Full Text
The Full Text of this article is available as a PDF (441.4 KB).
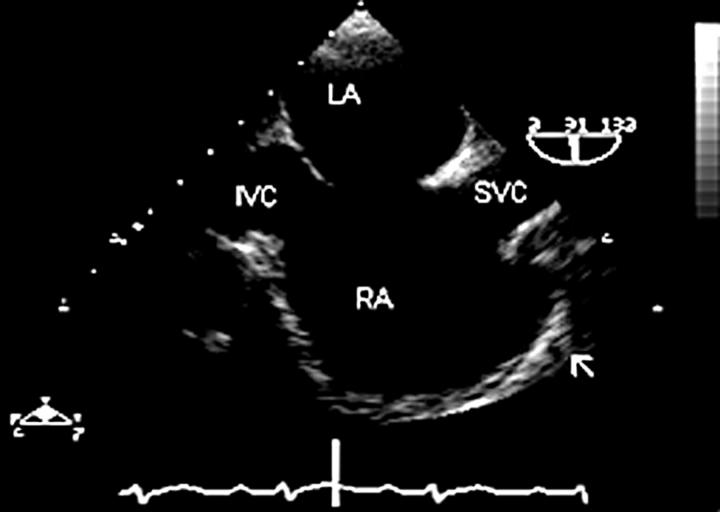
Figure 1 .
The atrial septum is seen in a vertical plane (90°), from the superior vena cava/right atrium junction to the inferior vena cava/right atrium junction. Right atrial morphology is well appreciated in this "bicaval view", including the broad, wide necked appendage (arrow). In this patient, the right atrium is dilated and there is a defect in the fossa ovalis region of the atrial septum (secundum atrial septal defect). IVC, inferior vena cava; SVC, superior vena cava; RA, right atrium; LA, left atrium.
Figure 2 .
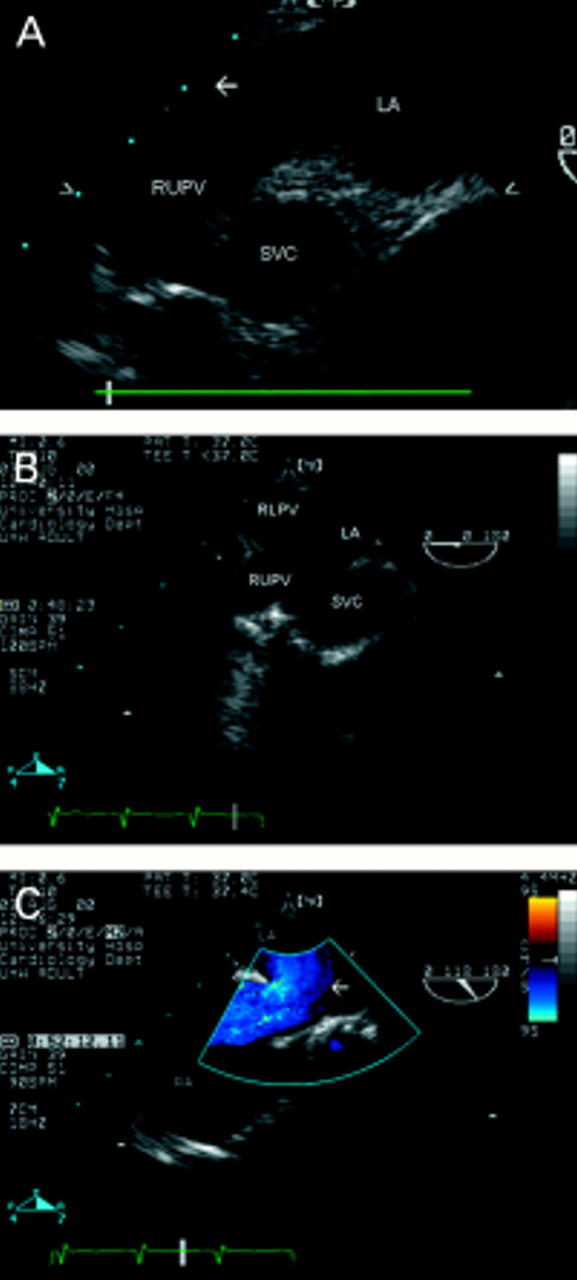
The relation between the superior vena cava (seen in short axis, superior to its junction with the right atrium) and the right sided pulmonary veins is seen from an upper oesophageal view, at 0° (A). The right upper pulmonary vein enters the left atrium "vertically", while the origin of the right lower pulmonary vein is seen in a horizontal orientation (arrow). In the same view (B), a communication is seen between the right upper and lower pulmonary veins, superior vena cava and left atrium, in a patient with sinus venosus atrial septal defect and partial anomalous pulmonary venous drainage. In the same patient, an oblique view, similar to the bicaval view (C), shows the superior vena cava overriding the upper atrial septum (arrow) at which point the superior sinus venosus defect, with a left-to-right shunt, can be seen. RUPV, right upper pulmonary vein; RLPV, right lower pulmonary vein; SVC, superior vena cava; RA, right atrium; LA, left atrium; IVC, inferior vena cava.
