Abstract
Upper GI tumours have a dismal prognosis. Only early diagnosis and accurate staging can optimize patient management.
Keywords: Oesophageal neoplasms, gastric neoplasms, adenocarcinoma, CT, endoscopic ultrasound, PET/CT
Introduction
Cancers of the oesophagus and stomach are among the most lethal of all malignancies. The majority of these neoplasms in Western countries are detected at an advanced stage due to the insidious nature of the onset of symptoms and their similarity in early stages to benign causes of dysphagia and dyspepsia. Only earlier diagnosis, more accurate staging methods, and more effective treatment protocols offer any hope of improving the dismal prognosis of these tumours [1, 2].
Oesophageal cancer
In the past, squamous cell carcinoma accounted for over 95% of oesophageal malignancies. Over the past two decades, however, there has been a dramatic increase of adenocarcinoma arising in columnar cell-lined Barrett’s mucosa, accounting for greater than 50% of all oesophageal cancers in some areas [1, 3].
Diagnosis
On double-contrast barium studies (Fig. 1), early squamous cell carcinomas of the oesophagus appear as small, sessile, polypoid lesions, with smooth or slightly lobulated contours; or as plaque-like lesions that often have flat, central ulcers that are best visualized in profile; or as a superficial, spreading lesion with a nodular appearance of the mucosa without a discrete mass. When early oesophageal cancer or superficial spreading cancer is suspected on barium examinations, endoscopic biopsy should be performed. Advanced squamous cell carcinomas may appear infiltrative, ulcerative, polypoid or less commonly varicoid [3, 4].
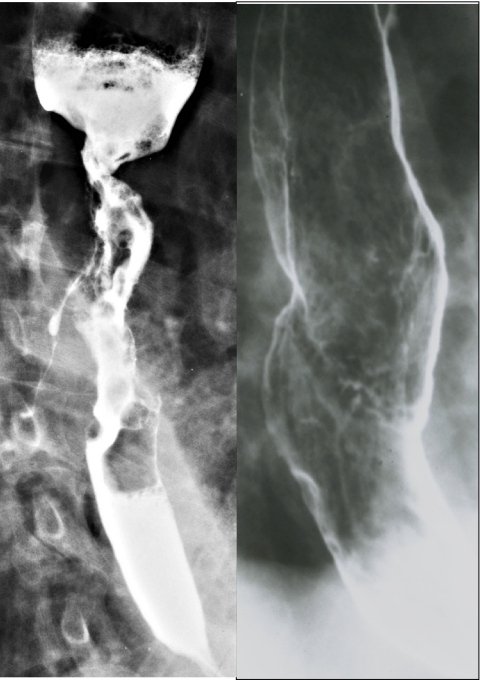
Figure 1.
Carcinoma of the oesophagus on double contrast barium studies. (a) Squamous cell carcinoma showing luminal narrowing, abrupt shelf-like borders, ulceration, circumferential growth and a fistula to the tracheobronchial tree. (b) Adenocarcinoma arising from Barrett’s mucosa causes a benign-appearing stricture associated with a plaque-like tumour of the mid-oesophagus.
Early adenocarcinoma arising from Barrett’s mucosa can manifest as small sessile polyps, plaque-like lesions, or superficial spreading lesions that cause focal nodularity of the mucosa without a discrete mass. These early cancers can also cause focal irregularity, flattening or nodularity of a pre-existing peptic stricture. Accordingly, early endoscopy and biopsy are necessary to exclude adenocarcinoma whenever any of these suspicious features develop in the region of a peptic stricture. Advanced adenocarcinoma of the oesophagus can appear infiltrating, polypoid, ulcerative, or, less commonly varicoid [1, 3].
Gastric cancer
Cancers of the antrum and body of the stomach have decreased in incidence in Western countries but the incidence of adenocarcinomas at the gastro-oesophageal junction have been dramatically rising. Early gastric cancer can only be found by screening asymptomatic, at risk patients.
Diagnosis
Early gastric cancer is limited to the mucosa (Fig. 2(a)) and submucosa, regardless of the presence or absence of lymph node involvement. Type 1 early gastric cancers are elevated lesions that protrude more than 5 mm into the lumen. Type 2 tumours appear as plaque-like elevations with mucosal nodularity, or shallow areas of ulceration, singly or severally. Type 3 early gastric cancers are excavated lesions resembling gastric ulcers but with irregular ulcer craters, clubbing, fusion, or amputation of radiating folds, and nodularity of adjacent mucosa [2, 5].
