Full Text
The Full Text of this article is available as a PDF (109.7 KB).
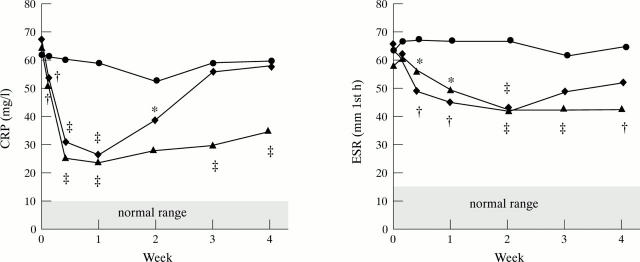
Figure 1 .
CRP and ESR measurements in a randomised placebo controlled trial of infliximab in RA. Patients were treated on day 0 with a single, two hour infusion of either placebo (circle), 1 mg/kg infliximab (diamond) or 10 mg/kg infliximab (triangle). Values are means of 24 patients at each point (25 for 1 mg/kg group). * p < 0.05; † p < 0.01; ‡ p < 0.001. Reproduced with modification from Lancet 1994;344:1105-10 by kind permission of the editor.
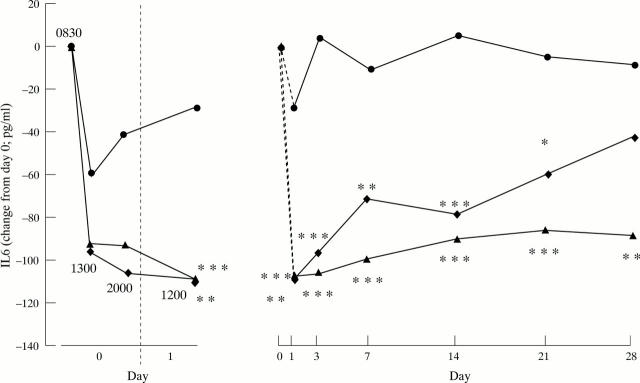
Figure 2 .
Effect of infliximab on circulating IL6 measurements in a randomised placebo controlled trial of infliximab in RA. Patients were treated on day 0 with a single, two hour infusion of either placebo (circle), 1 mg/kg infliximab (diamond) or 10 mg/kg infliximab (triangle). A detailed time/response profile on day 0 and 1 is shown on the left, with the mean sampling times indicated on the figure. Changes in circulating IL6 in the same three patient groups over the longer term are shown on the right. Each point represents the median circulating IL6 (pg/ml) concentrations. * p < 0.05; ** p < 0.01; *** p < 0.001 compared with placebo by ANOVA. Reproduced from J Immunol 1999;163:1521−8 by kind permission of the editor.
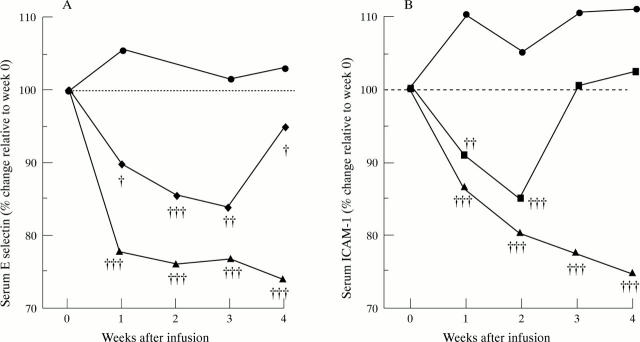
Figure 3 .
Decreases in (A) serum E selectin concentration and (B) serum ICAM-1 concentration after infliximab infusion in a randomised placebo controlled trial of infliximab in RA. Patients were treated on day 0 with a single, two hour infusion of either placebo (circle), 1 mg/kg infliximab (diamond) or 10 mg/kg infliximab (triangle). Values are expressed relative to median pre-infusion values (100%) in placebo treated (n = 24), 1 mg/kg (n = 23) and 10 mg/kg (n = 21) of infliximab treated patients. Significance was determined versus change in placebo treated group at different time points by Mann-Whitney U test: † = p < 0.05; †† = p < 0.01; ††† = p < 0.001. Reproduced with modification from Arthritis Rheum 1996;39:1082-91 by kind permission of the editor.
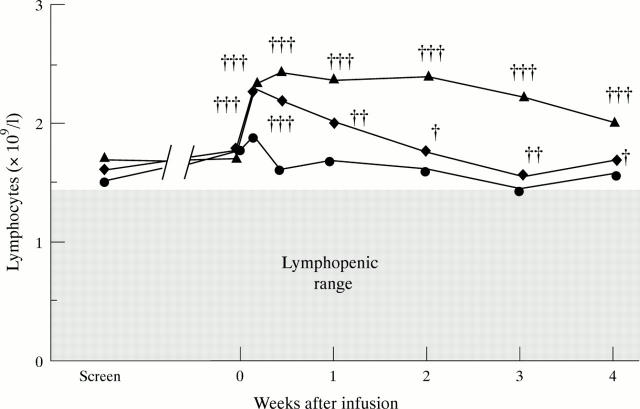
Figure 4 .
