevant guinea pig model.
M. tb. is an intracellular bacterial pathogen that resides and multiplies in host mononuclear phagocytes. In previous studies, we have proposed and provided evidence in support of the concept that extracellular proteins of intracellular parasites are potent immunoprotective molecules (8–13).† Extracellular proteins are released by the pathogens into their environment within the host cell. Those studies focused on Legionella pneumophila, the primary causative agent of Legionnaires' disease, and M. tb. Using the guinea pig model of Legionnaires' disease, the most relevant animal model of this disease, we have demonstrated that immunization with the major secretory protein of L. pneumophila induces extremely strong protective immunity against lethal aerosol challenge with highly virulent strains of L. pneumophila (9, 10). Using the guinea pig model of TB, the most relevant small animal model of pulmonary TB and one that closely resembles human disease, we have demonstrated that immunization with purified M. tb. major extracellular proteins, including the 30-kDa major secretory protein [MSP; a mycolyltransferase (14) also referred to as α-antigen and antigen 85B] induces substantial protective immunity against aerosol challenge with the highly virulent Erdman strain of M. tb. (13). Protection was manifest by decreased weight loss, mortality, lung damage, and growth of M. tb. in the lung and spleen of immunized guinea pigs compared with controls. Other investigators have shown that vaccines containing purified M. tb. extracellular proteins (15) or DNA encoding them (16–20) also induce protection in the mouse model of pulmonary TB.
Although immunization of guinea pigs with purified extracellular proteins induced statistically significant protective immunity against challenge with M. tb., the magnitude of protection, assessed by measuring the reduction in live M. tb. in the lung and spleen, was 1–1.5 logs less than that with BCG vaccine. A DNA vaccine encoding a major extracellular protein was even less potent. Whereas this vaccine yielded protection comparable to BCG in an inbred mouse strain (16), it failed to induce significant protection in the more demanding guinea pig model under conditions in which BCG was highly protective (17). This prompted us to attempt to enhance the protective immune response induced by extracellular proteins. Our initial efforts focused on administering the proteins in a large variety of nonliving formulations, including linking the proteins to molecules that had the potential to enhance their immunogenicity (mannosylated BSA, L. pneumophila MSP, and tetanus toxoid), administering the proteins with IL-12 and oligodeoxyribonucleotides containing immunostimulatory CpG motifs, and administering the proteins in slow-release capsules or microspheres or in liposomes. Although several of these interventions improved the potency of the vaccine, none yielded a vaccine comparable to BCG.
Lacking sufficient success with nonliving vaccine formulations, we turned to live delivery vehicles. We hypothesized that the optimal vehicle would have three characteristics: be capable of multiplying in the mammalian host, be nonpathogenic, and be capable of expressing and secreting M. tb. major extracellular proteins in native form, something that our previous studies had demonstrated requires a mycobacterial host (21). These considerations prompted us to use BCG as a delivery vehicle, the only organism known to us that met all of these criteria. Because BCG vaccine was also the standard that we were attempting to surpass, any enhancement in its potency would automatically meet our goal.
In this paper, we report on the protective efficacy of two different recombinant BCG strains expressing and secreting the M. tb. 30-kDa MSP. We demonstrate that the recombinant vaccines induce protective immunity against TB superior to that induced by the widely used commercially available parental BCG vaccines from which they are derived.
Materials and Methods
Bacteria.
Wild-type M. bovis BCG Tice was purchased from Organon, and wild-type M. bovis BCG Connaught (Conn) was provided by Connaught Laboratories. M. tb. Erdman strain (ATCC 35801) was passaged through outbred guinea pigs to maintain virulence, cultured on 7H11 agar, subjected to gentle sonication to obtain a single cell suspension, and frozen at −70°C for use in animal challenge experiments.
Preparation of rBCG30.
The plasmid pMTB30, a recombinant construct of the Escherichia coli/mycobacteria shuttle plasmid pSMT3, was prepared as described (21). The plasmid pMTB30 was engineered to express the recombinant M. tb. 30-kDa MSP (r30) from its own promoter. The plasmid was introduced by electroporation into BCG Tice in our laboratory and subsequently into BCG Conn at Connaught Laboratories. Both recombinant (rBCG30) strains were maintained in medium containing hygromycin at a concentration of 50 μg/ml. The expression and export of r30 were verified by SDS/PAGE and immunoblotting (21). The quantity of the 30-kDa MSP in culture filtrates was quantitated by densitometry by subculturing strains in 7H9 broth at a density of 5 × 105 colony-forming units (CFU)/ml, growing the strains for 14 days, collecting and concentrating the culture filtrate proteins, electrophoresing concentrates representing the equivalent of 5 × 109 bacterial cells on 12.5% polyacrylamide gels, staining with Coomassie blue, scanning the dried gels on an Arcus II scanner (Agfa) to produce a digitized image using Adobe PHOTOSHOP 5.0.2, and assigning each band an optical density value using NIH IMAGE 1.62. Plasmid stability in rBCG30 was assessed by subculturing the strain (1:10 dilution) six consecutive times for 4 weeks each in the absence of hygromycin and analyzing the filtrate of the third and sixth subculture.
