Abstract
Anopheles gambiae is the primary vector of human malaria in sub-Saharan Africa. Invasion of Anopheles salivary glands by Plasmodium sporozoites is a necessary step in the transmission of malaria and is likely to be mediated by specific receptor–ligand interactions. We are interested in identifying putative an A. gambiae salivary gland receptor or receptors for sporozoite invasion as a possible target for blocking malaria transmission. By using monoclonal antibodies against female-specific A. gambiae salivary gland proteins, two molecules, one of 29 kDa and one of 100 kDa, were identified and characterized with respect to the age and blood-feeding process of mosquitoes. In an in vivo bioassay, the monoclonal antibody against the 100-kDa protein inhibited Plasmodium yoelii sporozoite invasion of salivary glands ≥75%. These results show that A. gambiae salivary gland proteins are accessible to monoclonal antibodies that inhibit sporozoite invasion of the salivary glands and suggest alternate targets for blocking the transmission of malaria by this most competent of malaria vectors.
Despite long-standing chemotherapeutic intercession and vector control programs, malaria exacts a heavy burden on human health, with 300–500 million infections and 1.5–2.7 million deaths annually. The disease is transmitted from female Anopheles mosquitoes to humans through the sporozoite stage of the Plasmodium parasite in the course of a blood meal. The penultimate event before the infectious bite is invasion of the Anopheles salivary glands by the Plasmodium sporozoites. This critical period of the sporogonic cycle presents a rarely studied target for malaria transmission blocking strategies. Identifying and blocking the receptors necessary for sporozoite invasion of the salivary glands could prevent the transmission of malaria from the Anopheles vector to humans.
Earlier studies have indicated that the sporozoite–salivary gland interaction is species specific and receptor mediated, suggesting that the glands dictate the ability of sporozoites to recognize and invade the salivary glands (1). Biochemical characterization of salivary gland components has shown that the basal lamina and the female-specific distal and median lateral lobes are heavily glycosylated (2). In addition, it has been shown that lectins that bind salivary gland-associated carbohydrates block Plasmodium gallinaceum (avian malaria parasite) sporozoite invasion of Aedes aegypti salivary glands. Concurrently, polyclonal serum against A. aegypti salivary glands inhibited P. gallinaceum sporozoite invasion as compared with preimmune serum and saline controls (3).
To date, efforts to block the invasion of mosquito salivary glands by malaria sporozoites have been carried out with A. aegypti mosquitoes and P. gallinaceum parasites. Although this system serves as an excellent model because of the relative simplicity of raising large numbers of A. aegypti mosquitoes and the ease of studying P. gallinaceum, results cannot be easily applied to the natural transmission of human malaria for two reasons. First, Aedes mosquitoes do not transmit human malaria parasites, and second, there are biological differences between many of the mammalian malaria parasites and P. gallinaceum (4). The goal of this study was to investigate putative receptors for mammalian malaria sporozoites on the salivary glands of A. gambiae, the primary malaria vector in Africa (5). The proposed hypothesis is that blocking the interaction between sporozoites and female-specific A. gambiae salivary gland proteins with salivary gland-specific antibodies will inhibit or prevent sporozoites from invading A. gambiae salivary glands and thus, reduce transmission. Identification of sporozoite receptors would not only increase the understanding of the biology of Plasmodium in the Anopheles vector but also suggest new molecular targets for blocking the transmission of human malaria.
Materials and Methods
Mosquitoes and P. yoelii Infection.
A. gambiae (G-3 strain) and Anopheles stephensi (Dutch strain), obtained from the Laboratory of Parasitic Diseases (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda), were reared at 27 ± 1°C and 80 ± 5% relative humidity with 12-h cycles of alternating darkness and light. Adult mosquitoes were maintained on 10% (vol/vol) Karo Dark Corn Syrup with 0.05% p-aminobenzoic acid (6). Thawed P. yoelii (17XNL strain) was injected i.p. into a 6- to 8-week-old BALB/c mouse (Charles River Breeding Laboratories). When the parasitemia reached 3–5%, blood was collected and used to infect more BALB/c mice. The parasitemia was monitored daily by Giemsa-stained thin films. When the parasitemia reached 4–7% (4–5 days), the mice were anesthetized and fed to starved 4- to 5-day-old A. gambiae females.