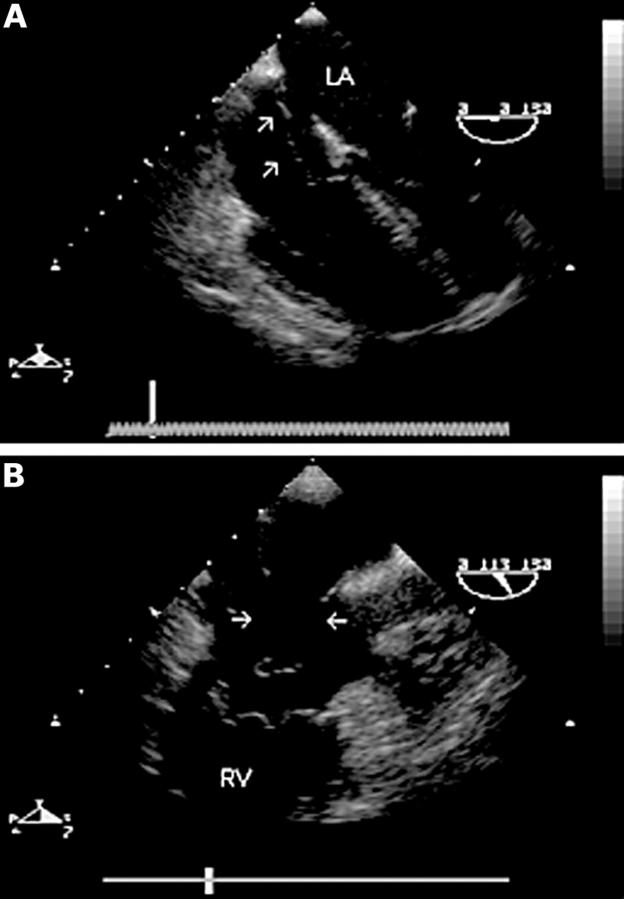
Figure 3 .
Mid oesophageal four chamber view (0°) (A) showing an atrial septal aneurysm and secundum defect. There is a mobile "wind sock" appearance of the mid portion of the atrial septum. A long axis view of the right heart (110°) (B) shows two large defects in the aneurysmal part of the septum (arrows). The entire defect was closed using a single percutaneous catheter based occlusion device. RV, right ventricle; LA, left atrium.
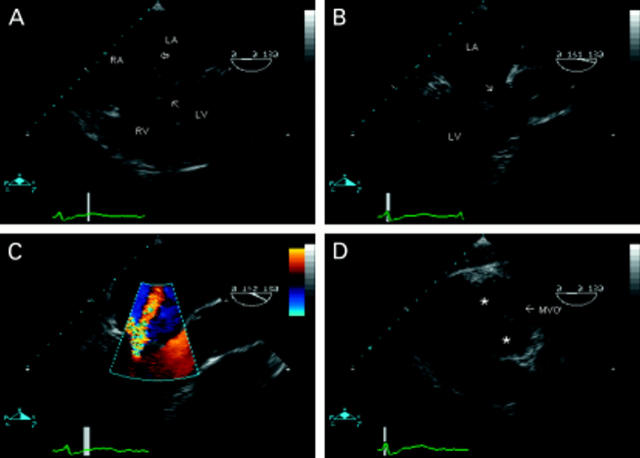
Figure 4 .