Preparation of Immunization Inocula.
Wild-type BCG and rBCG30 were cultured to midlog phase in 7H9 medium, pH 6.7 (Difco), at 37°C in 5% CO2/95% air as unshaken cultures under exactly the same conditions except that 50 μg/ml hygromycin was added to rBCG30 cultures. On the day of immunization, the bacteria were pelleted by centrifugation at 3,500 × g for 15 min, washed with PBS, and resuspended to a final concentration of 104 CFU/ml in PBS. r30 was purified as described (13, 21) from culture filtrates of recombinant Mycobacterium smegmatis 1–2c containing plasmid pMTB30 and demonstrated to be indistinguishable from the native protein (21).
Immunization of Animals.
The experiments used specific pathogen-free, 250- to 300-g outbred male Hartley strain guinea pigs from Charles River Breeding Laboratories. In the first two experiments, animals were immunized intradermally in groups of eight or nine with one of the following: (i) rBCG30 Conn, 103 CFU one time only (Time 0 weeks); (ii) wild-type BCG Conn, 103 CFU one time only (Time 0 weeks); (iii) purified r30, 100 μg in 100 μl of Syntex adjuvant formulation (SAF) (22), three times, 3 weeks apart (Time 0, 3, and 6 weeks); and (iv) SAF only (Sham-immunized), 100 μl three times, 3 weeks apart (Time 0, 3, and 6 weeks). An additional group of three sham-immunized animals was used as a skin test control. These and 3–6 other sham-immunized animals served as uninfected controls in the first two experiments. In the third experiment, animals were immunized intradermally in groups of 23 or 24 one time only with 103 CFU of BCG Conn, rBCG30 Conn, BCG Tice, or rBCG30 Tice. In addition, 18 animals were sham-immunized one time only with buffer only, of which six animals were used exclusively as a skin test control.
Cutaneous Delayed-Type Hypersensitivity (DTH) to r30.
Nine weeks after the start of the immunization protocol, guinea pigs were shaved over the back and injected intradermally with 10 μg of purified r30 in 100 μl of PBS. After 24 h, the diameter of induration was measured. (Sham-immunized animals that were skin-tested were not challenged with M. tb. to eliminate the possibility that the skin test itself might influence the outcome of the challenge study.) In experiment 3, only 12 animals in each group immunized with BCG or rBCG30 were skin-tested.
Protective Immunity to Aerosol Challenge.
Nine weeks after the first or only immunization and immediately after skin testing, animals were challenged with an aerosol generated from a 10-ml, single-cell suspension containing a total of 1 × 105 CFU (experiments 1 and 2) or 2 × 105 CFU (experiment 3) of M. tb. Erdman strain as described (13). These aerosol doses delivered ≈40 (experiments 1 and 2) or 80 (experiment 3) live bacilli to the lungs of each animal. The airborne route of infection was used because this is the natural route of infection for pulmonary TB. A large dose was used to induce measurable clinical illness in 100% of control animals within a relatively short time frame (10 weeks). Afterward, guinea pigs were housed individually in stainless-steel cages contained within a laminar-flow biohazard safety enclosure and allowed free access to standard laboratory food and water. The animals were observed for illness and weighed weekly for 10 weeks and then killed. The lungs, spleen, and liver of each animal were removed aseptically and inspected immediately for pathology, and the right lung and spleen were cultured for CFU of M. tb. (12).
Statistical Analysis.
Both parametric ANOVA methods and nonparametric Kruskal–Wallis (K-W) methods were used to compare the mean and median induration, log CFU, and lung pathology across immunization groups. For the ANOVA method, post hoc comparisons were judged statistically significant by using the Fisher–Tukey least significant difference criterion.
Results
Analysis of the Recombinant BCG Strains.
