Abstract
Cutaneous cancer is now the most common human malignancy and in the UK malignant melanoma comprises 11% of all skin cancers. Eighty percent of malignant melanoma is thought to be related to excessive exposure to sunlight, particularly in childhood. Although the least common skin cancer, malignant melanoma is the most deadly. In 1999 it killed over 1600 individuals in the UK and by 2004, in the USA, 55,100 new cases were anticipated. The incidence of this disease is increasing more rapidly than any other malignancy and in males there was a four-fold increase in incidence between 1971 and 1997 whilst there was a three-fold increase in women. Cutaneous melanoma is arguably the most widely metastasising neoplastic disease and it has a particularly unpredictable pattern of spread. Imaging has an important role in the management of this disease as the demonstration and delineation of metastases influences management and prognosis.
Keywords: Cutaneous cancer, melanoma, imaging
Introduction
The worldwide incidence of melanoma increased on average by 2.5% per year between 1990 and 1995, a rate higher than any other malignancy [1–3]. Although mortality rates are higher in men and are increasing, rates for women appear to have plateaued in the UK and USA [3]. The cause of this increase is uncertain but is thought to relate to a combination of increased exposure to ultraviolet radiation and improved detection through screening. The lifetime risk for developing malignant melanoma has increased from 1 in 1500 in 1930 to 1 in 75 in the year 2000 in the United States. The current lifetime risk of developing the disease in Australia is 1 in 25 [4]. It is more common in women in the UK with a lifetime risk of 1 in 117, whereas the UK incidence for men is 1 in 147. Although incidence rises with age it still has a high incidence in the young being the third most common cancer in 15–39 year olds.
Melanoma confined to the epidermis is effectively curable and thin lesions carry a >98% 5-year survival rate. However patients with primary tumours of >4 mm thickness have a <50% survival rate and the median survival for disseminated melanoma is just 7–8 months. The prognosis for patients with advanced visceral metastatic melanoma is particularly poor with a 5-year survival of 5%–14% [5].
Role of imaging at presentation
There is no evidence to support the routine use of chest radiography, ultrasound or computed tomography (CT) for screening patients with early stage melanoma.
A retrospective review of the staging chest radiographs of 876 asymptomatic patients with localized cutaneous melanoma found a true positive rate of just 0.1% for pulmonary metastases [6].
High frequency ultrasound examination may be employed in assessing regional draining lymph nodes and guiding fine needle aspiration. In some centres, AJCC stage I or II tumours are routinely investigated by sentinel node examination using lympho-scintigraphic guidance and there is now considerable evidence that these techniques are the most effective in detecting occult regional lymph node metastases [7]. Imaging of sentinel nodes by hybrid imaging using emission nuclear medicine techniques (SPECT) and low dose CT transmission techniques have further improved detection rates in identifying melanoma in regional lymph nodes [8].
It is the view of some authorities, however, that sentinel node examination and completion elective node dissection are techniques that have yet to show impact on overall survival and their application remains controversial [9].
Staging of malignant melanoma
The initial staging of malignant melanoma is surgical, defined after wide local excision (Table 1).
Table 1.
AJCC revised version of the melanoma TNM classification [16]
| T classification | Thickness | Ulceration status |
|---|---|---|
| T1 | ≤1.0 mm | a. without ulceration |
| and level II/III | ||
| b. with ulceration or | ||
| level IV/V | ||
| T2 | 1.01–2.0 mm | a. without ulceration |
| b. with ulceration | ||
| T3 | 2.01–4.0 mm | a. without ulceration |
| b. with ulceration | ||
| T4 | >4.0 mm | a. without ulceration |
| b. with ulceration | ||
| N classification | No. of metastatic nodes | Nodal metastatic mass |
| N1 | 1 node | a. micrometastasis a |
| b. macrometastasis b | ||
| N2 | 2–3 nodes | a. micrometastasis a |
| b. macrometastasis b | ||
| c. in transit | ||
| met(s)/satellite(s) | ||
| without metastatic | ||
| nodes | ||
| N3 | 4 or more metastatic nodes, | |
| or matted nodes, or in | ||
| transit met(s)/satellite(s) | ||
| with metastatic node(s) | ||
| M classification | Site | Serum lactate |
| dehydrogenase | ||
| M1a | Distant skin, subcutaneous, | Normal |
| or nodal metastases | ||
| M1b | Lung metastases | Normal |
| M1c | All visceral metastases | Normal |
| Any distant metastases | Elevated | |
aMicrometastases are diagnosed after sentinel or elective lymphadenectomy. bMacrometastases are defined as clinical detectable nodal metastases confirmed by therapeutic lymphadenectomy or when modal metastasis exhibits gross extracapsular extension.