SDS/PAGE Analysis of Salivary Gland Proteins.
Dissected salivary glands (two pairs per lane) extracted in SDS/PAGE sample buffer containing 10% (vol/vol) β-mercaptoethanol and heated at 95°C for 10 min were analyzed on a 5–20% gradient SDS/PAGE gel (7). The gel was silver stained (Rapid Ag Stain, ICN) according to the manufacturer's protocol and photographed with the Kodak 1D (New Haven, CT) system. To determine the effect of a blood meal on salivary gland protein expression, female A. gambiae mosquitoes were deprived of sugar water for 12 h and then fed human erythrocytes in an equal volume of heat-inactivated normal human serum (NHS) through a membrane feeder at 39°C for ≈1 h. Intact pairs of salivary glands were dissected from each mosquito immediately after they fed on the blood meal and at the indicated intervals after feeding and were then analyzed by SDS/PAGE.
Salivary Gland Protein Expression Analysis.
A. gambiae mosquitoes deprived of sugar water for 12 h were fed 1 mCi (1 Ci = 37 GBq) [35S]methionine (Trans 35S-Label, ICN), dried in a SpeedVac (Savant), and reconstituted in 300 μl of 10% (vol/vol) sugar water with 50 μl of red food coloring for 1 h through a membrane feeder heated to 39°C. Glands were dissected from radiolabeled mosquitoes at the indicated time points and were processed for SDS/PAGE and autoradiography. To analyze the protein content of the saliva, radiolabeled A. gambiae (4–7 days after emergence) were allowed to probe for 3 h through a membrane feeding apparatus containing distilled water. The contents of the feeder (water plus the saliva of mosquitoes that probed) were collected and dried in a SpeedVac. Intact salivary gland pairs were also dissected from mosquitoes before and after saliva collection to compare protein profiles to those of saliva.
Monoclonal Antibody Preparation and Immunoprecipitation Analysis.
BALB/c mice were immunized i.p. with 50 A. gambiae female salivary glands (sonicated and freeze-thaw extracted) emulsified with Freund's complete adjuvant (Sigma). Mice were boosted twice with 50 salivary glands emulsified in Freund's incomplete adjuvant (Sigma) at 20-day intervals, and spleen cells were fused with P3-X-Ag8.653 myeloma cells (ATCC CRL 1580). For immunoprecipitation, salivary glands from [35S]methionine-fed A. gambiae females were extracted in 1% Triton X-100 in the presence of a protease inhibitor mixture as described (8). These procedures resulted in the selection of two clones, 2A3 (IgG2a) and C26 (IgG1) for further investigation.
Immunoelectron Microscopy and Indirect Immunofluorescence Assay.
Female A. gambiae salivary glands were fixed for 10 min in 1% paraformaldehyde/0.2% glutaraldehyde in 0.1 M PBS, pH 7.4, and washed in 0.1 M PBS. Fixed glands were dehydrated in ethanol and embedded in LR Gold Resin (London Resin, Basingstoke, U.K.) at −20°C (9). Thin sections were cut with a diamond knife and placed on 300-mesh nickel grids. Sections were etched with saturated aqueous sodium metaperodiate and incubated with 1:100 dilutions (vol/vol) of salivary-gland-specific monoclonal antibodies 2A3 and C26 and a negative control, 6B6. Gland sections were then incubated with 15-nm goat anti-mouse IgG ImmunoGold (Janssen) diluted 1:20 (vol/vol) in PBS/Tween-20. Glands were stained with uranyl acetate and Reynold's lead citrate and were coated with carbon. Grids were examined with a JEOL 100 CX electron microscope.
To localize salivary gland proteins by indirect immunofluorescence assay, intact pairs of salivary glands were incubated in a drop of 0.1% Evans blue in PBS on a glass slide at room temperature for 10 min. The glands were carefully washed with PBS and used in an indirect immunofluorescent assay as described (10) by using a 1:10 dilution (vol/vol) of mouse ascites (2A3, C26, and 6B6 as a negative control) as the primary antibody and a 1:50 dilution (vol/vol) of fluorescein-conjugated sheep anti-mouse Ig (Amersham Pharmacia) as the secondary antibody.
ELISA Determination of in Vivo Binding of Monoclonal Antibodies to Salivary Gland Proteins.
