Full Text
The Full Text of this article is available as a PDF (172.8 KB).
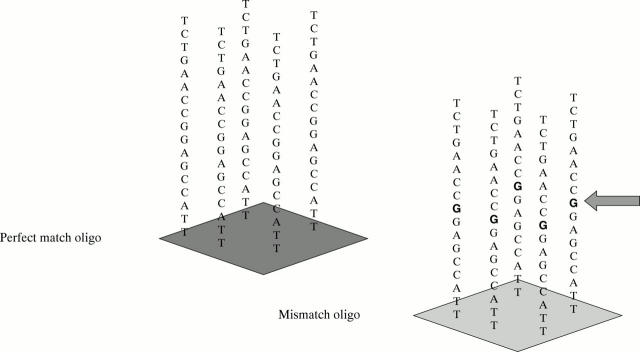
Figure 1 .
Chip design. A perfect match (PM) and an adjacent mismatch (MM) 25-mer DNA oligonucleotide probe pair is illustrated. The MM oligonucleotide has a single change at position 13.
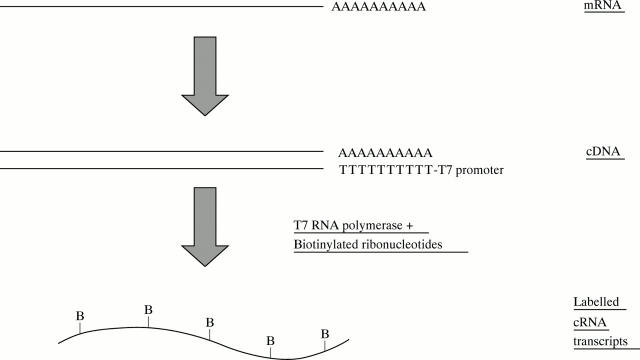
Figure 2 .
Sample preparation. A primer containing oligo-dT (T24) and a T7 RNA polymerase binding site is used for first strand cDNA synthesis. Double stranded cDNA is used for an in vitro transcription reaction, to make cRNA labelled with biotin-UTP and biotin-CTP.
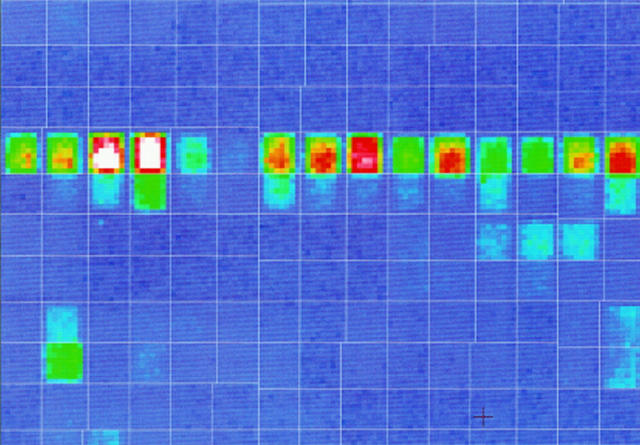
Figure 3 .
Genechip image. The figure shows a scanned image of a HuGeneFL chip.
Figure 4 .
Close view of genechip. Individual synthesis features or probe cells can be seen. Each 24 × 24 µm probe cell is imaged by the scanner as a series of 3 µm pixels. The software aligns a grid to the image after scanning. A row of brighter PM probes can be seen, above a row of corresponding MM probes.
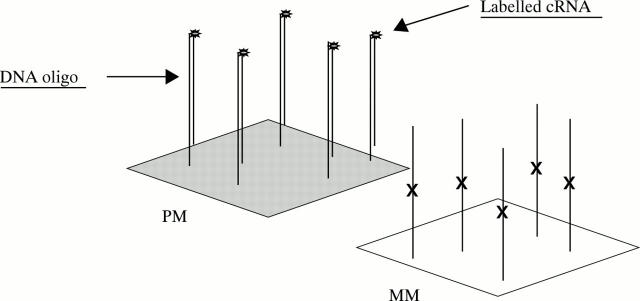
Figure 5 .