Imaging of metastases
Ultrasound, CT and magnetic resonance imaging (MRI) all have the ability to identify nodal and distant metastases but there is general agreement that their routine use in asymptomatic patients with melanoma is not indicated. However some centres now routinely perform CT studies of the chest, abdomen and pelvis on patients with tumours with a thickness <2 mm.
More recently, however, the application of fused positron emission tomography (PET) and CT imaging has led to the increased use of this technique in staging patients with malignant melanoma and a study of 250 patients with stages I–IV disease published recently found PET/CT to be significantly more accurate than PET alone and CT alone for the identification of visceral and non-visceral metastases [10].
Lymph node metastasis
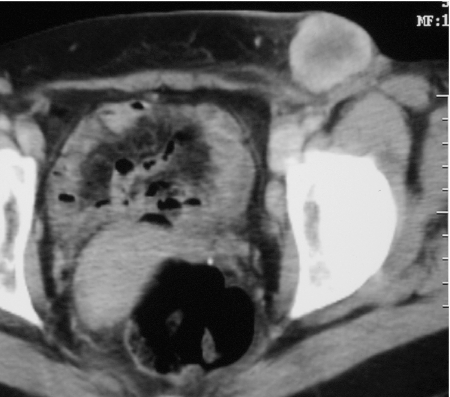
An autopsy series of 216 melanoma patients revealed lymph nodes to be the dominant site of metastatic disease with deposits present in almost three-quarters [11]. Lymph node metastases not accessible to clinical examination or ultrasound evaluation are usually imaged on CT with reliance being placed on established size criteria for the various nodal groups to define the likelihood of involvement (Fig. 1).
Figure 1.
Enhanced axial CT showing bulky lymph node metastases extending from the left inguinal into the external iliac chain.
The skin, subcutaneous fat and muscles
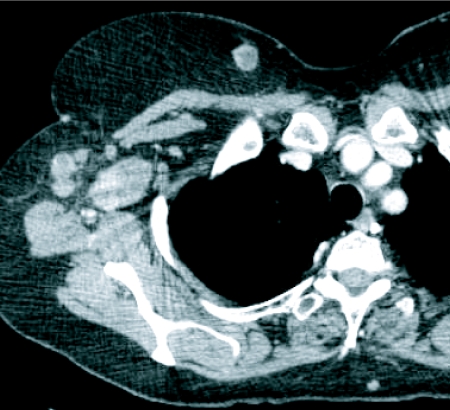
At autopsy over two-thirds of patients dying of malignant melanoma have metastases in the soft tissues [11] and subcutaneous and soft tissue metastases predominate (Fig. 2). The distribution of muscle metastases reflects the distribution of muscle mass with the lower limb therefore predominating as a site of disease spread.
Figure 2.
Enhanced CT image demonstrating melanoma metastases in breast and axillary nodes.
The lung
The lung is the most frequently affected organ at autopsy [11]. Chest radiography and thoracic CT form the mainstay of imaging for pulmonary metastases. Although a comparative study of PET versus CT and chest radiography found the latter two tests to be superior to PET in the detection of lung metastases [12], the fusion of the two methods has shown increased utility [10]. When they are found to be present, pulmonary metastases in melanoma usually number more than five [12].