Partial atrioventricular septal defect seen in a four chamber view (A). There is a primum atrial septal defect (unmarked) and also a small secundum defect (open arrow). There is loss of offsetting of the atrioventricular valves, which insert into the crest of the ventricular septum giving the appearance of thinning at this point (closed arrow). In an oblique, long axis view of the left ventricle (B), deformity of the anterior leaflet of the left atrioventricular ("mitral") valve is seen (arrow). Colour flow mapping in the same view (C) reveals valvar regurgitation through the commissural orifice, not through the apparent "cleft", which is intact. From the transgastric position (D), deep flexion of the probe produces images of the atrioventricular valves in cross section. The "mitral valve" orifice is marked (MVO). The anterior leaflet can be seen divided by a "cleft" into superior and inferior components (*). LA, left atrium; LV, left ventricle; MVO, left atrioventricular valve orifice.
Figure 5 .
Ebstein's anomaly. From a modified four chamber view at the gastro-oesophageal junction (A), the right atrium is seen to be severely dilated. The closed arrows indicate the level of the tricuspid annulus. There is atrialisation of a large part of the right ventricular cavity. The septal leaflet is displaced downward from the annulus (*) and the anterosuperior tricuspid valve leaflet is large and abnormally tethered to the right ventricular wall (open arrows). In B, colour flow mapping reveals very severe tricuspid regurgitation, through a regurgitant orifice well below the tricuspid valve annulus. Oblique views of the right sided structures are often helpful; C shows multiple abnormal "chordae" tethering the large anterosuperior leaflet to the right ventricular wall (*). In systole (D), severe tricuspid regurgitation is seen through two regurgitant orifices (that is, two flow convergence zones). RA, right atrium; RV, right ventricle; LV, left ventricle; RVOT, right ventricular outflow tract.
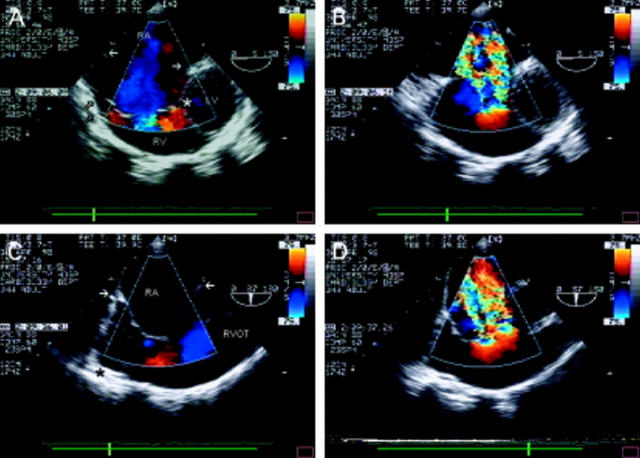
Figure 6 .