Hybridisation. Biotinylated cRNA, stained with streptavidin-phycoerythrin, binds preferentially to the PM oligonucleotide. Hybridisation is measured by calculation of PM-MM differences, and PM/MM ratios. The software makes a call of increased, decreased, or no change for each RNA, when comparing two chips.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akassoglou K., Bauer J., Kassiotis G., Pasparakis M., Lassmann H., Kollias G., Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998 Sep;153(3):801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K., Probert L., Kontogeorgos G., Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol. 1997 Jan 1;158(1):438–445. [PubMed] [Google Scholar]
- Andersson P. B., Goodkin D. E. Glucocorticosteroid therapy for multiple sclerosis: a critical review. J Neurol Sci. 1998 Sep 18;160(1):16–25. doi: 10.1016/s0022-510x(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Apodaca G., Rutka J. T., Bouhana K., Berens M. E., Giblin J. R., Rosenblum M. L., McKerrow J. H., Banda M. J. Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res. 1990 Apr 15;50(8):2322–2329. [PubMed] [Google Scholar]
- Baker D., Butler D., Scallon B. J., O'Neill J. K., Turk J. L., Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994 Sep;24(9):2040–2048. doi: 10.1002/eji.1830240916. [DOI] [PubMed] [Google Scholar]
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J., Rondot P., Catinot L., Falcoff E., Kirchner H., Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988 Oct;78(4):318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Begolka W. S., Vanderlugt C. L., Rahbe S. M., Miller S. D. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol. 1998 Oct 15;161(8):4437–4446. [PubMed] [Google Scholar]
- Bongioanni P., Mosti S., Moscato G., Lombardo F., Manildo C., Meucci G. Decreases in T-cell tumor necrosis factor alpha binding with interferon beta treatment in patients with multiple sclerosis. Arch Neurol. 1999 Jan;56(1):71–78. doi: 10.1001/archneur.56.1.71. [DOI] [PubMed] [Google Scholar]
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C. G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993 Oct 14;365(6447):642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Brocke S., Gijbels K., Allegretta M., Ferber I., Piercy C., Blankenstein T., Martin R., Utz U., Karin N., Mitchell D. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature. 1996 Jan 25;379(6563):343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- Chabot S., Williams G., Yong V. W. Microglial production of TNF-alpha is induced by activated T lymphocytes. Involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest. 1997 Aug 1;100(3):604–612. doi: 10.1172/JCI119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I. Y., Norris J. G., Benveniste E. N. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991 Apr 1;173(4):801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. M., Cossins J. A., Wells G. M., Corkill D. J., Helfrich K., Wood L. M., Pigott R., Stabler G., Ward G. A., Gearing A. J. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997 Apr;74(1-2):85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- Colton C. A., Keri J. E., Chen W. T., Monsky W. L. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993 Jun 15;35(3):297–304. doi: 10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- Correale J., Gilmore W., McMillan M., Li S., McCarthy K., Le T., Weiner L. P. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J Immunol. 1995 Mar 15;154(6):2959–2968. [PubMed] [Google Scholar]
- Cossins J. A., Clements J. M., Ford J., Miller K. M., Pigott R., Vos W., Van der Valk P., De Groot C. J. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997 Dec;94(6):590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- Crisi G. M., Santambrogio L., Hochwald G. M., Smith S. R., Carlino J. A., Thorbecke G. J. Staphylococcal enterotoxin B and tumor-necrosis factor-alpha-induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-beta and interleukin-10. Eur J Immunol. 1995 Nov;25(11):3035–3040. doi: 10.1002/eji.1830251108. [DOI] [PubMed] [Google Scholar]
