Abstract
The most important issues in pancreatic imaging are the detection and staging of pancreatic cancer, differentiation between cancer and focal pancreatitis, the characterization of cystic lesions and the search for neuroendocrine tumours. Magnetic resonance (MR) units (1.5 T) with strong gradients and a phased-array torso coil should be used, making breath-hold imaging possible in order to avoid motion artifacts. Standard imaging sequences are T1-weighted (T1w) gradient recalled-echo (GRE) with and without fat saturation. For T2-weighted (T2w) imaging, axial single-shot turbo spin-echo (TSE) and coronal/oblique MR cholangio-pancreatography (MRCP) pulse sequences are preferable. As contrast agents either gadolinium agents or mangafodipir trisodium are used. Dynamic gadolinium-enhanced T1w fatsat 3D GRE images are helpful to delineate vessel infiltration by adenocarcinoma and to assess the aetiology of cystic masses. Mangafodipir-enhanced MRI has been found to be superior to helical computed tomography (CT) in the detection of small cancers and in the delineation of liver metastases. In cases of an equivocal pancreatic mass the presence of the “duct penetrating sign” at MRCP (i.e., the duct traversing the mass) is suggestive of an inflammatory pseudotumour. Hypoattenuation due to focal fatty infiltration may mimic a tumour at CT, but in-phase and opposed-phased T1w imaging readily depicts the fat. Multi-detector CT has gained increasing popularity for pancreatic imaging because of the 3D visualization of the peripancreatic vessels. However, MR imaging is excellent in the delineation of small pancreatic tumours. Due to its superior soft tissue contrast, MR imaging is also the method of choice in the differential diagnosis between tumours and tumour-simulating conditions in patients with equivocal CT and to assess cystic lesions.
Keywords: Pancreas, MR imaging, carcinoma, gadolinium, mangafodipir
Introduction
Magnetic resonance (MR) imaging has three different tasks in pancreatic imaging. First, MR imaging should provide a definitive diagnosis in patients with equivocal findings at ultrasound or multidetector computed tomography (MDCT) whether there is a tumour present or absent. Second, it should provide correct staging of cancer in order to identify patients with advanced tumours that are unresectable. Third, MR imaging has gained popularity in the characterization of cystic lesions, which are found in increasing number during CT scanning.
MR imaging technique
MR imaging is usually performed with a 1.5 T MR unit, although recent experience with 3.0 T machines has indicated that increased field strength may further improve image quality. T1-weighted (T1w) gradient recalled-echo (GRE) images in-phase and opposed-phase provide good anatomic detail. Fat-saturated T1w GRE images have even greater lesion contrast, and should be used for post-contrast imaging. T2-weighted (T2w) turbo spin-echo (TSE) images with and without fat suppression provide good delineation of organ contour, the presence of peripancreatic inflammation, but less lesion contrast is obtained. Multi-slice MR cholangio-pancreatography (MRCP) pulse sequences (HASTE) are performed in case of obstruction of the pancreatic or common bile duct. Routinely, contrast material is administered if a mass is suspected in the pancreas. After bolus administration of 0.1 mmol /kg of a non-specific gadolinium chelate (gadopentetate dimeglumine, Schering, Germany; Gd-DTPA-BMA, GE Healthcare, Norway; Gd-DOTA, Guerbet, France, etc.) dynamic T1w GRE images with fat saturation are obtained. The acquisition of a 3D GRE pulse sequence is advantageous because of the higher resolution in the z-axis (slice thickness of 2–3 mm for a 3D pulse sequence instead of approximately 5 mm for a 2D GRE). Gadolinium chelates are the agent of choice in patients with pancreatic cancer for local vascular staging, for neuroendocrine tumours, and for cystic lesions. Alternatively, mangafodipir trisodium (Teslascan ®, GE Healthcare, Norway) is administered as an IV infusion at a dosage of 5 μmol /kg (0.5 ml /kg) body weight over 10–15 min. Twenty minutes after the start of the infusion of contrast material, T1w pulse sequences are repeated. The 20 min delay period can be used to obtain the MRCP and the T2w TSE sequences. Mangafodopir trisodium (formerly known as Mn-DPDP) is a contrast agent originally developed for MR imaging of the liver. Since 2001, mangafodipir trisodium has been licensed for use in pancreatic MR imaging in several EU countries.