A. gambiae females (5–6 days after emergence) were fed 200 μl of a 1:3 dilution (vol/vol) of monoclonal antibodies 2A3, C26, and 6B6 (mouse ascites) in washed human erythrocytes and heat-inactivated NHS through a membrane feeder. Unfed mosquitoes were removed and used as the control. Previous studies have shown that fed antibodies reach maximum hemolymph titers 3 h after feeding (11). One pair of whole, intact salivary glands was dissected from each of 15 mosquitoes 3 h post feeding and extracted by 10 freeze-thaw cycles followed by centrifugation (Brinkmann) at 10,000 rpm for 2 min. The pellet was resuspended in 400 μl of carbonate buffer (4 mM Na2C03/NaHCO3, pH 9.6), vortexed thoroughly, and used to coat ELISA plate wells in triplicate for each serial dilution. After blocking with 5% (vol/vol) milk in PBS/Tween-20, 100 μl of a 1:1000 dilution (vol/vol) of horseradish peroxidase-conjugated goat anti-mouse antibody (Bio-Rad) was added (37°C for 1 h) and developed with 100 μl of 2,2′-azinodi(3-ethylbenzthiazoline-6-sulfonate) (Kirkegaard & Perry Laboratories). The plate was read with an ELISA reader (Molecular Devices) at 405 nm.
In Vivo Sporozoite Invasion-Blocking Assay.
A. gambiae females were allowed to feed on an anesthetized BALB/c mouse infected with P. yoelii 17XNL for 30 min. Fed mosquitoes were kept at 24°C and 68% relative humidity with alternating light and dark cycles. At 5 days after feeding, mosquitoes were given a second, uninfected blood meal of washed human erythrocytes in an equal volume of heat-inactivated NHS to decrease mosquito mortality (12). A small sample of mosquito midguts was dissected 4 days later to determine oocyst infection rates. On day 10, after feeding on infected blood meal, mosquitoes were divided randomly among four cages and were fed monoclonal antibodies through a membrane feeder apparatus in a double-blinded fashion. Salivary glands from individual mosquitoes were removed 4–5 days after antibody feeding and were homogenized in microtissue grinders (Kontes) with 35 μl of 1% BSA in PBS on ice. A 10-μl aliquot was placed on a hemocytometer, and the mean number of sporozoites per infected salivary gland was determined with a phase-contrast microscope. The significance of the sporozoite counts was analyzed by using STATVIEW 5.0 and the two sample t test.
Results
Monoclonal Antibody Preparation and Immunoprecipitation.
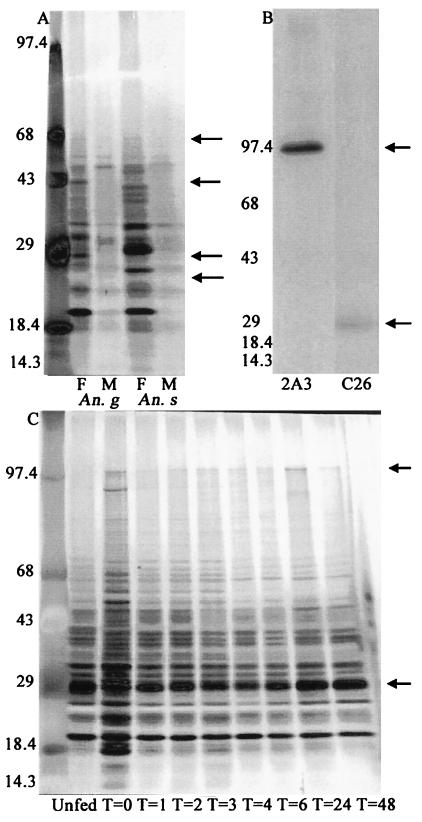
The two monoclonal antibodies (2A3 and C26) with the highest reactivity for female A. gambiae salivary gland proteins, as determined by ELISA, recognized proteins of 100 kDa and 29 kDa, respectively, in the female A. gambiae salivary gland (Fig. 1B). We limited the scope of our study to the 29-kDa and 100-kDa proteins of A. gambiae, primarily because the epitopes may be mosquito species specific, and moreover, A. gambiae is a major malaria vector. The expression of these two proteins in relation to the life cycle of female A. gambiae mosquitoes and any role they have in sporozoite recognition of salivary glands became the focus of our investigation.
Figure 1.