- Cuzner M. L., Davison A. N., Rudge P. Proteolytic enzyme activity of blood leukocytes and cerebrospinal fluid in multiple sclerosis. Ann Neurol. 1978 Oct;4(4):337–344. doi: 10.1002/ana.410040409. [DOI] [PubMed] [Google Scholar]
- Cuzner M. L., Gveric D., Strand C., Loughlin A. J., Paemen L., Opdenakker G., Newcombe J. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996 Dec;55(12):1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Dal Canto R. A., Shaw M. K., Nolan G. P., Steinman L., Fathman C. G. Local delivery of TNF by retrovirus-transduced T lymphocytes exacerbates experimental autoimmune encephalomyelitis. Clin Immunol. 1999 Jan;90(1):10–14. doi: 10.1006/clim.1998.4653. [DOI] [PubMed] [Google Scholar]
- Drulović J., Mostarica-Stojković M., Lević Z., Stojsavljević N., Pravica V., Mesaros S. Interleukin-12 and tumor necrosis factor-alpha levels in cerebrospinal fluid of multiple sclerosis patients. J Neurol Sci. 1997 Apr 15;147(2):145–150. doi: 10.1016/s0022-510x(96)05320-8. [DOI] [PubMed] [Google Scholar]
- Dubois B., D'Hooghe M. B., De Lepeleire K., Ketelaer P., Opdenakker G., Carton H. Toxicity in a double-blind, placebo-controlled pilot trial with D-penicillamine and metacycline in secondary progressive multiple sclerosis. Mult Scler. 1998 Apr;4(2):74–78. doi: 10.1177/135245859800400206. [DOI] [PubMed] [Google Scholar]
- Duchini A., Govindarajan S., Santucci M., Zampi G., Hofman F. M. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996 Oct;44(8):474–482. [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K., Eugster H. P., Bopst M., Constantinescu C. S., Lavi E., Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med. 1997 Jun 16;185(12):2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayo A., Mozo L., Suárez A., Tuñn A., Lahoz C., Gutiérrez C. Interferon beta-1b treatment modulates TNFalpha and IFNgamma spontaneous gene expression in MS. Neurology. 1999 Jun 10;52(9):1764–1770. doi: 10.1212/wnl.52.9.1764. [DOI] [PubMed] [Google Scholar]
- Genain C. P., Cannella B., Hauser S. L., Raine C. S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999 Feb;5(2):170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Genain C. P., Roberts T., Davis R. L., Nguyen M. H., Uccelli A., Faulds D., Li Y., Hedgpeth J., Hauser S. L. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijbels K., Galardy R. E., Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994 Dec;94(6):2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijbels K., Masure S., Carton H., Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992 Nov;41(1):29–34. doi: 10.1016/0165-5728(92)90192-n. [DOI] [PubMed] [Google Scholar]
- Gijbels K., Proost P., Masure S., Carton H., Billiau A., Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993 Nov 1;36(4):432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Banda M. J., Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996 Jan 1;156(1):1–4. [PubMed] [Google Scholar]
- Gottschall P. E., Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996 Mar-Jun;3(2-3):69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- Hartung H. P. Pathogenesis of inflammatory demyelination: implications for therapy. Curr Opin Neurol. 1995 Jun;8(3):191–199. doi: 10.1097/00019052-199506000-00007. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Doolittle T. H., Lincoln R., Brown R. H., Dinarello C. A. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 1990 Nov;40(11):1735–1739. doi: 10.1212/wnl.40.11.1735. [DOI] [PubMed] [Google Scholar]