Detection and staging of pancreatic cancer
Surgical resection remains the treatment of choice for pancreatic adenocarcinoma. To date, it is the only curative therapy . Unfortunately, up to 80% of patients present with locally advanced disease or distant metastases at the time of diagnosis, which precludes surgery. In most institutions, contrast-enhanced MDCT is now the standard technique for detection and staging of pancreatic cancer . However, even contrast-enhanced MDCT has some limitations in the detection of small cancers . On unenhanced MR images, small tumours are best detected on T1w breath-hold fat-suppressed GRE images as hypointense masses . If tumours involve the peripancreatic tissues, fat-suppressed T1w GRE images lack contrast between low-signal intensity tumour and suppressed fat signal of the peripancreatic fat. Delineation of tumours is difficult on T2w images, as they may appear iso- or only mildly hyperintense. To improve tumour detection, administration of contrast agents is mandatory .
At gadolinium-enhanced MR imaging, adenocarcinoma tend to be hypointense after contrast media administration (Fig. 1). Several studies have compared CT with contrast-enhanced MRI regarding tumour detection and staging . Gadolinium-enhanced dynamic breath-hold MR imaging proved to be at least equal to single-slice helical CT in lesion detection . There is only one recent study comparing MDCT with gadolinium-enhanced MRI, which showed MDCT to be superior to enhanced MRI for detection . In recent clinical trials, mangafodipir-enhanced MRI using a whole body-coil has been shown to be effective in the detection and staging of cancer . Romijn et al. reported that mangafodipir-enhanced MRI improved the detection rate for cancer, but not the accuracy for tumour staging. In our experience, mangafodipir improves the sensitivity for detection of small tumours (≤2 cm in size) . In our series, mangafodipir-enhanced MRI revealed all cases of small tumours, but helical CT missed 2/8 tumours, for a sensitivity of only 75%.
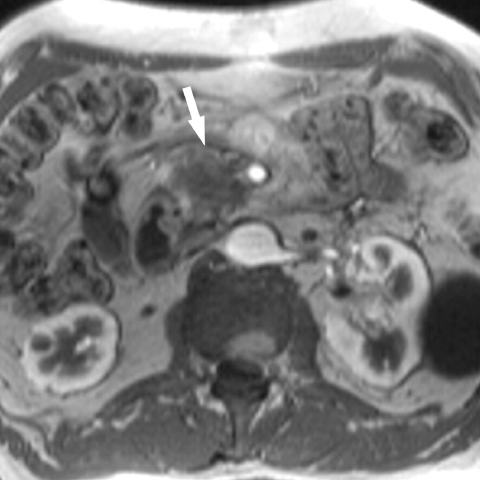
Figure 1.
Pancreatic cancer: gadolinium-enhanced T1w GRE image shows a hypointense mass in the pancreatic head, which abuts the superior mesenteric artery. The superior mesenteric vein is not visible, indicative of tumour compression.
For vascular staging MDCT imaging with 3D reformations are excellent to delineate arterial or venous encasement by tumour. Gadolinium-enhanced MRI at 1.5 T is inferior to MDCT in terms of spatial resolution and does not provide isotropic imaging to get high-quality 3D reformations. Just recently, with the development of 3.0 T imaging, interpolated 3D GRE images have become available, which allow the entire organ to be scanned at 1–1.5 mm slice thickness, which is a good basis for 3D reconstruction of data sets.
Focal pancreatitis
Differentiation between cancer and pancreatitis is difficult both with CT and MRI. Calcification is rarely present in pancreatic cancer. The most reliable sign with contrast-enhanced CT or gadolinium-enhanced MRI is the detection of a focal and defined mass. At MRCP, the duct penetrating sign may contribute to characterization: a tumour mass does not contain pancreatic ductal structures, whereas focal pancreatitis may display numerous dilated side branches traversing the mass . In general, differentiation between cancer and focal pancreatitis is difficult with a single imaging modality and may sometimes require the use of contrast-enhanced CT, MRI, and ERCP with biopsy.
Neuroendocrine tumours
Tumours other than ductal adenocarcinoma are infrequent, with cystadenomas, cystadenocarcinomas, islet cell tumours, and metastases the most common. Islet cell tumours are of neuroendocrine origin. Tumours are classified as hyperfunctioning or non-functioning, depending on the synthesis of hormones, such as insulin, gastrin, glucagon, somatostatin, etc. Insulinomas and gastrinomas are the most common types of neuroendocrine tumours. They tend to be small (70% of insulinomas are <1.5 cm in diameter), multiple, and hypervascularized (Fig. 2). Multi-phasic contrast-enhanced helical CT and dynamic gadolinium-enhanced MRI have been successfully applied to delineate even small tumours . However, the detection rate for bi-phasic helical CT and dynamic GRE MR imaging for neuroendocrine tumours is only 74%–79% (MRI) and 69%–73% (CT). In our experience, MDCT and contrast-enhanced MRI are complementary methods for the detection of neuroendocrine tumours. However, large comparative studies that include MDCT techniques with 3D reconstructions will be needed to resolve this issue.