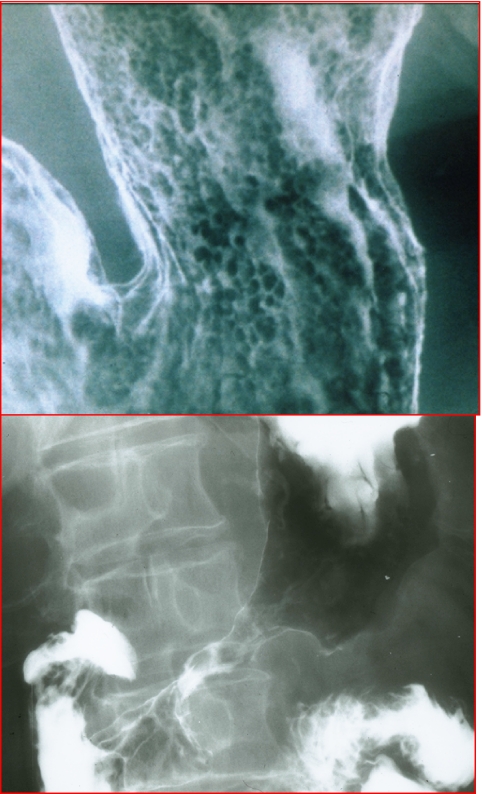
Figure 2.
Adenocarcinoma of the stomach. (a) Early gastric cancer with mucosal nodularity. (b) Advanced gastric cancer with narrowing and rigidity of the antral wall due to mural infiltration of scabrous tumour.
Type 1 advanced gastric cancer is large polypoid or fungating lesion that has irregular lobulation and measures 3 cm or larger in greatest diameter. In Type 2 advanced gastric cancer, the bulk of the tumour has been replaced by ulceration. These tumours have discrete, sharply defined borders. Type 3 advanced gastric cancers have mixed morphology with both infiltrative and ulcerative components. The ulceration does not have discrete borders however. Type 4 advanced gastric cancers are diffusely infiltrating lesions that are associated with marked proliferation of fibrotic tissue and desmoplasia producing the so-called linitis plastica appearance (Fig. 2(b)) [2, 5, 6].
Staging
Once the diagnosis of oesophageal or gastric cancer is established, accurate staging is essential in planning the surgical approach, in deciding whether neoadjuvant chemotherapy or radiation therapy is necessary, and in determining the risk of tumour recurrence and overall prognosis [7–17].
A number of imaging examinations have proven useful for upper gastrointestinal tumour staging:
(1) Multi-detector computed tomography (MDCT)
(2) Magnetic resonance imaging (MRI)
(3) Endoluminal MRI
(4) Transabdominal ultrasound
(5) Endoscopic ultrasound
(6) Intraoperative ultrasound
(7) Positron emission tomography
(8) PET/CT
T staging
T staging (Fig. 3) assesses the depth of tumour invasion into the wall of the oesophagus and stomach, surrounding adventitia, serosa, fat, and adjacent organs. Endoscopic ultrasound is superior to endoscopic MR in depicting the depth of mural invasion (Fig. 4) for oesophago–gastric neoplasms and both modalities are superior to MDCT (Figs 5 and 6) and conventional MR. PET and PET/CT have only a limited role in this aspect of tumour staging [7–17].
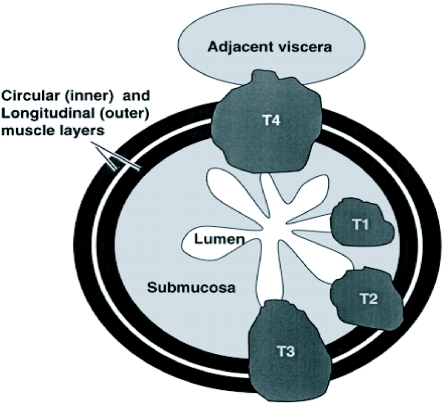
Figure 3.
Schematic showing T staging of oesophageal and gastric cancer. T1, tumour extends into submucosa; T2, tumour extends into muscularis propria; T3, tumour extends through the muscularis propria into the subserosa; T4, tumour extends directly into other organs or tissues.