- Hewson A. K., Smith T., Leonard J. P., Cuzner M. L. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res. 1995 Aug;44(8):345–349. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issazadeh S., Ljungdahl A., Höjeberg B., Mustafa M., Olsson T. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J Neuroimmunol. 1995 Sep;61(2):205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- Jenkins H. G., Ikeda H. Tumour necrosis factor causes an increase in axonal transport of protein and demyelination in the mouse optic nerve. J Neurol Sci. 1992 Mar;108(1):99–104. doi: 10.1016/0022-510X(92)90194-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin N., Mitchell D. J., Brocke S., Ling N., Steinman L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J Exp Med. 1994 Dec 1;180(6):2227–2237. doi: 10.1084/jem.180.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiotis G., Bauer J., Akassoglou K., Lassmann H., Kollias G., Probert L. A tumor necrosis factor-induced model of human primary demyelinating diseases develops in immunodeficient mice. Eur J Immunol. 1999 Mar;29(3):912–917. doi: 10.1002/(SICI)1521-4141(199903)29:03<912::AID-IMMU912>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., Kojima K., Lesslauer W., Rinner W., Lassmann H., Wekerle H. TNF-alpha receptor fusion protein prevents experimental auto-immune encephalomyelitis and demyelination in Lewis rats: an overview. J Neuroimmunol. 1997 Feb;72(2):163–168. doi: 10.1016/s0165-5728(96)00183-x. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Shimamoto Y. Human tumor necrosis factor-alpha augments experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1991 Nov;34(2-3):159–164. doi: 10.1016/0165-5728(91)90125-q. [DOI] [PubMed] [Google Scholar]
- Körner H., Riminton D. S., Strickland D. H., Lemckert F. A., Pollard J. D., Sedgwick J. D. Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. J Exp Med. 1997 Nov 3;186(9):1585–1590. doi: 10.1084/jem.186.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., Keere F. V., Hsu A. L., Baron J. L., Haas W., Raine C. S., Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med. 1997 Jul 21;186(2):307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S. Array of hope. Nat Genet. 1999 Jan;21(1 Suppl):3–4. doi: 10.1038/4427. [DOI] [PubMed] [Google Scholar]
- Leppert D., Ford J., Stabler G., Grygar C., Lienert C., Huber S., Miller K. M., Hauser S. L., Kappos L. Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain. 1998 Dec;121(Pt 12):2327–2334. doi: 10.1093/brain/121.12.2327. [DOI] [PubMed] [Google Scholar]
- Leppert D., Waubant E., Bürk M. R., Oksenberg J. R., Hauser S. L. Interferon beta-1b inhibits gelatinase secretion and in vitro migration of human T cells: a possible mechanism for treatment efficacy in multiple sclerosis. Ann Neurol. 1996 Dec;40(6):846–852. doi: 10.1002/ana.410400606. [DOI] [PubMed] [Google Scholar]
- Liedtke W., Cannella B., Mazzaccaro R. J., Clements J. M., Miller K. M., Wucherpfennig K. W., Gearing A. J., Raine C. S. Effective treatment of models of multiple sclerosis by matrix metalloproteinase inhibitors. Ann Neurol. 1998 Jul;44(1):35–46. doi: 10.1002/ana.410440110. [DOI] [PubMed] [Google Scholar]
- Liu J., Marino M. W., Wong G., Grail D., Dunn A., Bettadapura J., Slavin A. J., Old L., Bernard C. C. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998 Jan;4(1):78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- Lockhart D. J., Dong H., Byrne M. C., Follettie M. T., Gallo M. V., Chee M. S., Mittmann M., Wang C., Kobayashi M., Horton H. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996 Dec;14(13):1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Maeda A., Sobel R. A. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996 Mar;55(3):300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Maimone D., Gregory S., Arnason B. G., Reder A. T. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991 Apr;32(1):67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- Martino G., Consiglio A., Franciotta D. M., Corti A., Filippi M., Vandenbroeck K., Sciacca F. L., Comi G., Grimaldi L. M. Tumor necrosis factor alpha and its receptors in relapsing-remitting multiple sclerosis. J Neurol Sci. 1997 Nov 6;152(1):51–61. doi: 10.1016/s0022-510x(97)00142-1. [DOI] [PubMed] [Google Scholar]