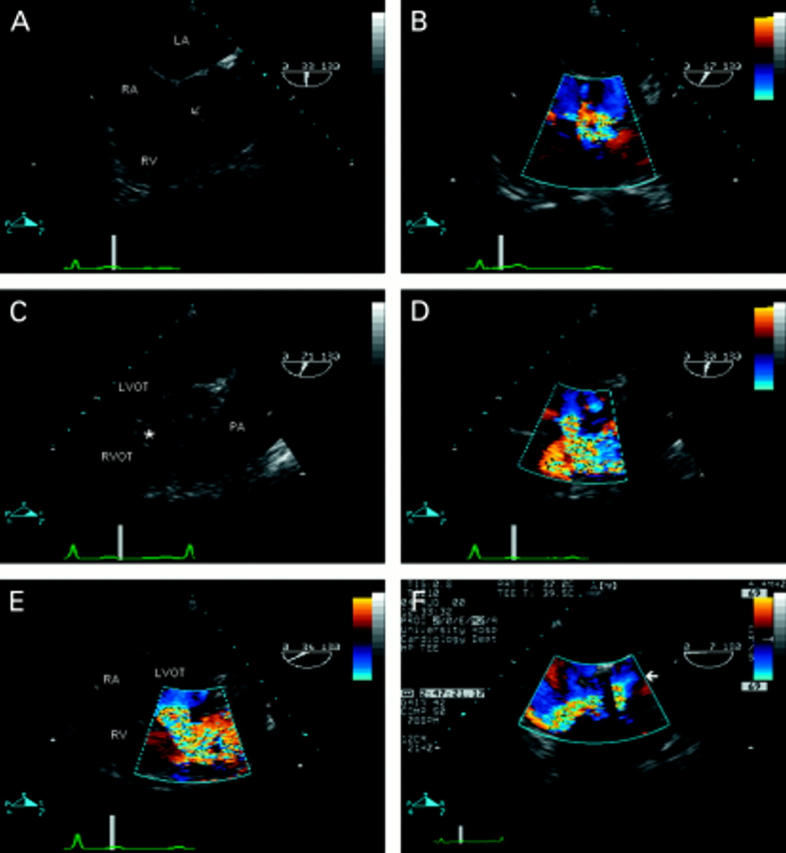
Ventricular septal defect (VSD) seen in the transverse plane of the left ventricular outflow tract (A). There is a defect in the perimembraneous region (arrow), partially plugged by tricuspid valve tissue. Colour flow mapping (B) reveals the presence of a left-to-right shunt—note the flow convergence zone in the left ventricular outflow tract—confirming the diagnosis of perimembraneous VSD. (C) Oblique view showing the right ventricular outflow tract in its long axis, in a patient with aortic valve endocarditis—note the vegetation seen in the left ventricular outflow tract. There is a defect seen between the two outflow tracts (*). The right ventricular infundibulum is hypertrophied, resulting in the appearance of subpulmonary stenosis, and the pulmonary artery is dilated. The diagnosis of doubly committed VSD is confirmed by colour flow mapping (D). (E) The same defect in the short axis view. Note that the defect is between left ventricular outflow tract and right ventricular outlet rather than between left ventricular outflow tract and right ventricular inlet as with perimembraneous defects (B). The importance of the flow convergence zone is demonstrated in F. The four chamber view with flexion reveals the left ventricular outflow tract in a patient with two previous aortic valve replacements. An apparent jet of tricuspid regurgitation is seen in the right atrium. However, note that this flow originates from the left ventricular outflow tract (arrow)—that is, there is a communication between the left and right ventricle. The dropout within the colour map is caused by artefact from the aortic valve replacement. RA, right atrium; RV, right ventricle; LV, left ventricle; RVOT, right ventricular outflow tract; LVOT, left ventricular outflow tract; LA, left atrium; PA, pulmonary artery.
Figure 7 .
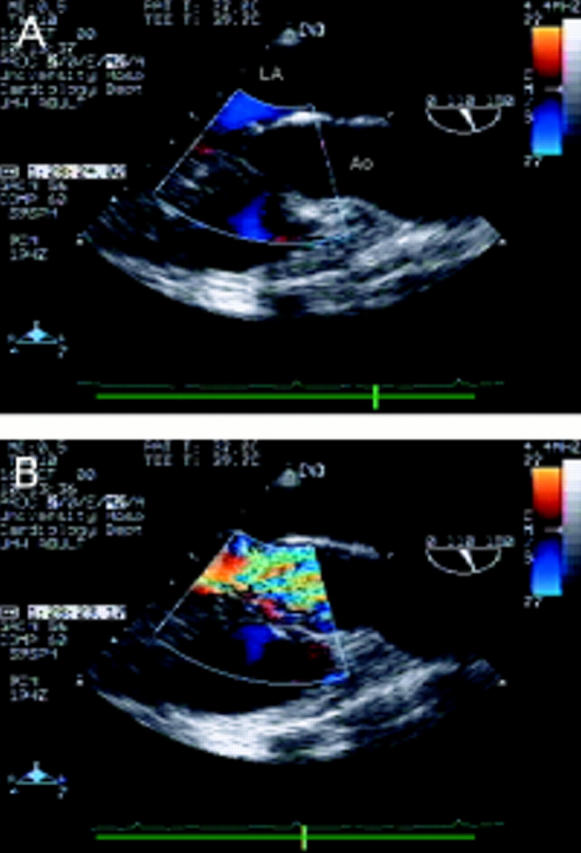
Long axis view of the left ventricular outflow tract (within the colour flow mapping box) and aortic root (A). A membrane is seen attached to the anterior mitral valve leaflet and the ventricular septum causing severe narrowing of the left ventricular outflow tract approximately 1 cm below the aortic valve. There is pronounced left ventricular hypertrophy. Colour flow mapping (B) in the same view reveals turbulence and a flow convergence zone proximal to the aortic valve. LA, left atrium; Ao, aorta.