Changes in circulating lymphocyte counts after infliximab infusion in a randomised placebo controlled trial of infliximab in RA. Values are the median counts in placebo treated (circle) (n = 24), 1 mg/kg (diamond) (n = 23) and 10 mg/kg (triangle) (n = 21) of infliximab treated patients. Circulating numbers of lymphocytes in patients with active RA were measured before and after treatment. Significance was versus change in placebo treated group at different time points by Mann-Whitney U test: † = p < 0.05; †† = p < 0.01; ††† = p < 0.001. Reproduced with modification from Arthritis Rheum 1996;39:1082-91 by kind permission of the editor.
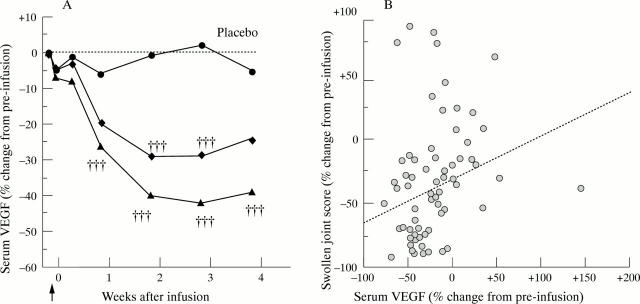
Figure 5 .
(A) Decrease in serum VEGF concentration after a single infusion of infliximab in a randomised placebo controlled trial of infliximab in RA. Serum VEGF levels were measured by enzyme linked immunosorbent assay in 69 patients with active RA who received a single infusion of either placebo or infliximab (indicated by arrow). Values are the median counts in placebo treated (circle), 1 mg/kg (diamond) and 10 mg/kg (triangle) of infliximab treated patients. Values were expressed as the % change from preinfusion for each patient, before calculation of median changes for each treatment group. Data were analysed by Mann-Whitney U test for comparison between treatment groups († = p < 0.05; †† = p < 0.01; ††† = p < 0.001). p Values for comparisons between multiple groups were adjusted using the Bonferroni correction. (B) Correlation between changes in serum VEGF concentrations and changes in swollen joint scores. Kendall's coefficient of rank correlation, calculated using % change in serum VEGF and swollen joint scores three weeks after infusion of infliximab is 0.245 (p < 0.01). Reproduced with modification from Arthritis Rheum 1998;41:1258-65 by kind permission of the editor.
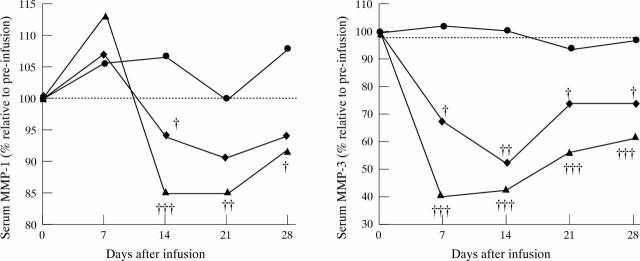
Figure 6 .
MMP-1 and MMP-3 levels are decreased after infliximab treatment in a randomised placebo controlled trial of infliximab in RA. MMP-1 and MMP-3 levels were determined in serum by a double antibody sandwich ELISA in placebo (circle) (n = 21), 1 mg/kg (diamond) (n = 19), 10 mg/kg (triangle) (n = 17) infliximab treated RA patients before infusion (day 0) and 7, 14, 21 and 28 days after infusion. Values are expressed relative to pre-infusion values (100%). Statistical analysis between the infliximab treated groups and placebo at day 7, 14, 21 and 28 was performed using the Mann-Whitney U test († = p < 0.05; †† = p < 0.01; ††† = p < 0.001). Reproduced with modification from Br J Rheumatol 1997;36:643-50 by kind permission of the editor.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan F. M., Browne K. A., Green P. A., Jaspar J. M., Maini R. N., Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-alpha (cA2) therapy. Br J Rheumatol. 1997 Jun;36(6):643–650. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Charles P., Elliott M. J., Davis D., Potter A., Kalden J. R., Antoni C., Breedveld F. C., Smolen J. S., Eberl G., deWoody K. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999 Aug 1;163(3):1521–1528. [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Bondeson J., Brennan F. M., Foxwell B. M., Maini R. N. The rationale for the current boom in anti-TNFalpha treatment. Is there an effective means to define therapeutic targets for drugs that provide all the benefits of anti-TNFalpha and minimise hazards? Ann Rheum Dis. 1999 Nov;58 (Suppl 1):I27–I31. doi: 10.1136/ard.58.2008.i27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz H. M., Antoni C., Valerius T., Repp R., Grünke M., Schwerdtner N., Nüsslein H., Woody J., Kalden J. R., Manger B. In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol. 1996 Feb 15;156(4):1646–1653. [PubMed] [Google Scholar]
- Paleolog E. M., Hunt M., Elliott M. J., Feldmann M., Maini R. N., Woody J. N. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1082–1091. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- Paleolog E. M., Young S., Stark A. C., McCloskey R. V., Feldmann M., Maini R. N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998 Jul;41(7):1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Taylor P. C., Breedveld F. C., Smeets T. J., Daha M. R., Kluin P. M., Meinders A. E., Maini R. N. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]