(A) Silver-stained SDS/PAGE gel of male (M) and female (F) A. gambiae and A. stephensi salivary gland extracts. (B) Immunoprecipitation of [35S]methionine-labeled A. gambiae salivary gland extracts with monoclonal antibodies 2A3 and C26. (C) Silver-stained SDS/PAGE gel of salivary glands isolated from A. gambiae females at the indicated time points (T = hours) after a blood meal. Arrows in A indicate the position of the 25-kDa, 29-kDa, 42-kDa, and 67-kDa proteins, and those in B and C indicate the position of the 29-kDa and 100-kDa proteins. Numbers on the left indicate molecular mass standards.
SDS/PAGE Analysis of Salivary Gland Proteins.
Salivary gland extracts were analyzed by using SDS/PAGE to investigate the species and sex specificity, the age-dependent expression, and the effect of a blood meal on salivary gland protein expression. The protein expression profiles of 4-day-old A. gambiae and A. stephensi female and male salivary glands show that males and females express some salivary gland proteins of similar molecular masses. There are, however, a number of proteins of approximately 25 kDa, 29 kDa, 42 kDa, and 67 kDa (Fig. 1A) that are present in female salivary glands but conspicuously absent in male salivary glands when equal numbers of glands are compared. A. stephensi female and male salivary glands were run as a comparison to show that the differences in salivary gland protein profiles are consistent between sexes, regardless of Anopheles species. When the salivary gland protein profiles of A. gambiae females are compared with those of A. stephensi females, it can be seen that some proteins are present across species lines, notably those of apparent molecular masses of 21 kDa, 25 kDa, and 32 kDa. However, many proteins of noncorresponding molecular masses are also detected in the two species compared (Fig. 1A).
Analysis of salivary glands from female A. gambiae of different ages, maintained on sugar water, also revealed that the 29-kDa protein is not expressed until 3 days after emergence, and thereafter it is expressed in abundant amounts (not shown).
The blood-feeding process initiates malaria infection in both the vertebrate and mosquito hosts and is essential to female oviposition. It is therefore reasonable to assume that this process may induce protein expression changes in the mosquito to facilitate the feeding process and aid in digestion of the blood meal and the accompanying physiological changes. When compared with equal numbers of sugar-fed female A. gambiae salivary glands, salivary glands dissected immediately after a blood meal showed a marked increase in a number of high and low molecular mass proteins. At 1 h after ingestion of a blood meal, the salivary gland protein profile, as revealed by silver staining, is once again similar to that of sugar-fed mosquitoes. The most notable difference seen was the expression of the 100-kDa protein in response to a blood meal in mosquitoes 4 days after emergence that otherwise do not express the 100-kDa protein. The expression of the 29-kDa salivary gland protein changes little in response to a blood meal and was comparable to that of sugar-fed mosquitoes (Fig. 1C).
Immunoelectron and Immunofluorescence Localization.
To localize the binding sites of the two monoclonal antibodies specific to A. gambiae female salivary glands, immunoelectron microscopy was used. 2A3 and C26 monoclonal antibodies were found to bind exclusively to the median and lateral lobes of female A. gambiae salivary glands. The monoclonal antibody C26, directed against the 29-kDa protein, showed extensive decoration of salivary glands (Fig. 2B). In contrast, the monoclonal antibody 2A3, which is specific for a 100-kDa protein, showed sparse localization dispersed throughout the female salivary gland (Fig. 2A). These results suggest that the 29-kDa protein is more abundant in female A. gambiae salivary glands than the 100-kDa protein.
Figure 2.
Immunoelectron and indirect immunofluorescence microscopy of female A. gambiae salivary glands. A and B show the binding of 2A3 and C26 to the distal lateral lobes of the salivary glands respectively (×15,600 magnification). C and D show the diffuse dispersion of the 29-kDa and 100-kDa proteins on the female-specific lobes of the salivary glands as revealed by immunofluorescence assay.
We also used an indirect immunofluorescence assay to localize these proteins further in the context of the whole gland. Both C26 and 2A3 bind in diffuse patterns to the median and lateral lobes of paraformaldehyde-fixed female salivary glands, indicating that these two proteins have similarly diffuse dispersion throughout the salivary gland (Fig. 2 C and D).
Biosynthetic Expression of Salivary Gland Proteins.