- Matusevicius D., Navikas V., Söderström M., Xiao B. G., Haglund M., Fredrikson S., Link H. Multiple sclerosis: the proinflammatory cytokines lymphotoxin-alpha and tumour necrosis factor-alpha are upregulated in cerebrospinal fluid mononuclear cells. J Neuroimmunol. 1996 May;66(1-2):115–123. doi: 10.1016/0165-5728(96)00032-x. [DOI] [PubMed] [Google Scholar]
- Matyszak M. K., Perry V. H. Delayed-type hypersensitivity lesions in the central nervous system are prevented by inhibitors of matrix metalloproteinases. J Neuroimmunol. 1996 Sep;69(1-2):141–149. doi: 10.1016/0165-5728(96)00082-3. [DOI] [PubMed] [Google Scholar]
- McRae B. L., Picker L. J., van Seventer G. A. Human recombinant interferon-beta influences T helper subset differentiation by regulating cytokine secretion pattern and expression of homing receptors. Eur J Immunol. 1997 Oct;27(10):2650–2656. doi: 10.1002/eji.1830271026. [DOI] [PubMed] [Google Scholar]
- McRae B. L., Semnani R. T., Hayes M. P., van Seventer G. A. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J Immunol. 1998 May 1;160(9):4298–4304. [PubMed] [Google Scholar]
- Miller A., Shapiro S., Gershtein R., Kinarty A., Rawashdeh H., Honigman S., Lahat N. Treatment of multiple sclerosis with copolymer-1 (Copaxone): implicating mechanisms of Th1 to Th2/Th3 immune-deviation. J Neuroimmunol. 1998 Dec 1;92(1-2):113–121. doi: 10.1016/s0165-5728(98)00191-x. [DOI] [PubMed] [Google Scholar]
- Mokhtarian F., Shi Y., Shirazian D., Morgante L., Miller A., Grob D. Defective production of anti-inflammatory cytokine, TGF-beta by T cell lines of patients with active multiple sclerosis. J Immunol. 1994 Jun 15;152(12):6003–6010. [PubMed] [Google Scholar]
- Monteyne P., Sindic C. J. Data on cytokine mRNA expression in CSF and peripheral blood mononuclear cells from MS patients as detected by PCR. Mult Scler. 1998 Jun;4(3):143–146. doi: 10.1177/135245859800400311. [DOI] [PubMed] [Google Scholar]
- Myers L. W., Ellison G. W., Merrill J. E., El Hajjar A., St Pierre B., Hijazin M., Leake B. D., Bentson J. R., Nuwer M. R., Tourtellotte W. W. Pentoxifylline is not a promising treatment for multiple sclerosis in progression phase. Neurology. 1998 Nov;51(5):1483–1486. doi: 10.1212/wnl.51.5.1483. [DOI] [PubMed] [Google Scholar]
- Nataf S., Louboutin J. P., Chabannes D., Fève J. R., Muller J. Y. Pentoxifylline inhibits experimental allergic encephalomyelitis. Acta Neurol Scand. 1993 Aug;88(2):97–99. doi: 10.1111/j.1600-0404.1993.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Navikas V., He B., Link J., Haglund M., Söderström M., Fredrikson S., Ljungdahl A, Höjeberg J., Qiao J., Olsson T. Augmented expression of tumour necrosis factor-alpha and lymphotoxin in mononuclear cells in multiple sclerosis and optic neuritis. Brain. 1996 Feb;119(Pt 1):213–223. doi: 10.1093/brain/119.1.213. [DOI] [PubMed] [Google Scholar]
- Noronha A., Toscas A., Jensen M. A. Interferon beta decreases T cell activation and interferon gamma production in multiple sclerosis. J Neuroimmunol. 1993 Jul;46(1-2):145–153. doi: 10.1016/0165-5728(93)90244-s. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Seboun E., Hauser S. L. Genetics of demyelinating diseases. Brain Pathol. 1996 Jul;6(3):289–302. doi: 10.1111/j.1750-3639.1996.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Powell M. B., Mitchell D., Lederman J., Buckmeier J., Zamvil S. S., Graham M., Ruddle N. H., Steinman L. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2(6):539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- Probert L., Akassoglou K., Pasparakis M., Kontogeorgos G., Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Bonetti B., Cannella B. Multiple sclerosis: expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Rev Neurol (Paris) 1998 Sep;154(8-9):577–585. [PubMed] [Google Scholar]
- Renno T., Krakowski M., Piccirillo C., Lin J. Y., Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995 Jan 15;154(2):944–953. [PubMed] [Google Scholar]