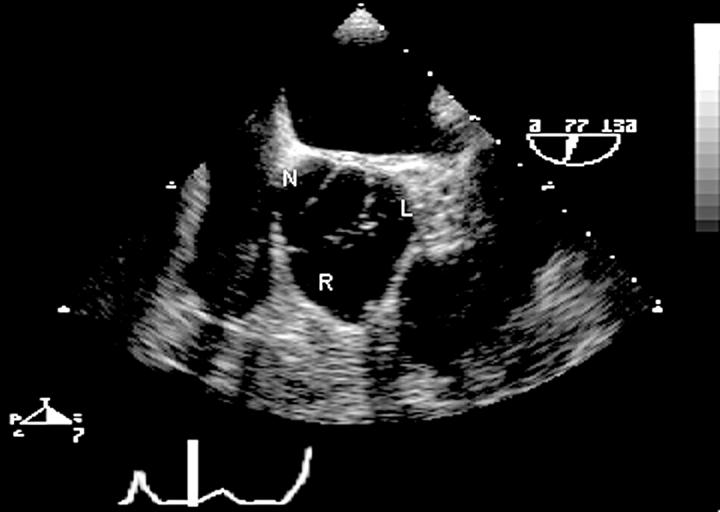
Figure 8 .
Short axis view at the aortic valve leaflet tips showing many characteristics of congenital bicuspid aortic valve: there is fusion of the left (L) and right (R) cusps. The raphe gives the appearance of a commissure dividing the valve into three leaflets when closed. The valve orifice during systole is oval shaped rather than triangular. The sinuses are asymmetric and unequal in size. The aortic valve area can be measured accurately by planimetry in this view. R, right coronary cusp; L, left coronary cusp; N, non-coronary cusp.
Figure 9 .
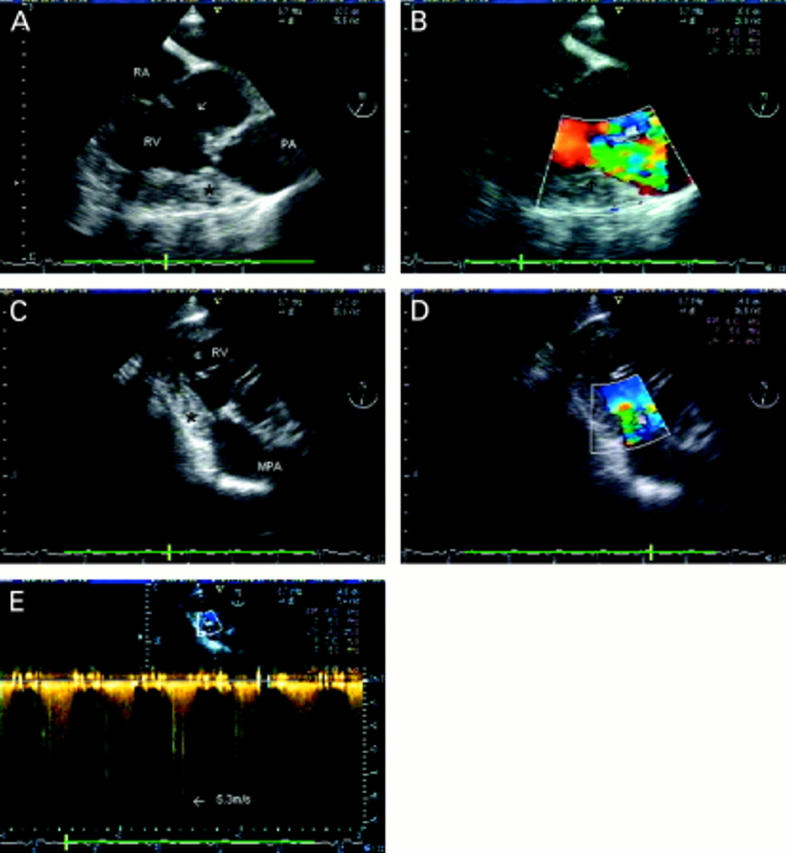
Mid cavity right ventricular obstruction. (A) Short axis view just below the aortic valve. A large perimembraneous ventricular septal defect is seen (arrow) between the left ventricular outflow tract and inlet part of the right ventricle. There is severe right ventricular hypertrophy and a critical obstruction in mid-cavity (*). The pulmonary valve leaflets cannot be seen; the level of the annulus is indicated (PA). In a similar view with colour flow mapping (B), flow convergence and turbulence are seen within the right ventricular cavity