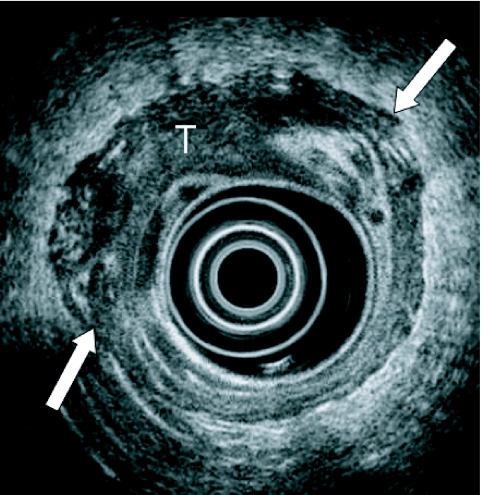
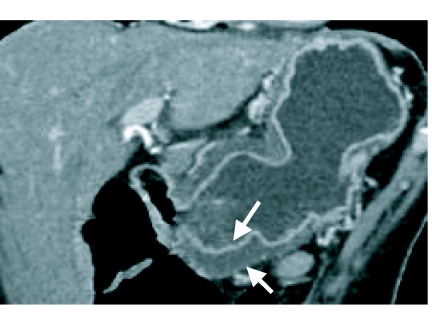
Figure 4.
Endoscopic ultrasound demonstrates a T3 oesophageal neoplasm (T) that has invaded beyond the muscularis propria (arrows).
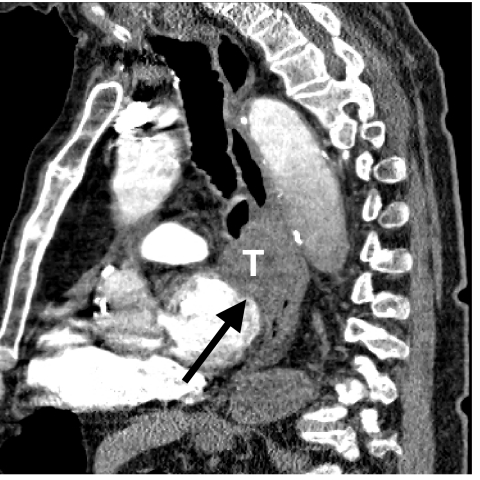
Figure 5.
Sagittal reformatted image discloses a T4 tumour (T) invading the mediastinum and left atrial wall (arrow).
Figure 6.
Coronal reformatted image of the stomach discloses a T2 tumour that is causing mural thickening of the gastric antrum (arrows) but no penetration beyond the muscularis propria.
N staging
CT and MR detection of malignant lymphadenopathy has traditionally been based on size criteria. Lymph nodes greater than 1 cm are considered abnormal (Fig. 7). Unfortunately size criteria are based only on statistical probability. In reality, many nodes smaller than 1 cm are malignant, and nodes larger than 1 cm are caused by reaction to a number of benign inflammatory conditions. Accordingly, CT and MR cannot reliably differentiate benign from malignant adenopathy [7–17].
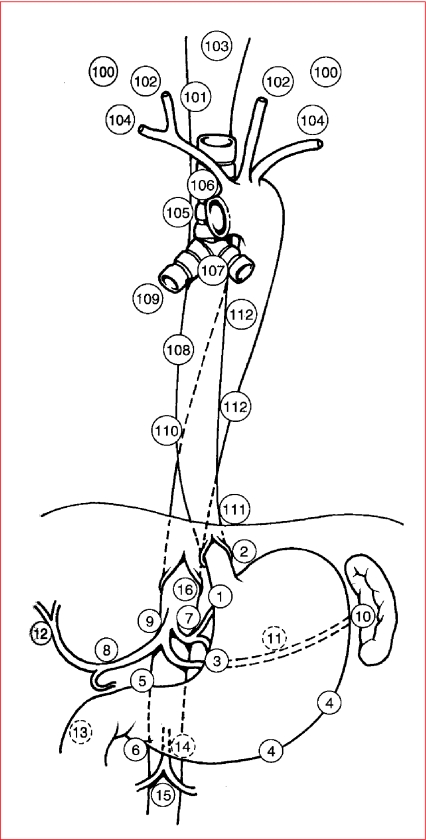
Figure 7.
N staging of oesophago-gastric neoplasm. Schematic diagram depicting the common sites of lymph node metastases in oesophageal and gastric cancers.