- Rep M. H., Hintzen R. Q., Polman C. H., van Lier R. A. Recombinant interferon-beta blocks proliferation but enhances interleukin-10 secretion by activated human T-cells. J Neuroimmunol. 1996 Jul;67(2):111–118. doi: 10.1016/0165-5728(96)00060-4. [DOI] [PubMed] [Google Scholar]
- Revel M., Chebath J., Mangelus M., Harroch S., Moviglia G. A. Antagonism of interferon beta on interferon gamma: inhibition of signal transduction in vitro and reduction of serum levels in multiple sclerosis patients. Mult Scler. 1995;1 (Suppl 1):S5–11. [PubMed] [Google Scholar]
- Richards P. T., Cuzner M. L. Proteolytic activity in CSF. Adv Exp Med Biol. 1978;100:521–527. doi: 10.1007/978-1-4684-2514-7_38. [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Albrecht M., Kitze B., Weber T., Tumani H., Broocks A., Lüer W., Helwig A., Poser S. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann Neurol. 1995 Jan;37(1):82–88. doi: 10.1002/ana.410370115. [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Weber F., Günther A., Martin S., Bitsch A., Broocks A., Kitze B., Weber T., Börner T., Poser S. Pentoxifylline, a phosphodiesterase inhibitor, induces immune deviation in patients with multiple sclerosis. J Neuroimmunol. 1996 Feb;64(2):193–200. doi: 10.1016/0165-5728(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. A., Dencoff J. E., Correa N., Jr, Reiners M., Ford C. C. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996 Jun;46(6):1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- Rott O., Cash E., Fleischer B. Phosphodiesterase inhibitor pentoxifylline, a selective suppressor of T helper type 1- but not type 2-associated lymphokine production, prevents induction of experimental autoimmune encephalomyelitis in Lewis rats. Eur J Immunol. 1993 Aug;23(8):1745–1751. doi: 10.1002/eji.1830230802. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick R. A., Ransohoff R. M., Peppler R., VanderBrug Medendorp S., Lehmann P., Alam J. Interferon beta induces interleukin-10 expression: relevance to multiple sclerosis. Ann Neurol. 1996 Oct;40(4):618–627. doi: 10.1002/ana.410400412. [DOI] [PubMed] [Google Scholar]
- Sean Riminton D., Körner H., Strickland D. H., Lemckert F. A., Pollard J. D., Sedgwick J. D. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J Exp Med. 1998 May 4;187(9):1517–1528. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K. W., Farooq M., Norton W. T., Raine C. S., Brosnan C. F. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990 Jan 1;144(1):129–135. [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Papierz W., Glabiński A., Kohno T. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol. 1995 Feb;56(2):135–141. doi: 10.1016/0165-5728(94)00139-f. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cross A. H. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991 Nov;30(5):694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Farooq M., Norton W. T., Brosnan C. F. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J Immunol. 1991 Sep 1;147(5):1522–1529. [PubMed] [Google Scholar]
- Selmaj K., Shafit-Zagardo B., Aquino D. A., Farooq M., Raine C. S., Norton W. T., Brosnan C. F. Tumor necrosis factor-induced proliferation of astrocytes from mature brain is associated with down-regulation of glial fibrillary acidic protein mRNA. J Neurochem. 1991 Sep;57(3):823–830. doi: 10.1111/j.1471-4159.1991.tb08225.x. [DOI] [PubMed] [Google Scholar]
- Sharief M. K., Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991 Aug 15;325(7):467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Sharief M. K., Thompson E. J. In vivo relationship of tumor necrosis factor-alpha to blood-brain barrier damage in patients with active multiple sclerosis. J Neuroimmunol. 1992 May;38(1-2):27–33. doi: 10.1016/0165-5728(92)90087-2. [DOI] [PubMed] [Google Scholar]