in systole, at the site of the obstruction (arrow). In the transgastric view, a long axis view of the right ventricle can be obtained, which shows the relation of the obstruction (*) to the main pulmonary artery (C) and allows good alignment for colour flow (D) and continuous wave Doppler (E). A peak gradient of 118 mm Hg was measured. RA, right atrium; RV, right ventricle; MPA, main pulmonary artery; PA, pulmonary annulus.
Figure 10 .
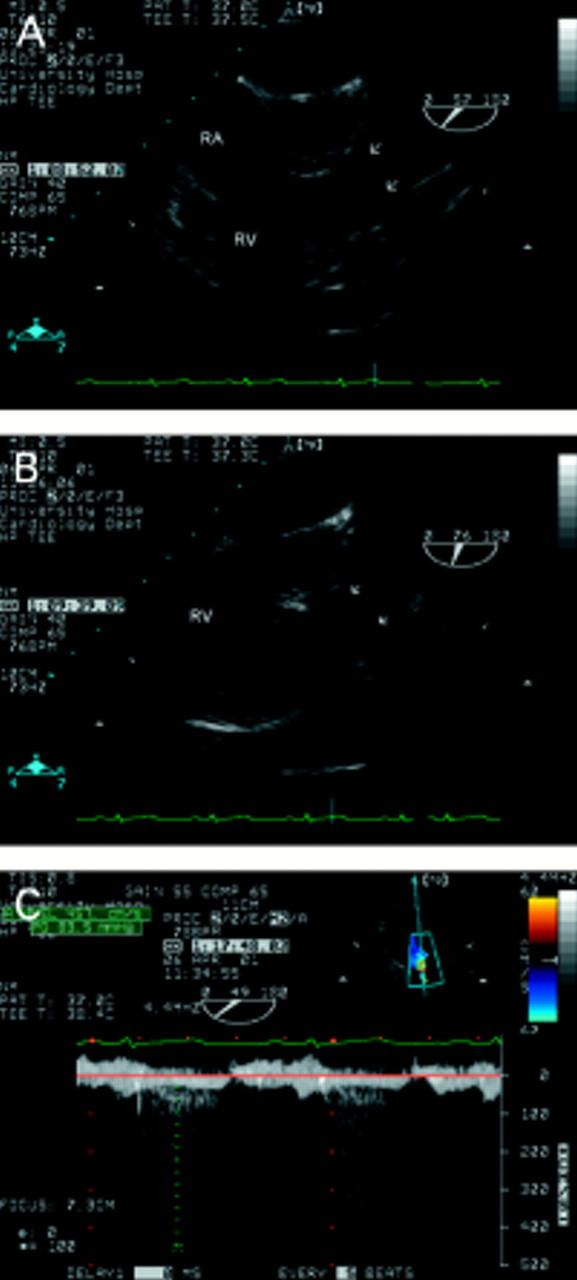
Pulmonary stenosis in a patient who had been misdiagnosed as having a ventricular septal defect 30 years earlier. The short axis view (A) shows the triangular orifice of a tricuspid aortic valve (see fig 8). Doming of the pulmonary leaflets (arrows) is seen in A and B. This relatively subtle feature is easy to miss but was indicative of severe pulmonary stenosis in this case. There is "post-stenotic" dilatation of the main pulmonary artery. The right ventricular wall is hypertrophied but the infundibulum is not narrowed. The transgastric view (C) allows good alignment of the Doppler signal with flow. A peak gradient of 84 mm Hg was obtained in this case. RA, right atrium; RV, right ventricle.