Endoscopic ultrasound is superior to MDCT, conventional MR and endoscopic MR in the depiction of local adenopathy. Tissue aspiration can also be performed during endoscopic ultrasound. PET/CT is superb for detecting regional and distant adenopathy [7–17].
M staging
Once oesophageal and gastric cancer have become invasive, there are five major routes of metastases that can be assessed with imaging: (1) direct invasion (Fig. 8); (2) lymphatic permeation and dissemination; (3) hematogenous embolization (Fig. 9); (4) transperitoneal seeding; (5) intraluminal implantation [1, 2].
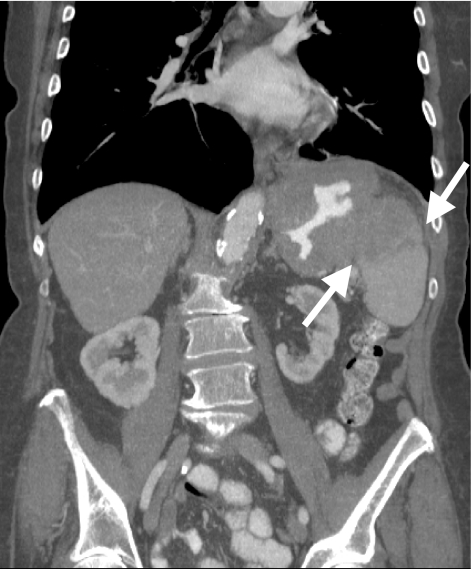
Figure 8.
Coronal reformatted image shows direct invasion (arrows) of a gastric cancer into the spleen via the gastrosplenic ligament.
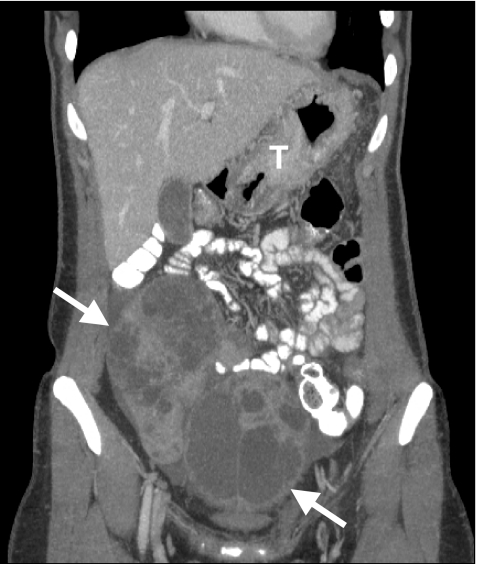
Figure 9.
Coronal reformatted image shows large Krukenberg tumours of the ovaries (arrows) due to hematogenous metastases from a gastric cancer (T).
MDCT is the standard means of M staging in most situations. It is superior to MR in depicting mediastinal, hilar, pulmonary, pericardial, pleural, omental, mesenteric and peritoneal disease. PET/CT appears to be the most accurate means of globally evaluating the chest and abdominal cavities for metastatic tumour (Fig. 10). Intraoperative ultrasound appears to be the most sensitive technique in the depiction of liver metastases [18, 19].
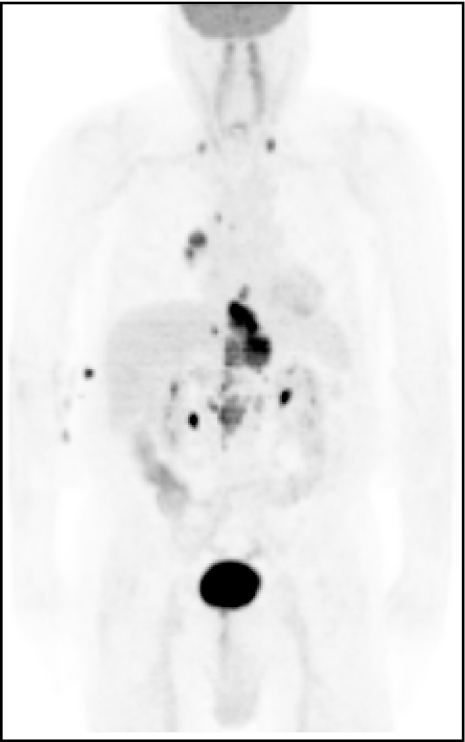
Figure 10.
PET scan showing a tumour of the oesophago-gastric junction with metastatic disease to the chest and neck.