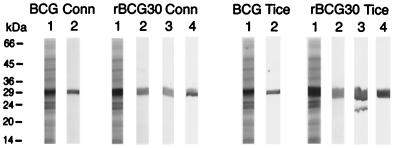
Two live vaccines expressing and secreting the M. tb. 30-kDa MSP in native form were prepared by transformation of wild-type BCG Conn and Tice strains with a recombinant plasmid (pMTB30) engineered to express r30 from its own promoter (21). For both BCG strains, the wild-type expressed a low level of the endogenous 30-kDa MSP (Fig. 1, Left and Right, BCG Conn and Tice lanes 1 and 2). In contrast, the same number of rBCG30 expressed and secreted a relatively high level of r30 (Fig. 1, Left and Right, rBCG30 Conn and Tice lanes 1 and 2). By densitometry, rBCG30 Conn secreted 2.0-fold and rBCG30 Tice secreted 5.4-fold more of the 30-kDa MSP than their parental counterparts. N-terminal amino acid sequencing revealed that r30 was processed in the same way as the native protein. DNA sequence analysis of the gene encoding the homologous 30-kDa protein in both BCG Conn and BCG Tice showed that the two full-length BCG 30-kDa proteins have identical amino acid sequence and differ from the full-length M. tb. 30-kDa protein by only 2 amino acids at a single site (M. tb. → BCG: 285 Asn → Lys; 286 Ala → Pro).
Figure 1.
rBCG30 secretes a large amount of r30 even in the absence of selective pressure. Culture filtrate proteins of the wild-type and recombinant BCG Conn strains (Left) and Tice strains (Right) grown for 4 weeks were subjected to SDS/PAGE (lane 1) or immunoblot analysis (lanes 2–4). The rBCG30 strains were subcultured in the presence of hygromycin three times over 12 weeks (lanes 2) or in the absence of hygromycin either three times over 12 weeks (lanes 3) or six times over 24 weeks (lanes 4). By densitometry, the relative amounts of the 30-kDa protein in the four strains were BCG Conn, 1.00; rBCG30 Conn, 2.03; BCG Tice, 1.10; rBCG30 Tice, 5.98.
To determine whether rBCG30 expresses r30 in the absence of selective pressure, such as would occur in the vaccinated mammal, we studied expression in the presence and absence of hygromycin, an antibiotic to which the recombinant plasmid conferred resistance. When subcultured six times (4 weeks each) over a 24-week period in the presence of hygromycin, broth cultures of the rBCG30 strains maintained a steady level of r30 expression (not shown). When subcultured six times over a 24-week period in the absence of hygromycin, the same cultures showed no decrease in r30 expression, indicating that the recombinant plasmid is stably maintained in the absence of selective pressure (Fig. 1, Left and Right, rBCG30 Conn and Tice lanes 3 and 4). This suggested that, after vaccination, recombinant bacteria would continue to express r30 in the mammalian host in the absence of selective pressure.
Cutaneous DTH to r30.
To assess the capacity of the recombinant vaccines to induce a cell-mediated immune response to r30, we assessed the animals for cutaneous DTH to r30 9 weeks after immunization. Data from three independent experiments are shown in Fig. 2. Experiments 1 and 2 involved only the Conn strain, and experiment 3 involved both the Conn and Tice strains. As expected, the sham-immunized animals had essentially no induration (1 mm is the lowest score possible because the needle injection itself results in 1-mm induration) in each experiment. Surprisingly, because wild-type BCG expresses an endogenous 30-kDa protein highly homologous with r30, animals immunized with wild-type BCG also exhibited a weak cutaneous response to r30. In contrast, animals immunized with r30 or rBCG30 exhibited marked induration that was significantly higher than in the sham-immunized or wild-type BCG immunized animals (P < 0.0001 for difference between BCG Conn and rBCG30 Conn groups in experiments 1–3 combined and P < 0.0001 for difference between BCG Tice and rBCG30 Tice in experiment 3 by both ANOVA and K-W methods). In experiment 3, in which the Conn and Tice strains were compared, animals immunized with rBCG30 Tice exhibited significantly greater induration in response to r30 than animals immunized with rBCG30 Conn (P < 0.02 by both ANOVA and K-W methods) even though the parental strains exhibited comparably low levels of induration. This likely reflected the much greater expression and secretion of r30 by the rBCG30 Tice strain.
Figure 2.
rBCG30-immunized animals exhibit strong, cutaneous DTH to r30. Guinea pigs were sham-immunized (Sham) or immunized with purified r30, wild-type BCG (Conn or Tice, as indicated), or rBCG30 (Conn or Tice, as indicated) and skin-tested with an intradermal injection of r30. The extent of induration was measured after 24 h. Data are the mean diameter + SE. In experiments 1, 2, and 3, differences between the BCG Conn and rBCG30 Conn were significant at P = 0.03, P = 0.01, and P < 0.0007, respectively, by ANOVA or K-W methods (P < 0.0001 for all three experiments combined). In experiment 3, differences between BCG Tice and rBCG30 Tice were significant at P < 0.0001 and differences between rBCG30 Conn and rBCG30 Tice were significant at P < 0.02 by either statistical method.
Protective Efficacy of rBCG30.