Figure 11 .
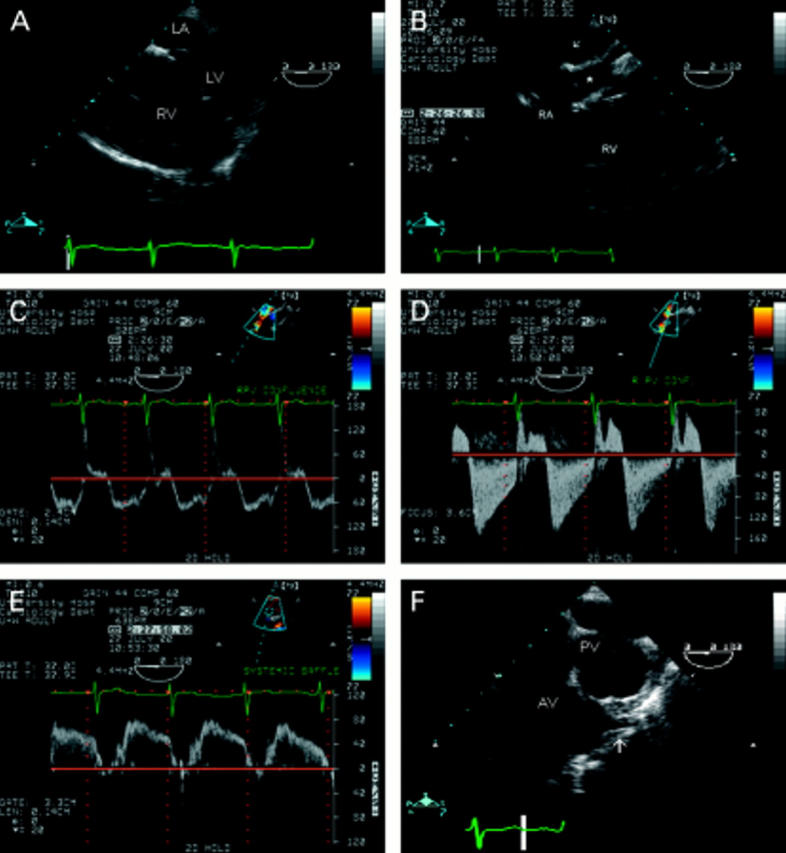
Transposition of the great arteries with Mustard procedure. The thick walled, dilated right ventricle and thin walled, compressed left ventricle (A) are typical of conditions in which the right ventricle is the systemic ventricle. The appearance of the posterior structures is complex (B). The pulmonary veins drain into a confluence (arrow) and then the right atrium. The systemic venous return is baffled (*) across to the left atrium. Colour and spectral Doppler interrogation of venous inflows must be scrupulous. Pulse wave Doppler at the pulmonary venous confluence shows a normal velocity and laminar (C) but, at the junction of the confluence and the right atrium there is turbulence, increased velocity indicating a small gradient (10 mm Hg), and an abnormal flow pattern (D). Flow within the systemic venous baffle was normal (E). Both great arteries are seen in parallel (F). The aorta is anterior and the origin of the left coronary artery well visualised. LA, left atrium; LV, left ventricle; RA, right atrium; PV, pulmonary valve; AV, aortic valve.
Figure 12 .