To follow the expression of salivary gland proteins, A. gambiae male and female mosquitoes were fed [35S]methionine (1 h) to determine the time of maximum incorporation of [35S]methionine into the proteins of the salivary glands. Equal numbers of salivary glands were dissected from mosquitoes at different time points and were analyzed by SDS/PAGE and autoradiography. The incorporation of [35S]methionine into salivary glands was detected immediately after feeding, with maximum expression 6 h after ingestion of [35S]methionine in sugar water (Fig. 3A). Male A. gambiae incorporated [35S]methionine into their salivary gland proteins at barely detectable levels on prolonged exposure of gels. Based on these results, the incorporation of [35S]methionine into salivary gland proteins was used to elucidate further the age-dependent expression profiles as well as the stability of expressed proteins. In all of the subsequent studies, salivary glands were dissected 6 h after [35S]methionine feeds.
Figure 3.
In vivo [35S]methionine labeling of A. gambiae salivary proteins. Gland extracts were run on SDS/PAGE and exposed to X-ray film. A depicts salivary glands dissected at the indicated times (T = hours) after male (M) and female (F) mosquitoes (4 days after emergence) fed on [35S]methionine. B shows in vivo labeling (6 h) of salivary gland proteins in A. gambiae females of the indicated adult age groups.
To investigate salivary gland protein expression profiles during the adult life of the mosquito, A. gambiae females of different age groups were fed on [35S]methionine under identical conditions. Salivary gland proteins are expressed in increasing amounts until the 7th day after emergence, with maximum expression on day 7. Whereas the expression of the 29-kDa protein was detectable in all of the age groups, the expression of the 100-kDa protein was not seen until mosquitoes were 6 days old, and the protein was expressed at maximum levels on the 7th day after emergence (Fig. 3B).
Sporozoite infection of salivary glands can occur at various times throughout the adult mosquito life. Thus, a putative salivary gland sporozoite receptor would have to be expressed throughout the life of the mosquito after the first infected blood meal. To investigate the stability of the 100-kDa and 29-kDa salivary gland proteins, 7-day-old A. gambiae females were fed [35S]methionine. Beginning 6 h after feeding, and at 48-h intervals thereafter, equal numbers of salivary glands were dissected until day 23 after emergence and were then analyzed by SDS/PAGE and autoradiography. The labeled proteins remained generally stable throughout the life of the mosquito. Notably, the radiolabeled 100-kDa and 29-kDa salivary gland proteins could be seen from day 7 through day 23 after emergence (not shown).
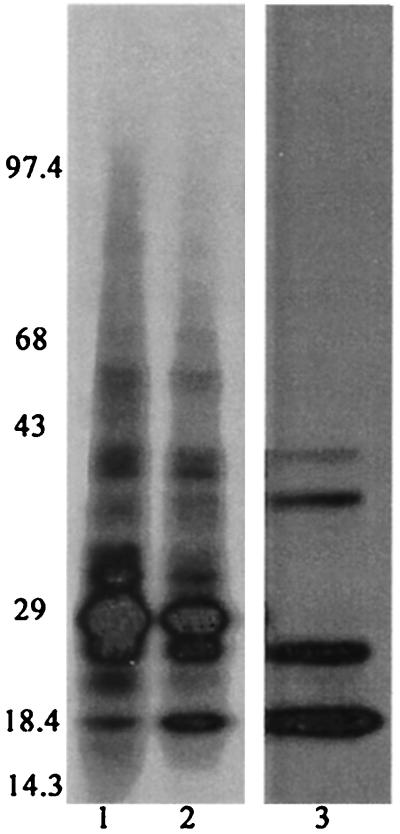
Finally, a putative sporozoite receptor on the A. gambiae salivary gland would be expected to be a component of the gland, and not secreted in the saliva. Analysis of radiolabeled whole salivary glands and radiolabeled saliva from 4- to 7-day-old A. gambiae females revealed a number of protein bands in the saliva; however the 29-kDa and 100-kDa proteins were not among the secreted proteins (Fig. 4).
Figure 4.
Analysis of [35S]methionine-labeled salivary gland extracts and saliva from A. gambiae females. Lane 1 shows salivary gland extracts from mosquitoes that had probed. Lane 2 shows salivary gland extracts from mosquitoes before probing. Lane 3 shows saliva collected from mosquitoes.