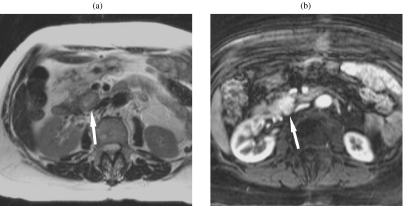
Figure 2.
Insulinoma. (a) The T2w TSE images show a round mass in the head, which is minimally hyperintense. (b) The hypervascular tumour is much clearer on the gadolinium-enhanced T1w image.
Cystic masses
Post-inflammatory pseudocysts are by far the most common cystic masses in the pancreas, with primary cystic neoplasms the second most common. They are classified as either serous (or microcystic) cystadenomas, which are always benign, and mucinous (macrocystic) cystadenoma (Fig. 3), which is a premalignant condition. Intraductal papillary mucinous tumour (IPMT), which grows intraductally, represents a spectrum of benign, borderline, or malignant conditions. IPMTs growing in the main duct (Fig. 4) are more likely to be malignant than branch duct types. The superior tissue contrast of MRI compared to CT is favourable for the definition of cysts and septations on T2w TSE images (Fig. 3). Gadolinium-enhanced MR imaging may demonstrate thick, irregular septations and solid components suspicious for malignant degeneration . Mangafodipir-enhanced MRI does not add much information in this respect. It lacks specificity, because neither solid nor cystic tumour components are enhanced.
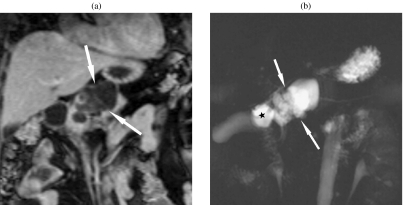
Figure 3.
Mucinous cystadenoma. (a) The MRCP image shows a macrocystic mass. (b) Septations are seen on a gadolinium-enhanced T1w GRE image. There are no solid nodules present, which would indicate malignancy.
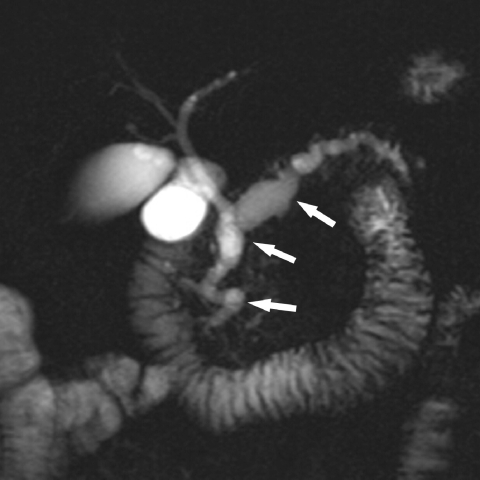
Figure 4.
Intraductal papillary mucinous tumour. MRCP shows a severely dilated pancreatic duct typical of an IPMT of main duct type.
Pseudomasses
There are two main types of pseudomasses, which may mimic a tumour of the pancreas. Focal fatty infiltration of the pancreas can appear as a hypodense mass at contrast-enhanced CT . T1w chemical shift MR imaging can be used to characterize this pseudolesion. On T1w in-phase images, the lesion shows normal or even higher signal intensity than the surrounding parenchyma. On opposed-phase GRE images, there is a typical drop in signal intensity, which confirms the presence of fat . Homogenous enhancement of the parenchyma after administration of mangafodipir confirms the diagnosis of focal lipomatosis.
There are also variations of the lateral contour of the pancreatic head, which may raise the suspicion of tumour with contrast-enhanced CT. Ross et al. have found lobulations of the pancreatic head close to the gastro-duodenal artery in more than one-third of patients. These lobulations may have an anterior, lateral, or posterior orientation. Typically, the lobules should be isodense to the adjacent parenchyma on contrast-enhanced CT . If in doubt, mangafodipir-enhanced MRI can rule out the presence of a mass.
Conclusions
Contrast-enhanced MRI is an excellent problem-solving tool in patients with suspected pancreatic cancer and equivocal findings at MDCT. Mangafodipir trisodium increases reader confidence in the detection or exclusion of small pancreatic tumours. MRI is the method of choice for detection and characterization of cystic pancreatic masses.