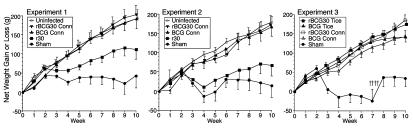
To determine the capacity of the recombinant vaccines to induce protective immunity, we challenged immunized and control animals by aerosol with the highly virulent Erdman strain of M. tb. and monitored the subsequent course of infection. As an objective indicator of illness, we assessed weight loss, a major physical sign of TB in humans and a hallmark of TB in the guinea pig model of this chronic infectious disease. In each of the first two experiments, involving only the BCG Conn strain, uninfected controls and animals immunized with either rBCG30 or wild-type BCG gained weight normally after challenge (Fig. 3). Indeed, there were no significant differences in weight gain among these three groups. In contrast, sham-immunized animals and, to a lesser extent, r30 immunized animals exhibited diminished weight gain. Compared with uninfected controls, sham-immunized animals in the two experiments lost or failed to gain an average of 156 g, equivalent to 19% of their total body weight, and r30-immunized animals lost or failed to gain an average of 87 g, equivalent to 10% of their total body weight by the end of the 10-week observation period. Thus, animals immunized with either the wild-type or recombinant BCG Conn strain were completely protected from weight loss.
Figure 3.
Animals immunized with recombinant or wild-type BCG are protected against weight loss after challenge with M. tb. Animals in the immunization groups described in Fig. 2 were challenged with M. tb. by aerosol and weighed weekly for 10 weeks. In experiments 1 and 2, an additional group of control animals was not challenged but weighed weekly (Uninfected controls). Data are the mean net weight gain or loss ± SE for each group of animals compared with their weight immediately before challenge. In experiment 3, daggers (†) indicate deaths of four sham-immunized animals during week 8.
In the third experiment, in which the Conn and Tice strains were compared and the animals were challenged with a 2-fold higher dose of M. tb. than in experiments 1 and 2, animals immunized with either of the two wild-type or recombinant strains gained weight normally after challenge, although both recombinant strains exhibited slightly greater weight gain than their parental counterparts after week 7.
In the third experiment, four (33%) of the 12 sham-immunized control animals died before the conclusion of the 10-week observation period (week 8), likely reflecting the greater challenge dose. In contrast, none (0%) of the 94 BCG or rBCG30 immunized animals died (P < 0.009 for difference between sham and each of the other groups and P < 0.0001 for difference between sham and all BCG/rBCG30 groups by χ2, Exact Permutation version). Thus, although the guinea pig model is not primarily a mortality model and deaths are infrequent during the first 10 weeks after challenge, animals immunized with either BCG or rBCG30 were protected against death.
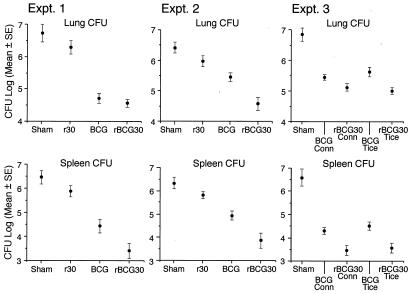
To assess the capacity of the recombinant vaccines to restrict the growth of M. tb. in tissues of challenged guinea pigs, we assayed the number of M. tb. in the lung, the primary site of infection, and spleen, a major site of bacterial dissemination, at the end of the 10-week observation period (Fig. 4). As expected, sham-immunized animals had the highest bacterial load in both organs. As demonstrated previously, animals immunized with r30 had significantly fewer organisms in the lung and spleen—≈0.5 logs fewer in each organ—than the sham-immunized animals in each experiment. Also as observed previously, BCG-immunized animals had significantly fewer organisms in their organs, 1 log fewer in the lung and 1.2 logs fewer in the spleen on average than r30-immunized animals and 1.5 logs fewer in the lung and 1.7 logs fewer in the spleen on average than sham-immunized animals, a level of protection comparable to that observed in previous experiments in this model. Remarkably, and of greatest importance, rBCG30-immunized animals had still fewer organisms in their lungs and spleens than BCG-immunized animals. The difference was especially noteworthy in the spleen, where the rBCG30 groups averaged 1.1 log fewer CFU than the BCG group. In the lung, the differences in CFU were smaller and more variable, averaging 0.5 logs. On statistical analysis by using both ANOVA and nonparametric methods (Table 1), differences between the BCG and rBCG30 groups in CFU in the spleen were statistically significant in both experiments, and the difference in CFU in the lung was significant in experiment 2. Statistical tests using two-way factorial ANOVA methods to compare means demonstrated that the mean CFU counts of the four immunization groups (Sham, r30, BCG, and rBCG30) in experiment 1 were not significantly different from the mean CFU counts of these groups in experiment 2 and that it, therefore, was appropriate to combine the data. On analysis of the combined data, differences between the BCG and rBCG30 groups in CFU in both the lung and spleen were statistically significant (Table 1).
Figure 4.