The central nervous system
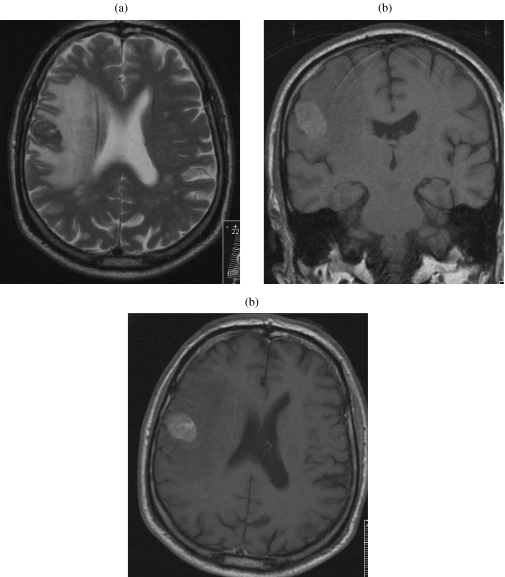
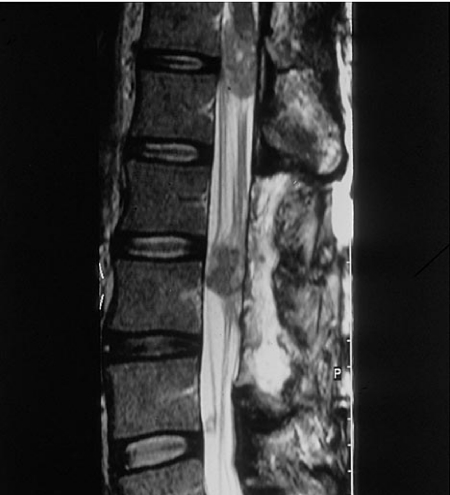
Autopsy data show that 49%–73% of patients with disseminated melanoma have cerebral metastases [13]. Cerebral metastases tend to occur late in the disease, carrying the gravest prognosis of all visceral sites with a median survival of 4 months [14]. Cerebral metastases show characteristic signal features on MRI due to the presence of iron in the melanin within the deposits (Fig. 3). Typically these lesions are usually multiple at presentation and average 1–4 cm in size. Intra-medullary or lepto-meningeal metastases are not uncommon in metastatic melanoma (Fig. 4).
Figure 3.
Cranial MR series showing single melanoma metastasis in the posterior right frontal lobe. (a) A low signal lesion and extensive oedema on T2 weighting with increase signal on T1 weighting due to high melanin content (b). There is avid enhancement after contrast (c).
Figure 4.
Multiple low signal lepto-meningeal deposits shown on T2 weighted unenhanced sagittal MRI.
Liver and biliary system
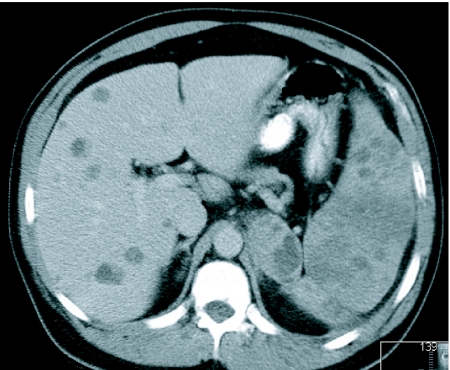
The liver is the third most common site of disease at autopsy being present in 58% of patients dying from melanoma [11]. The majority of liver metastases from malignant melanoma are of low attenuation on CT when compared to the normal parenchyma (Fig. 5). Portal venous phase scanning alone fails to detect 14% of liver deposits. Melanoma accounts for more than half of metastases to the gallbladder [15] and autopsy data show an incidence of gallbladder metastases from melanoma to be 9%–20% with a lower frequency for bile duct deposits. They are usually clinically occult (Fig. 6).
Figure 5.
Multiple hypo-dense hepatic deposits on axial enhanced CT. Extensive splenic disease is also demonstrated as well as a large deposit in the left adrenal.
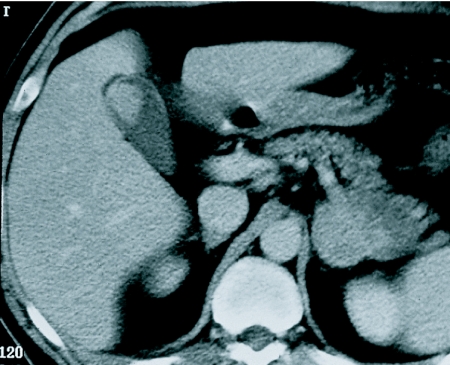
Figure 6.
Melanoma tumour nodule in the gall bladder. Note also the metastasis in the left adrenal.
The spleen
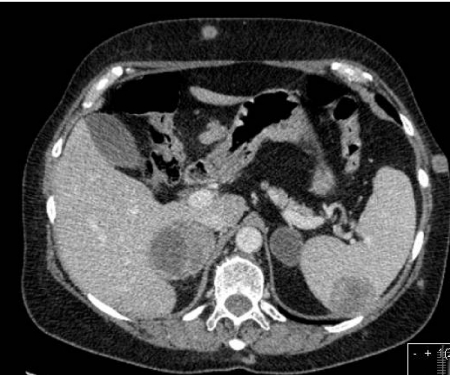
The spleen is a rare site for metastatic disease in cancer patients, with an incidence of 5% at autopsy. However, the two tumours most frequently implicated in splenic metastases are ovarian carcinoma and malignant melanoma with 30% of melanoma patients having splenic involvement at autopsy (Figs 5 and 7) [11].