In Vivo Accessibility of Monoclonal Antibodies to Salivary Glands and Inhibition of Sporozoite Invasion.
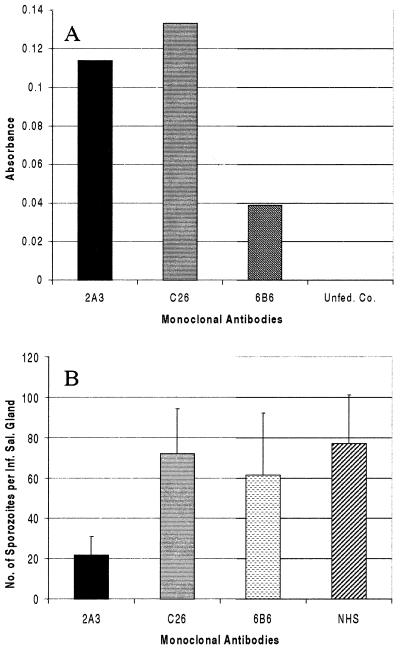
An ELISA-based assay was used to demonstrate the binding of blood-fed monoclonal antibodies to salivary glands in female A. gambiae mosquitoes (6 days after emergence). As shown in Fig. 5A, much higher binding was detected for the monoclonal antibodies 2A3 and C26 than for the negative control monoclonal antibody, 6B6. These results demonstrate that the fed monoclonal antibodies can transverse through the midgut wall and reach their salivary gland target via circulation through the hemocoel.
Figure 5.
In vivo binding of fed monoclonal antibodies to salivary glands (A) and in vivo blocking of sporozoite invasion of salivary glands (B). Female A. gambiae were fed monoclonal antibodies in a blood meal, and salivary gland-bound antibodies were detected by ELISA (A). The graph shows the average absorbance of three replicates with two salivary gland equivalents per well. B shows the combined results of three in vivo sporozoite blocking assays. P values as compared with the NHS control are: 2A3, 0.0604 (n = 51); C26, 0.8829 (n = 50); 6B6, 0.6865 (n = 42); NHS, (n = 71). The third experiment was performed with ammonium sulfate-precipitated mouse ascites to eliminate any inhibitory ascites factors.
An in vivo sporozoite invasion-blocking assay was developed to determine whether the identified 100-kDa and 29-kDa female A. gambiae salivary gland proteins may have a role in the invasion of salivary glands by Plasmodium sporozoites. A. gambiae were blood fed on mice infected with P. yoelii 17XNL. At 9 days after the infectious blood meal, midguts were dissected to determine infection rates, and the numbers of oocysts were counted. There was no significant difference in the percentage of oocyst infection (87.5%, 75%, and 75%) between each of the three experiments. The mosquitoes were then fed monoclonal antibodies to the aforementioned salivary gland proteins on the 10th day after infection. One whole, intact pair of salivary glands was dissected and homogenized from each mosquito on days 4 and 5 after monoclonal antibody feeding, and the number of sporozoites per salivary gland pair was counted. The negative controls were fed 6B6, a nonsalivary gland-specific monoclonal antibody, and the controls were fed heat-inactivated NHS. Mosquitoes fed 2A3 had 73% fewer sporozoites (P = 0.0604) than controls fed NHS (Fig. 5B). Mosquitoes fed C26 and the negative control antibody (6B6) showed no significant differences compared with controls. Salivary gland infection rates were not significantly different between any of the treatment groups (67–71%, 50–65.2%, 34.5–41.67%) in the three experiments.
Discussion
In this study, we characterized female-specific salivary gland proteins of A. gambiae mosquitoes and attempted to determine whether these proteins play a role in facilitating sporozoite invasion of the salivary glands. When whole female and male A. gambiae salivary gland protein expression profiles are compared, there are a few proteins common between the two sexes. However, there are many proteins that are not shared between the two sexes. Previous studies have shown that Plasmodium sporozoites preferentially migrate to mosquito salivary glands and that salivary gland factors may be responsible for sporozoite recognition and subsequent invasion (1, 13). Therefore, the different protein content of female and male A. gambiae salivary glands visible in Fig. 1A may represent one or more proteins necessary for sporozoite recognition, survival, and further transmission to a host.