References
- 1.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697–701. doi: 10.1148/radiology.199.3.8637990. [DOI] [PubMed] [Google Scholar]
- 3.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebramariam A. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher JG, Wiersema MJ, Farrell MA, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81–90. doi: 10.1148/radiol.2291020582. [DOI] [PubMed] [Google Scholar]
- 5.Prokesch R, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB Jr. Isoattenuating pancreatic carcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–8. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 6.Kelekis NL, Semelka RC. MRI of pancreatic tumors. Eur Radiol. 1997;7:875–86. doi: 10.1007/s003300050221. [DOI] [PubMed] [Google Scholar]
- 7.Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol. 2002;12:2998–3008. doi: 10.1007/s00330-002-1531-y. [DOI] [PubMed] [Google Scholar]
- 8.Schima W, Ba-Ssalamah A, Plank C, et al. Das Pankreas: Teil 2. Tumore. Radiologe. 2006;46:421–38. doi: 10.1007/s00117-006-1372-9. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa T, Haradome H, Hachiya J, et al. Pancreatic ductal adenocarcinoma: preoperative assessment with helical CT versus dynamic MR imaging. Radiology. 1997;202:655–62. doi: 10.1148/radiology.202.3.9051012. [DOI] [PubMed] [Google Scholar]
- 10.Nishiharu T, Yamashita Y, Abe Y, et al. Local extension of pancreatic carcinoma: assessment with thin-section helical CT versus with breath-hold fast MR imaging-ROC analysis. Radiology. 1999;212:445–52. doi: 10.1148/radiology.212.2.r99au09445. [DOI] [PubMed] [Google Scholar]
- 11.Trede M, Rumstadt B, Wendl K, et al. Ultrafast magnetic resonance imaging improves the staging of pancreatic tumors. Ann Surg. 1997;226:393–407. doi: 10.1097/00000658-199710000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grenacher L, Klauss M, Dukic L, et al. Hochauflösende Bildgebung beim Pankreaskarzinom: prospektiver Vergleich von MRT und 4-Zeilen-Spiral-CT. Fortschr Röntgenstr. 2004;176:1624–33. doi: 10.1055/s-2004-813642. [DOI] [PubMed] [Google Scholar]
- 13.Rieber A, Tomczak R, Nüssle K, Klaus H, Brambs HJ. MRI with mangafodipir trisodium in the detection of pancreatic tumours: comparison with helical CT. Br J Radiol. 2000;73:1165–9. doi: 10.1259/bjr.73.875.11144793. [DOI] [PubMed] [Google Scholar]
- 14.Romijn MG, Stoker J, van Eijck CH, van Muiswinkel JM, Torres CG, Lameris JS. MRI with mangafodipir trisodium in the detection and staging of pancreatic cancer. J Magn Reson Imaging. 2000;12:261–8. doi: 10.1002/1522-2586(200008)12:2<261::aid-jmri8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Schima W, Függer R, Schober E, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir-enhanced MRI and contrast-enhanced helical hydro-CT. AJR. 2002;179:717–24. doi: 10.2214/ajr.179.3.1790717. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa T, Sou H, Araki T, et al. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–16. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163–71. doi: 10.1148/radiology.216.1.r00jl26163. [DOI] [PubMed] [Google Scholar]
- 18.Procacci C, Megibow AJ, Carbognin G, et al. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19:1447–63. doi: 10.1148/radiographics.19.6.g99no011447. [DOI] [PubMed] [Google Scholar]
- 19.Procacci C, Carbognin G, Biasiutti C, Guarise A, Ghirardi C, Schenal G. Intraductal papillary mucinous tumors of the pancreas: spectrum of CT and MR findings with pathologic correlation. Eur Radiol. 2001;11:1939–51. doi: 10.1007/s003300100823. [DOI] [PubMed] [Google Scholar]
- 20.Katz DS, Hines J, Math KR, Nardi PM, Mindelzun RE, Lane MJ. Using CT to reveal fat-containing abnormalities of the pancreas. AJR. 1999;172:393–6. doi: 10.2214/ajr.172.2.9930790. [DOI] [PubMed] [Google Scholar]
- 21.Isserow JA, Siegelman ES, Mammone J. Focal fatty infiltration of the pancreas: MR characterization with chemical shift imaging. AJR. 1999;173:1263–5. doi: 10.2214/ajr.173.5.10541101. [DOI] [PubMed] [Google Scholar]
- 22.Ross BA, Jeffrey RB Jr, Mindelzun RB. Normal variations in the lateral contour of the head of the pancreas mimicking neoplasm: evaluation with dual-phase helical CT. AJR. 1996;166:799–801. doi: 10.2214/ajr.166.4.8610553. [DOI] [PubMed] [Google Scholar]