Animals immunized with rBCG30 have significantly fewer M. tb. bacilli in the lung and spleen than animals immunized with BCG. At the end of the 10-week observation period, challenged animals were killed, and CFU of M. tb. in the lung and spleen were assayed. Data are the mean ± SE for all animals in a group (see Table 1). The lower limit of detection was 2.0 logs/organ (1 CFU on a plate seeded with an undiluted 1% sample of an organ, i.e., 100 μl of a total sample volume of 10 ml). In experiment 3, one spleen culture from the rBCG30 Conn and three spleen cultures from the rBCG30 Tice group had 0 CFU on plates seeded with undiluted samples. For statistical purposes, these three organs were scored as 2.0 logs.
Table 1.
Key statistical analyses of CFU in lung and spleen
| Experiment | Comparison | Lung
|
Spleen
|
||
|---|---|---|---|---|---|
| P(ANOVA) | P(K-W) | P(ANOVA) | P(K-W) | ||
| 1 | Sham (9) vs. BCG Conn (8) | <0.0001 | 0.0005 | <0.0001 | 0.0015 |
| BCG Conn (8) vs. rBCG30 Conn (8) | NS | NS | 0.015 | 0.048 | |
| 2 | Sham (9) vs. BCG Conn (9) | 0.0008 | 0.002 | 0.0004 | 0.001 |
| BCG Conn (9) vs. rBCG30 Conn (9) | 0.002 | 0.03 | 0.004 | 0.015 | |
| 1 + 2 | Sham (18) vs. BCG Conn (17) | <0.0001 | 0.000002 | <0.0001 | 0.000005 |
| BCG Conn (17) vs. rBCG30 Conn (17) | 0.01 | 0.01 | 0.0002 | 0.002 | |
| 3 | Sham (12) vs. BCG Conn (23) | <0.0001 | <0.000001 | <0.0001 | <0.000001 |
| Sham (12) vs. BCG Tice (24) | <0.0001 | 0.00006 | <0.0001 | 0.00001 | |
| BCG Conn (23) vs. rBCG30 Conn (23) | 0.085 | 0.03 | 0.004 | 0.005 | |
| BCG Tice (24) vs. rBCG30 Tice (24) | 0.001 | 0.004 | 0.0009 | 0.003 | |
Number of animals per group is shown in parentheses. NS, not significant.
In experiment 3, in which animals received a higher challenge dose, the results for the wild-type and recombinant Conn strains were similar to the results for the wild-type and recombinant Tice strains, respectively, and also similar to the results for these strains in experiments 1 and 2. Again, the animals immunized with rBCG30 strains had an average of 0.5 logs fewer CFU in the lung and nearly 1 log fewer in the spleen than animals immunized with their parental counterparts, and these differences were statistically significant (Table 1).
Paralleling the differences in CFU, on gross inspection at necropsy, the lungs and livers of animals immunized with either BCG or rBCG30 of either strain had much fewer and smaller lesions than the sham-immunized animals, and most important, the animals immunized with rBCG30 of either strain had much fewer and smaller lesions than animals immunized with their wild-type counterpart (Fig. 5). Lesions were particularly scant in the organs of animals immunized with rBCG30 Tice. In addition, on visual inspection of the lung surfaces, lungs of rBCG30-immunized animals exhibited significantly less pathology (defined as the percentage of lung surface area that was abnormal because of discoloration, chiefly from inflammation or hemorrhage, or the presence of tubercles) than lungs of BCG-immunized animals [21 ± 4% vs. 34 ± 5%, mean ± SE, for the Conn strain in experiments 1 and 2 (P < 0.05 by both ANOVA and K-W methods); 42 ± 5% vs. 57 ± 4% for the Conn strain in experiment 3 (P < 0.02); and 18 ± 3% vs. 34 ± 5% for the Tice strain in experiment 3 (P < 0.001)].
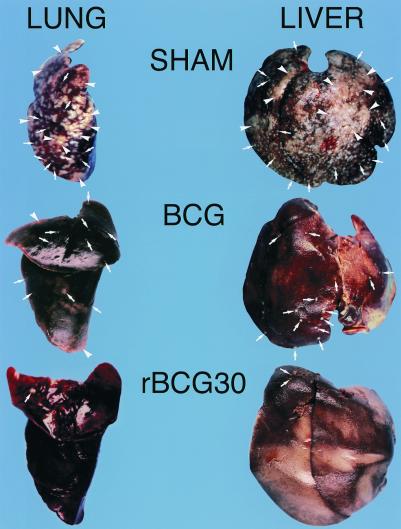
Figure 5.