Monoclonal antibodies made against female A. gambiae salivary glands identified two proteins, one with a molecular mass of 29 kDa and one with a molecular mass of 100 kDa. Because these proteins are expressed in a sex-specific fashion, they were characterized with respect to the life of the adult mosquito to determine whether the expression of these proteins corresponds with the biological and physiological needs of the A. gambiae vector and for Plasmodium sporozoite invasion. The 29-kDa protein was found to be abundant as revealed by intense labeling with [35S]methionine and immunoelectron microscopy. Expression was detected on the 3rd day after emergence, with maximum expression on day 7. The expression of the 29-kDa protein was not significantly affected by blood feeding, nor is it a component of the saliva. In sharp contrast to the 29-kDa protein, the 100-kDa salivary gland protein was not detectable by silver staining until after blood feeding. Similarly to the 29-kDa protein, the 100-kDa protein was expressed at maximum levels on the 7th day after emergence, remained stable throughout the life of the mosquito, and is not a component of the saliva.
These findings prompted us to speculate and test a hypothesis that these two proteins are putative candidates for sporozoite recognition. The sporozoites recognize the female-specific lobes of the salivary glands (14, 15). Both the 29-kDa and 100-kDa salivary gland proteins are expressed on these lobes and fit the logical parameters we considered for a putative salivary gland receptor for sporozoites.
With this information in mind, we developed an in vivo sporozoite invasion-blocking assay to ascertain the role of the 29-kDa and 100-kDa proteins in salivary gland infection. Before we could implement this assay, we determined, by ELISA, that monoclonal antibodies fed to mosquitoes in a blood meal are indeed capable of binding salivary glands. In the sporozoite blocking assay, infected mosquitoes were fed the monoclonal antibodies on the 10th day after infection. This time period corresponds with the release of P. yoelii sporozoites into the hemocoel (12), and a large majority of the free sporozoites would be expected to still be circulating in the hemolymph. The results show that the mosquitoes fed 2A3 displayed a 73% reduction in the average number of sporozoites per infected salivary gland. Mosquitoes fed the C26 monoclonal antibody showed little difference in the average number of sporozoites per infected salivary gland when compared with controls. Because the salivary gland infection rates between treatment and control groups were similar, it is highly unlikely that antibody 2A3 inhibits the ability of sporozoites to enter salivary glands. Perhaps a more reasonable explanation is that the antibodies reduce the available number of target sites that participate either in the binding or entry of sporozoites.
Studies investigating the possibility of blocking sporozoite invasion of mosquito salivary glands used a fixed number of sporozoites injected intrathoracically along with anti-salivary gland antibodies (3). Although assays performed in this manner may serve to evaluate more accurately the role of salivary gland proteins in sporozoite invasion, we feel that our assay better emulates the natural course of malaria transmission, but with it comes the inherent variability of any biological system. This variability can be attributed to a number of possible factors. The number of oocysts on the midgut of an infected mosquito is highly variable, as it was in our experiments; thus, the number of sporozoites released is consequently highly variable as well. In addition, the release of sporozoites from the oocyst is an ongoing process (16), and it is possible that sporozoites were released and invaded the salivary glands after the monoclonal antibodies had lost their potency and the ability to bind their targets or before the monoclonal antibodies were fed to the mosquitoes. It is also possible that not all of the salivary gland targets were bound by presumed insufficient concentrations of monoclonal antibody fed in a blood meal. The number of 29-kDa and 100-kDa antigens present on the salivary glands has not been quantitated; thus, it is not possible to determine whether saturation occurs with the concentration of monoclonal antibody present in the hemocoel. Nonetheless, the monoclonal antibody 2A3 consistently resulted in fewer sporozoites in the salivary glands (average reduction of 73% in three experiments), which approaches the level of statistical significance. Taking into account all of the biological considerations above and the fact that a single epitope-specific antibody was an effective inhibitor, further studies with a monospecific polyclonal antibody must be undertaken for in vivo blocking assays. Finally, we believe that sporozoite recognition and invasion may involve more than one salivary gland component. Thus, although our assay is more applicable to natural malaria transmission (and perhaps possible vaccine applications) than other methods, further work and refinements are necessary.