- Sommer N., Löschmann P. A., Northoff G. H., Weller M., Steinbrecher A., Steinbach J. P., Lichtenfels R., Meyermann R., Riethmüller A., Fontana A. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995 Mar;1(3):244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- Sommer N., Martin R., McFarland H. F., Quigley L., Cannella B., Raine C. S., Scott D. E., Löschmann P. A., Racke M. K. Therapeutic potential of phosphodiesterase type 4 inhibition in chronic autoimmune demyelinating disease. J Neuroimmunol. 1997 Oct;79(1):54–61. doi: 10.1016/s0165-5728(97)00111-2. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996 May 3;85(3):299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Steinman L. Some misconceptions about understanding autoimmunity through experiments with knockouts. J Exp Med. 1997 Jun 16;185(12):2039–2041. doi: 10.1084/jem.185.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüve O., Dooley N. P., Uhm J. H., Antel J. P., Francis G. S., Williams G., Yong V. W. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann Neurol. 1996 Dec;40(6):853–863. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]
- Suen W. E., Bergman C. M., Hjelmström P., Ruddle N. H. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997 Oct 20;186(8):1233–1240. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin V., Renno T., Bourbonnière L., Peterson A. C., Rodriguez M., Owens T. Increased severity of experimental autoimmune encephalomyelitis, chronic macrophage/microglial reactivity, and demyelination in transgenic mice producing tumor necrosis factor-alpha in the central nervous system. Eur J Immunol. 1997 Apr;27(4):905–913. doi: 10.1002/eji.1830270416. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Peterson J., Ransohoff R. M., Rudick R., Mörk S., Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998 Jan 29;338(5):278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Uhm J. H., Dooley N. P., Oh L. Y., Yong V. W. Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia. 1998 Jan;22(1):53–63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Catz I., Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B-cell epitope and a model of its features. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11061–11065. doi: 10.1073/pnas.92.24.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg D. O., Fordham S. A., Cowden W. B., Ramshaw I. A. Cytokines and murine autoimmune encephalomyelitis: inhibition or enhancement of disease with antibodies to select cytokines, or by delivery of exogenous cytokines using a recombinant vaccinia virus system. Scand J Immunol. 1995 Jan;41(1):31–41. doi: 10.1111/j.1365-3083.1995.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Wodicka L., Dong H., Mittmann M., Ho M. H., Lockhart D. J. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997 Dec;15(13):1359–1367. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- Woodroofe M. N., Cuzner M. L. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993 Nov;5(6):583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Chabot S., Stuve O., Williams G. Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology. 1998 Sep;51(3):682–689. doi: 10.1212/wnl.51.3.682. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Krekoski C. A., Forsyth P. A., Bell R., Edwards D. R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998 Feb;21(2):75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- van Oosten B. W., Barkhof F., Truyen L., Boringa J. B., Bertelsmann F. W., von Blomberg B. M., Woody J. N., Hartung H. P., Polman C. H. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996 Dec;47(6):1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- van Oosten B. W., Rep M. H., van Lier R. A., Scholten P. E., von Blomberg B. M., Pflughaupt K. W., Hartung H. P., Adèr H. J., Polman C. H. A pilot study investigating the effects of orally administered pentoxifylline on selected immune variables in patients with multiple sclerosis. J Neuroimmunol. 1996 May;66(1-2):49–55. doi: 10.1016/0165-5728(96)00019-7. [DOI] [PubMed] [Google Scholar]