Animals immunized with rBCG30 have fewer and smaller lesions in their lung and liver than animals immunized with BCG. These photographs depict a representative, formalin-treated left lung and liver from a sham-, BCG Tice-, and rBCG30 Tice-immunized animal. The organs of the sham-immunized animal are peppered with tubercles (arrows), many of which have coalesced into large lesions (arrowheads). The organs of the BCG and rBCG30 immunized animals have progressively fewer and smaller lesions.
Discussion
This study describes the first experimental vaccines against TB more potent than current commercially available vaccines in a susceptible animal model. As is universal in studies of TB vaccine efficacy, the major parameter used to assess efficacy in our study was CFU in animal organs. The rBCG30 vaccines induced a 0.5-log reduction in CFU in the lung and a 1-log reduction in CFU in the spleen above and beyond the 2- to 2.5-log reduction in CFU in these organs induced by the parental BCG strains.
In our previous studies in the guinea pig model (12, 13), in which the effect of a vaccine was compared with sham-immunized controls, a reduction in CFU of a magnitude of 0.5–1 log was correlated with significant reductions in overt clinical manifestations of disease including weight loss and mortality. In the present study, however, one highly effective vaccine in the guinea pig model was compared with an even more effective one. Because parental BCG already fully protects against weight loss and death in this model, these parameters of disease could not be used to distinguish between the BCG and rBCG30 vaccines. Nevertheless, differences in CFU clearly did reflect differences in disease status that were readily apparent at necropsy. Thus, the 0.5-log reduction in CFU in the lung reflected significantly less lung pathology, and the 1-log reduction in CFU in the spleen reflected an approximately 10-fold reduction in tubercles in the liver and spleen in the rBCG30-immunized animals compared with the BCG-immunized animals.
Our study provides additional evidence in support of the hypothesis that extracellular proteins of intracellular parasites are key immunoprotective molecules. This hypothesis has three main elements (9, 13). First, the hypothesis holds that extracellular proteins, by virtue of their release by the pathogen into the intracellular milieu of the host cell, are available for processing and presentation to the immune system as fragments bound to MHC molecules on the host cell surface. These peptide–MHC complexes serve to alert the immune system to the presence within the host cell of an otherwise hidden invader, enabling the immune system to mount an appropriate antimicrobial attack. Second, the hypothesis holds that effective immunization with extracellular proteins is able to induce a population of immune cells that recognize the same peptide–MHC complexes at some future time when the complexes are displayed on host cells invaded by the relevant intracellular pathogen. The immune cells thus are able to target the infected host cells and either activate them with cytokines, thereby enabling them to restrict growth of the intracellular pathogen, or lyse them, thereby denying the pathogen the intracellular milieu in which it thrives. Third, the hypothesis holds that among the extracellular proteins, the major ones, i.e., those produced most abundantly, will figure most prominently as immunoprotective molecules because they generally would provide the richest display of peptide–MHC complexes to the immune system. In this regard, it is noteworthy that the M. tb. 30-kDa MSP is not only the most abundant protein released in broth cultures of M. tb., amounting to nearly one-fourth the total extracellular protein (13), but this protein is also abundantly released into the phagosome of M. tb. in infected human macrophages (23) and among the most abundant proteins of all types, both cell-associated and extracellular, synthesized by M. tb. in human macrophages (24).
A T cell epitope-mapping study of the M. tb. 30-kDa MSP in outbred guinea pigs revealed multiple immunoreactive regions of the protein (25). Interestingly, immunoreactive epitopes identified in this study frequently overlapped with immunoreactive epitopes of the highly homologous M. bovis BCG Tokyo 30-kDa protein identified on the basis of mapping studies in healthy tuberculin-positive and BCG-vaccinated humans (25).
Remarkably, immunization with rBCG30 induced protection superior to BCG even though wild-type BCG expresses and secretes a highly homologous endogenous 30-kDa MSP that differs from the M. tb. 30-kDa protein by only 2 contiguous amino acids. Hence, the improved protection of the recombinant strains is unlikely to be due to the small amino acid difference between the recombinant and endogenous proteins. More likely, it is due to the enhanced expression of the recombinant protein. If so, then the abundant expression obtained by using a high-copy-number plasmid likely was critical to the success of the recombinant vaccines.
Immunization of animals with rBCG30 induced a cell-mediated immune response to r30, as evidenced by the development of strong, cutaneous DTH to the protein. However, this result by itself does not correlate with strong protective immunity because immunization with purified r30 alone also induced a strong, cutaneous DTH response to r30 but relatively weak protective immunity. In this regard, it is instructive that in a separate experiment (not shown), immunization with the combination of wild-type BCG and purified r30 also induced a strong, cutaneous DTH response to r30 but did not induce protective immunity superior to immunization with BCG alone. Taken together, these experiments indicate that immunization with rBCG30 induces a qualitatively different immune response to r30 than immunization with the purified protein. Deciphering the nature of that qualitative difference could shed light on the immunologic basis for protective immunity to TB and reveal key correlates of protective immunity, about which nothing currently is known. Consistent with the idea that the nature of the immune response to the 30-kDa protein may be a critical factor in protective immunity to TB, in studies comparing patients with active TB with healthy PPD-positive household contacts, the contacts, who were presumed to have protective immunity against TB, were found to exhibit strong lymphocyte blastogenic responses and IFN-γ production in response to the 30-kDa protein but to have low levels of antibody to the protein, whereas the opposite was true for patients with active TB, who were presumed to lack protective immunity (26, 27).