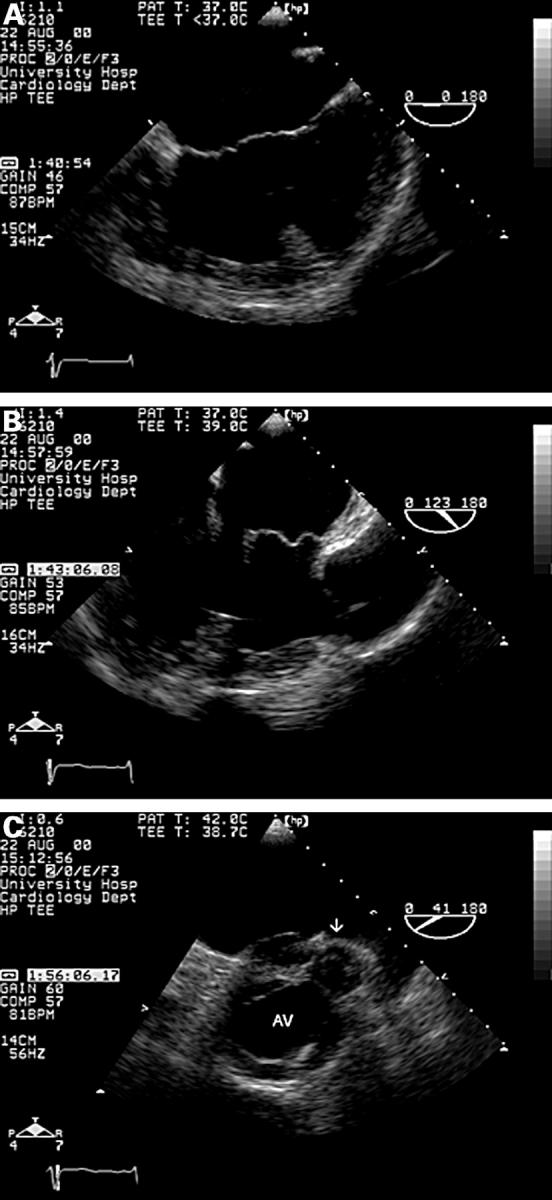
Complex congenital heart disease illustrating many of the points in the text. In the mid oesophageal 0° view (A), a single common atrium is seen. The atrioventricular valves are aligned and there is a large inlet ventricular septal defect (essentially producing a single ventricle) consistent with a common atrioventricular valve/complete atrioventricular septal defect. (B) Longitudinal image plane (120°). The aorta is seen anteriorly, connected to an anteriorly placed ventricle of indeterminate morphology. The pulmonary valve/artery cannot be seen. (C) Short axis view of the aortic valve and pulmonary valve (arrow). The ventriculo-arterial valves are arranged in a parallel fashion, with the aorta anterior, indicating malposed great arteries. The aortic valve orifice is triangular, suggesting a tricuspid valve. The pulmonary valve is small and the pulmonary artery was atretic in this case. The pulmonary valve orifice is oval, typical of a bicuspid (or unicuspid) valve. AV, aortic valve.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hirsch R., Kilner P. J., Connelly M. S., Redington A. N., St John Sutton M. G., Somerville J. Diagnosis in adolescents and adults with congenital heart disease. Prospective assessment of individual and combined roles of magnetic resonance imaging and transesophageal echocardiography. Circulation. 1994 Dec;90(6):2937–2951. doi: 10.1161/01.cir.90.6.2937. [DOI] [PubMed] [Google Scholar]
- Houston A., Hillis S., Lilley S., Richens T., Swan L. Echocardiography in adult congenital heart disease. Heart. 1998 Nov;80 (Suppl 1):S12–S26. doi: 10.1136/hrt.80.2008.12s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumper O. Imaging the heart in adult congenital heart disease. Heart. 1998 Dec;80(6):535–536. doi: 10.1136/hrt.80.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan M. J., Becker A. E., Macartney F. J., Jiménez M. Q., Shinebourne E. A., Anderson R. H. Nomenclature and classification of congenital heart disease. Br Heart J. 1979 May;41(5):544–553. doi: 10.1136/hrt.41.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren C., O'Sullivan J. J. Survival with congenital heart disease and need for follow up in adult life. Heart. 2001 Apr;85(4):438–443. doi: 10.1136/heart.85.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]