References
- 1.Gore RM, Yaghmai V. Esophageal cancer. In: Bragg DG, Rubin P, Hricak H, editors. Oncologic Imaging. 2nd ed. Philadelphia: Saunders; 2002. pp. 359–90. [Google Scholar]
- 2.Gore RM, Miller FH. Stomach cancer. In: Bragg DG, Rubin P, Hricak H, editors. Oncologic Imaging. 2nd ed. Philadelphia: Saunders; 2002. pp. 391–418. [Google Scholar]
- 3.Lee SS, Ha HK, Shin YM, et al. Superficial esophageal cancer: esophagogastric findings correlated with histopathologic findings. Radiology. 2005;236:535–44. doi: 10.1148/radiol.2362040748. [DOI] [PubMed] [Google Scholar]
- 4.Iyer RB, Silverman PM, Tamm EP, et al. Diagnosis, staging, and follow-up of esophageal cancer. AJR. 2003;181:785–93. doi: 10.2214/ajr.181.3.1810785. [DOI] [PubMed] [Google Scholar]
- 5.Iyer R, Dubrow R. Imaging upper gastrointestinal malignancy. Semin Roentgenol. 2006;41:113–20. doi: 10.1053/j.ro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Carrascosa P, Capunay C, Ulla M, et al. Elevated gastric lesions: virtual gastroscopy. Abdom Imaging. 2006;31:261–7. doi: 10.1007/s00261-005-0373-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum SJ, Stergar H, Antoch G, et al. Staging and follow-up of gastrointestinal tumors with PET/CT. Abdom Imaging. 2006;31:25–35. doi: 10.1007/s00261-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 8.Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy-systematic review. Radiology. 2005;236:841–51. doi: 10.1148/radiol.2363041042. [DOI] [PubMed] [Google Scholar]
- 9.Umeoka S, Koyama S, Togashi K, et al. Esophageal cancer: evaluation with triple-phase dynamic CT—initial experience. Radiology. 2006;239:777–83. doi: 10.1148/radiol.2393050222. [DOI] [PubMed] [Google Scholar]
- 10.Panebianco V, Grazhdani H, Iafrate F, et al. 3D CT protocol in the assessment of the esophageal neoplastic lesions: can it improve TNM staging? Eur Radiol. 2006;16:414–21. doi: 10.1007/s00330-005-2851-5. [DOI] [PubMed] [Google Scholar]
- 11.Hur J, Park M-S, Lee JH, et al. Diagnostic accuracy of multidetector row computed tomography in T- and N- staging of gastric cancer with histopathologic correlation. J Comput Assist Tomogr. 2006;30:272–377. doi: 10.1097/00004728-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lim JS, Yun MJ, Kim M-J, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143–56. doi: 10.1148/rg.261055078. [DOI] [PubMed] [Google Scholar]
- 13.Kim HY, Kim AY, Oh ST, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879–85. doi: 10.1148/radiol.2363041101. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Eun HW, Goo DE, et al. Imaging of various gastric lesions with 2D MPR and CT gastrography performed with MDCT. Radiographics. 2006;26:1101–18. doi: 10.1148/rg.264055089. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt GP, Haug AR, Schoenberg SO, et al. Whole-body MRI and PET-CT in the management of cancer patients. Eur Radiol. 2006;16:1216–25. doi: 10.1007/s00330-006-0183-8. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Ito K, Matsunaga N, et al. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstrusction: CT-histologic correlation. AJR. 2005;185:1152–8. doi: 10.2214/AJR.04.0651. [DOI] [PubMed] [Google Scholar]
- 17.Inamoto K, Kouzai K, Ueeda T, et al. CT virtual endoscopy of the stomach: comparison study with gastric fiberscopy. Abdom Imaging. 2005;30:473–9. doi: 10.1007/s00261-004-0278-0. [DOI] [PubMed] [Google Scholar]
- 18.Van Westreenen HL, Plukker JTM, Cobben DCP, et al. Prognostic value of the standard uptake value in esophageal cancer. AJR. 2006;185:436–40. doi: 10.2214/ajr.185.2.01850436. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt GP, Haug AR, Schoenberg SO, et al. Whole-body MRI and PET-CT in the management of cancer patients. Eur Radiol. 2006;16:1216–25. doi: 10.1007/s00330-006-0183-8. [DOI] [PubMed] [Google Scholar]