In our previous studies, immunization with the relevant major extracellular protein in an adjuvant induced highly potent protection against challenge with L. pneumophila but relatively modest protection against M. tb. (9, 13). This likely reflects the fact that humans are an accidental host for L. pneumophila, which infects primarily aquatic protozoa, but the primary host for M. tb. As such, M. tb. has evolved powerful mechanisms to resist host antimicrobial defenses, and an exceptionally potent vaccine may be required to combat it.
Remarkably, insertion of only a single recombinant M. tb. protein into wild-type BCG provided a substantial increase in its protective efficacy. Whether the insertion of additional M. tb. proteins will further boost the protective immune response remains to be determined.
That immunization with rBCG30 induces protection superior to BCG in guinea pigs bodes well for human studies of the protective efficacy of the recombinant vaccine. TB in the guinea pig strongly resembles TB in humans clinically, immunologically, and pathologically. The guinea pig, like the human but unlike the mouse and rat, (i) is susceptible to low doses of aerosolized M. tb.; (ii) exhibits strong, cutaneous DTH to tuberculin; and (iii) displays Langhans giant cells and caseation in pulmonary lesions (28). However, guinea pigs are much more susceptible to disease after infection (100% develop disease vs. only 10% of immunocompetent humans). That the recombinant vaccine induces a higher level of protection than BCG in a highly susceptible animal model gives reason for optimism that it also will induce a higher level of protection than BCG in humans.
Aside from the issue of potency, a recombinant BCG vaccine akin to the one described here likely would share several important advantages and disadvantages of conventional BCG vaccine. On the plus side, the vaccine likely would share BCG's excellent safety record. BCG's chief risk is disseminated disease, which occurs in ≈1 in a million vaccinees (29). This complication occurs in immunocompromised persons, and, hence, the vaccine is not recommended for many such individuals, including those with established HIV infection. Also on the plus side, the recombinant BCG vaccine, like conventional BCG, should be inexpensive to produce because costly purification or synthesis procedures are not required. Cost is a particularly important consideration because TB is of greatest prevalence in the developing world, where budgets for vaccines are very limited.
On the negative side, the recombinant vaccine would share the major drawback of conventional BCG vaccine—interference with the tuberculin skin test, still the gold standard for detecting M. tb. infection. In the future, this disadvantage of BCG vaccines may be alleviated by diagnostic tests capable of distinguishing between BCG vaccination and M. tb. infection. In any case, if the recombinant vaccine proves highly efficacious in humans, its benefits would seem to outweigh this disadvantage.
Whereas other studies have demonstrated protective immunity against various pathogens by using recombinant BCG expressing cell-associated antigens (30–37), ours demonstrates protective immunity by using a recombinant BCG vaccine expressing an extracellular protein, and protection was remarkably strong. On the basis of our study, in addition to providing potent protection against TB, a recombinant BCG vaccine abundantly expressing extracellular proteins of other mycobacteria may provide potent protection against diseases caused by these organisms, including M. leprae, the agent of leprosy, M. avium-intracellulare, a major opportunistic pathogen in immunocompromised individuals, and M. bovis, the primary agent of TB in cattle and other domesticated animals. In addition, a recombinant BCG vaccine abundantly expressing extracellular proteins of nonmycobacterial intracellular pathogens may provide potent protection against the diseases caused by these organisms.
If a recombinant BCG vaccine against TB akin to the ones described here is more potent than conventional BCG vaccines in humans, it could have a tremendous impact on human health. Even a modest improvement in potency over BCG vaccine would translate into hundreds of thousands of saved lives.
Acknowledgments
We are grateful to Chalermchai Chaloyphian for technical assistance, Jeffrey Gornbein for assistance with statistical analyses, and the staff of the University of California at Los Angeles Division of Laboratory Animal Medicine for assistance with the care of animals under challenging conditions. We thank Robin Harkness of Aventis Pasteur for providing BCG Conn and Kathryn Horwitz, Lawrence Horwitz, and Andrew Saxon for critical review of the manuscript. This work was supported by National Institutes of Health Grant AI31338.
Abbreviations
- BCG
bacillus Calmette–Guérin
- TB
tuberculosis
- M. tb.