Figure 7.
Axial CT section demonstrating bilateral adrenal metastasis as well as a deposit in the spleen.
Adrenals
The adrenal gland is the most frequently invaded endocrine organ. Adrenal metastases have a mean diameter of 4 cm and are usually unilateral in clinical series but bilateral at autopsy (Figs 5 and 7) [11].
Other sites
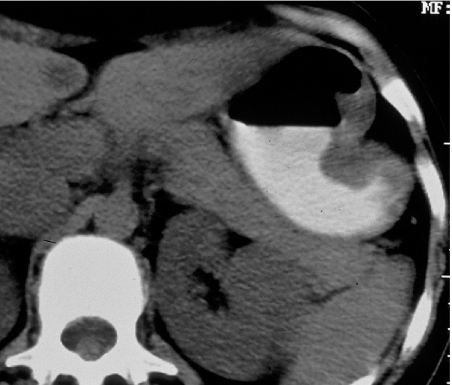
Melanoma is the most common blood-borne metastasis to the gastro-intestinal tract and may present anywhere from mouth to anus. In the stomach and small bowel they often promote significant haemorrhage (Fig. 8). Melanoma may also involve the pancreas, kidneys, uterus and heart. Bone metastases are rare until late in the disease and are typically osteolytic.
Figure 8.
Enhanced axial CT showing melanoma metastasis protruding into the gastric lumen.
Conclusion
Cutaneous malignant melanoma has a predilection for young adults. Early stage disease is curable with surgery. The majority of patients at first metastatic relapse will exhibit disease confined to a single organ, however not infrequently the disease will be widely disseminated with any number and combination of metastatic sites being involved. The imaging demonstration of widespread metastatic disease is important in determining treatment and in predicting outcome.
References
- 1.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiological studies. Cancer Causes Control. 2001;12:69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society; 2004. Available from http://www.cancer.org/downloads/STT/CAFF/-finalPWSecured.pdf
- 3.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality 1973–1995. A report card for the US. Cancer. 1998;82:1197–207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol. 1996;34:839–47. doi: 10.1016/s0190-9622(96)90041-9. [DOI] [PubMed] [Google Scholar]
- 5.Tomsu K, Von Eschen KB, Lee M. A meta-analysis of median survival of patients with stage IV melanoma. Proc Am Soc Clin Oncol. 1997;16:1784. [Google Scholar]
- 6.Terhune MH, Swanson N, Johnson TM. Use of chest radiography in the initial evaluation of patients with localised melanoma. Arch Dermatol. 1998;134:569–72. doi: 10.1001/archderm.134.5.569. [DOI] [PubMed] [Google Scholar]
- 7.Cochran AJ, Balda BR, Starz H, et al. The Augsburg consensus: techniques of lymphatic mapping, sentinel lymphadenectomy, and completion lymphadenectomy in cutaneous malignancies. Cancer. 2000;89:236–41. [PubMed] [Google Scholar]
- 8.Schillaci O. Hybrid SPECT/CT: a new era for SPECT imaging? Eur J Nucl Med Mol Imaging. 2005;32:521–4. doi: 10.1007/s00259-005-1760-9. [DOI] [PubMed] [Google Scholar]
- 9.Meirion TJ, Patocskai EJ. The argument against sentinel node biopsy for malignant melanoma. BMJ. 2006;321:3–4. doi: 10.1136/bmj.321.7252.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhardt MJ, Alexius YJ, Huber A, et al. Diagnostic performances of whole-body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–87. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 11.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–10. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 12.Krug B, Dietlein M, Groth W, et al. Fluor-18-fluorodeoxyglucose positron emission tomography (FDG-PET) in malignant melanoma. Acta Radiol. 2000;41:446–52. doi: 10.1080/028418500127345668. [DOI] [PubMed] [Google Scholar]
- 13.de la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983;43:3427–33. [PubMed] [Google Scholar]
- 14.Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 15.Backman H. Metastases of malignant melanoma in the gastrointestinal tract. Geriatrics. 1969;24:112–20. [PubMed] [Google Scholar]
- 16.Singh-Ranger G. The American Joint Committee on Cancer staging (AJCC) atlas, by Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP. Int Semin Surg Oncol. 2006;3:34. [Google Scholar]