Ultrastructural studies have shown that sporozoites invading salivary glands may encounter one receptor at the basal lamina of the salivary glands and another at the plasma membrane of the secretory cells (15). Other studies have indicated that the salivary gland components are highly glycosylated (2) and that specific lectins can inhibit sporozoite invasion of the salivary glands (3). In this study, we did not attempt to determine whether the 29-kDa or 100-kDa A. gambiae salivary gland proteins were part of the basal lamina or the secretory cells. We only confirmed that they are part of the salivary gland structure and not a component of the saliva. If it is assumed that the basal lamina is impermeable to antibodies, the binding of the two monoclonal antibodies to the salivary glands suggests that the 29-kDa and 100-kDa proteins are most likely components of the basal lamina. Further studies are needed to determine the precise ultrastructural location of these proteins and any posttranslational modifications.
As previously stated, A. gambiae is responsible for the devastating effects of malaria in sub-Saharan Africa, a region that bears the greatest burden of the world's malaria problem. As vector control becomes more difficult because of insecticide resistance and the fact that limited funds need to be continuously redistributed to address the diversifying health needs of regions with a high malaria endemicity, new control strategies are required. Effectively blocking malaria transmission will rein in the prevalence of the disease, and established measures may make it possible finally to control malaria. Although the results of this study are preliminary, they do demonstrate that A. gambiae salivary gland proteins can be additional putative targets for new transmission blocking strategies and warrant a detailed molecular investigation.
Acknowledgments
We thank Jeff Vaughan, Darin Kongkasuriyachai, Mrinal Bhattacharyya, Lucy Bartels-Andrews, and Sabra Klein for their helpful discussions and insight. These studies were supported in part by National Institutes of Health Grants AI38403 and AI47089 (to N.K.) and AI35827 (to H.F.).
Abbreviation
- NHS
normal human serum
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250472597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250472597
References
- 1.Rosenberg R. Am J Trop Med Hyg. 1985;34:687–691. doi: 10.4269/ajtmh.1985.34.687. [DOI] [PubMed] [Google Scholar]
- 2.Perrone J B, DeMaio J, Spielman A. Insect Biochem Mol Biol. 1986;16:313–318. [Google Scholar]
- 3.Barreau C, Touray M, Pimenta P F, Miller L H, Vernick K D. Exp Parasitol. 1995;81:332–343. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- 4.McCutchan T F, Kissinger J C, Touray M G, Rogers M J, Li J, Sullivan M, Braga E M, Krettli A U, Miller L H. Proc Natl Acad Sci USA. 1996;93:11889–11894. doi: 10.1073/pnas.93.21.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Service M W. In: Bruce-Chwatt's Essential Malariology. Gilles H M, Warrell D A, editors. London: Edward Arnold; 1993. [Google Scholar]
- 6.Peters W, Ramkaran A E. Ann Trop Med Parasitol. 1980;74:275–282. [PubMed] [Google Scholar]
- 7.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Wizel B, Kumar N. Proc Natl Acad Sci USA. 1991;88:9533–9537. doi: 10.1073/pnas.88.21.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aikawa M, Atkinson C T. Adv Parasitol. 1990;29:151–214. doi: 10.1016/s0065-308x(08)60106-2. [DOI] [PubMed] [Google Scholar]
- 10.Barreau C, Conrad J, Fischer E, Lujan H D, Vernick K D. Insect Biochem Mol Biol. 1999;29:515–526. doi: 10.1016/s0965-1748(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan J A, Wirtz R A, do Rosario V E, Azad A F. Am J Trop Med Hyg. 1990;42:10–16. doi: 10.4269/ajtmh.1990.42.10. [DOI] [PubMed] [Google Scholar]
- 12.Sinden R E. In: Molecular Biology of Insect Disease Vectors: A Methods Manual. Crampton J M, Beard C B, Louis C, editors. London: Chapman and Hall; 1997. [Google Scholar]
- 13.Golenda C F, Starkweather W H, Wirtz R A. J Histochem Cytochem. 1990;38:475–481. doi: 10.1177/38.4.2181019. [DOI] [PubMed] [Google Scholar]
- 14.Sterling C R, Aikawa M, Vanderberg J P. J Parasitol. 1973;59:593–605. [PubMed] [Google Scholar]
- 15.Pimenta P F, Touray M, Miller L. J Eukaryotic Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 16.Sinden R E. Nature (London) 1974;252:314. doi: 10.1038/252314a0. [DOI] [PubMed] [Google Scholar]