Mycobacterium tuberculosis
- MSP
major secretory protein
- CFU
colony-forming units
- DTH
delayed-type hypersensitivity
Footnotes
Breiman, R. F. & Horwitz, M. A. (1987) Clin. Res. 35, 469A.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250480397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250480397
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. J Am Med Assoc. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Cohn D L, Bustreo F, Raviglione M C. Clin Infect Dis. 1997;24:S121–S130. doi: 10.1093/clinids/24.supplement_1.s121. [DOI] [PubMed] [Google Scholar]
- 3.Fine P E M. Rev Infect Dis. 1989;11, Suppl. 2:S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- 4.Clemens J D, Chuong J H, Feinstein A P. J Am Med Assoc. 1983;249:2362–2369. [PubMed] [Google Scholar]
- 5.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. J Am Med Assoc. 1994;271:698–702. [PubMed] [Google Scholar]
- 6.Rodriguez L C, Diwan V K, Wheeler J G. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz M A. Immunologist. 1997;5:15–20. [Google Scholar]
- 8.Blander S J, Horwitz M A. J Exp Med. 1989;169:691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blander S J, Szeto L, Shuman H A, Horwitz M A. J Clin Invest. 1990;86:817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blander S J, Horwitz M A. J Immunol. 1991;147:285–291. [PubMed] [Google Scholar]
- 11.Blander S J, Horwitz M A. J Clin Invest. 1993;91:717–723. doi: 10.1172/JCI116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal P G, Horwitz M A. Infect Immunol. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz M A, Lee B-W E, Dillon B J, Harth G. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 15.Brandt L, Elhay M, Rosenkrands I, Linblad E B, Andersen P. Infect Immunol. 2000;68:791–795. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C, Orme I M, Baldwin S, D'Souza C, et al. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Liu M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Infect Immunol. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W B. Infect Immunol. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Howard A, Kelley C, Delogu G, Collins F, Morris S. Infect Immunol. 1999;67:4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delogu G, Howard A, Collins F, Morris S L. Infect Immunol. 2000;68:3097–3102. doi: 10.1128/iai.68.6.3097-3102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harth G, Lee B-Y, Horwitz M A. Infect Immunol. 1997;65:2321–2328. doi: 10.1128/iai.65.6.2321-2328.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison A C, Byars N E. J Immunol Methods. 1986;95:157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- 23.Harth G, Lee B-Y, Wang J, Clemens D L, Horwitz M A. Infect Immunol. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee B-Y, Horwitz M A. J Clin Invest. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B-Y, Horwitz M A. Infect Immunol. 1999;67:2665–2670. doi: 10.1128/iai.67.5.2665-2670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres M, Mendez-Sampiero P, Jimenez-Zumaido L, Teran L, Camarena A, Quezada R, Ramos E, Sada E. Clin Exp Immunol. 1994;96:75–78. doi: 10.1111/j.1365-2249.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres M, Herrera T, Villareal H, Rich E A, Sada E. Infect Immunol. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefford M J. In: The Mycobacteria: A Sourcebook. Kubica G P, Wayne L G, editors. New York: Dekker; 1984. pp. 947–977. [Google Scholar]
- 29.Lotte A, Wasz-Höckert O, Poisson N, Dumitrescu D. Bull Int Union Tuberc. 1978;53:121–123. [PubMed] [Google Scholar]
- 30.Stover C K, Bansal G P, Hanson M S, Burlein J E, Palaszynski S R, Young J F, Koenig S, Young D B, Sadziene A, Barbour A L. J Exp Med. 1993;178:197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langerman S, Palaszynski S, Sadziene A, Stover C K, Koenig S. Nature (London) 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 32.Langerman S, Palaszynski S, Burlein J E, Koenig S, Hanson M S, Briles D E, Stover C K. J Exp Med. 1994;180:2277–2286. doi: 10.1084/jem.180.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connell N D, Medina-Acosta E, McMaster W R, Bloom B, Russel D. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelhak S, Louzir H, Timm J, Blel L, Benlasfar Z, Lagranderie M, Gheorghiu M, Dellagi K, Gicquel B. Microbiology. 1995;141:1585–1592. doi: 10.1099/13500872-141-7-1585. [DOI] [PubMed] [Google Scholar]
- 35.Streit J A, Recker T J, Donelson J E, Wilson M E. Exp Parasitol. 2000;94:33–41. doi: 10.1006/expr.1999.4459. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto S, Yukitake H, Kanbara H, Yamada T. J Exp Med. 1998;188:845–854. doi: 10.1084/jem.188.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Fennelly G, Miller C, Tarara R, Saxe I, Bloom B, McChesney M. J Inf Dis. 1997;176:1445–1453. doi: 10.1086/514140. [DOI] [PubMed] [Google Scholar]