Abstract
BACKGROUND—Mammalian Toll-like receptor (TLR) proteins are pattern recognition receptors for a diverse array of bacterial and viral products. Gram negative bacterial lipopolysaccharide (LPS) activates cells through TLR4, whereas the mycobacterial cell wall glycolipids, lipoarabinomannan (LAM) and mannosylated phosphatidylinositol (PIM), activate cells through TLR2. Furthermore, short term culture filtrates of M tuberculosis bacilli contain a TLR2 agonist activity, termed soluble tuberculosis factor (STF), that appears to be PIM. It was recently shown that stimulation of RAW264.7 murine macrophages by LPS, LAM, STF, and PIM rapidly activated NF-κB, AP1, and MAP kinases. RESULTS—This study shows that signalling by TLR2 and TLR4 also activates the protein kinase Akt, a downstream target of phosphatidylinositol-3'-kinase (PI-3-K). This finding suggests that activation of PI-3-K represents an additional signalling pathway induced by engagement of TLR2 and TLR4. Subsequently, the functional responses induced by the different TLR agonists were compared. LPS, the mycobacterial glycolipids, and the OspC lipoprotein (a TLR2 agonist) all induced macrophages to secrete tumour necrosis factor α (TNFα), whereas only LPS could induce nitric oxide (NO) secretion. Human alveolar macrophages also exhibited a distinct pattern of cellular response after stimulation with TLR2 and TLR4 agonists. Specifically, LPS induced TNFα, MIP-1β, and RANTES production in these cells, whereas the TLR2 agonists induced only MIP-1β production. CONCLUSION—Together, these data show that different TLR proteins mediate the activation of distinct cellular responses, despite their shared ability to activate NF-κB, AP1, MAP kinases, and PI-3-K.
Full Text
The Full Text of this article is available as a PDF (181.9 KB).
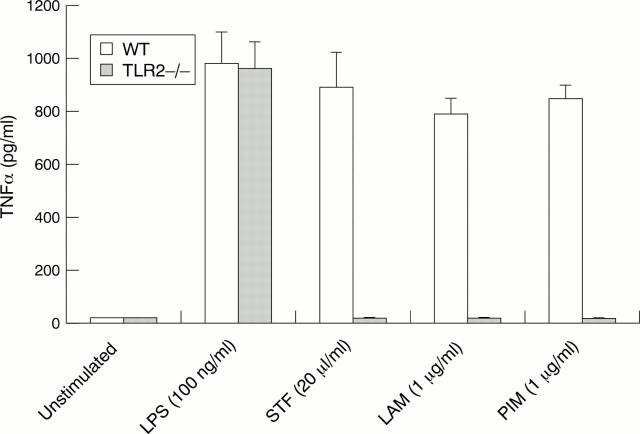
Figure 1 .
TLR2 is required for macrophage activation by mycobacterial glycolipids. Adherent peritoneal macrophages elicited by thioglycollate were collected from TLR2−/− and C57BL/6 (WT) mice and stimulated for 24 hours with LPS (100 ng/ml), STF (20 µl/ml), LAM (1 µg/ml), and PIM (1 µg/ml). Supernatants were collected and concentrations of secreted TNFα were determined by ELISA. Assays were performed in triplicate and repeated on three separate occasions. A single representative experiment is shown and data are expressed as mean values (SEM). LPS = lipopolysaccharide; STF = soluble tuberculosis factor; LAM = lipoarabinomannan; PIM = phosphatidylinositol dimannoside.
Figure 2 .
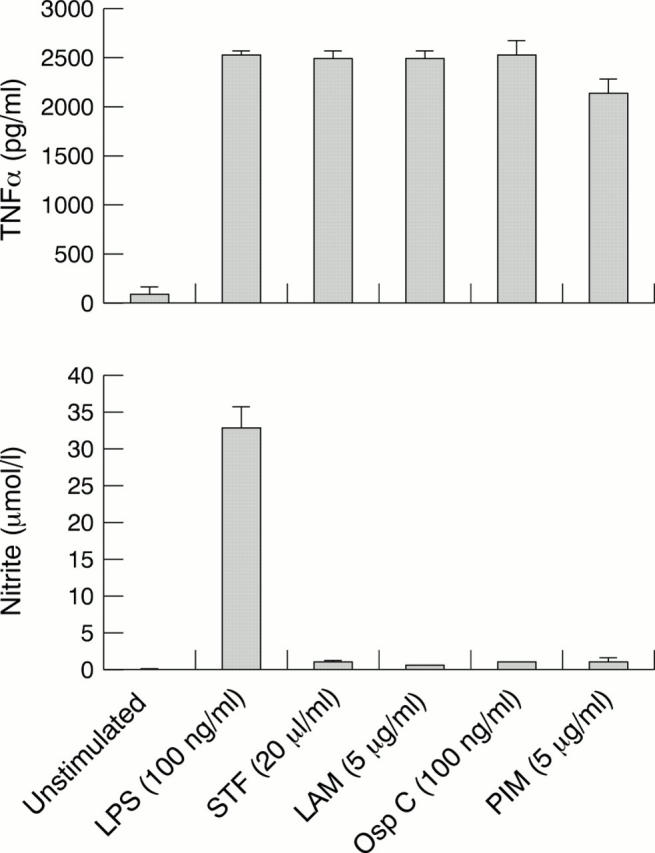
TLR2 agonists fail to induce nitric oxide production. LPS and the mycobacterial glycolipids were analysed for their abilities to induce TNFα and nitric oxide (NO) production in the murine macrophage-like cell line RAW264.7. NO levels were indirectly determined by measuring the levels of the stable NO catabolite nitrite in the culture supernatants of stimulated macrophages. Cells were stimulated for 24 hours with LPS (100 ng/ml), STF (20 µl/ml), LAM (5 µg/ml), lipoprotein OspC (100 ng/ml), and PIM (5 µg/ml). Supernatants were collected and analysed for the presence of nitrite using the Greiss assay, and for TNFα by ELISA. Assays were performed in triplicate and repeated on three separate occasions. A single representative experiment is shown and data are expressed as mean values (SEM). LPS = lipopolysaccharide; STF = soluble tuberculosis factor; LAM = lipoarabinomannan; PIM = phosphatidylinositol dimannoside.
Figure 3 .
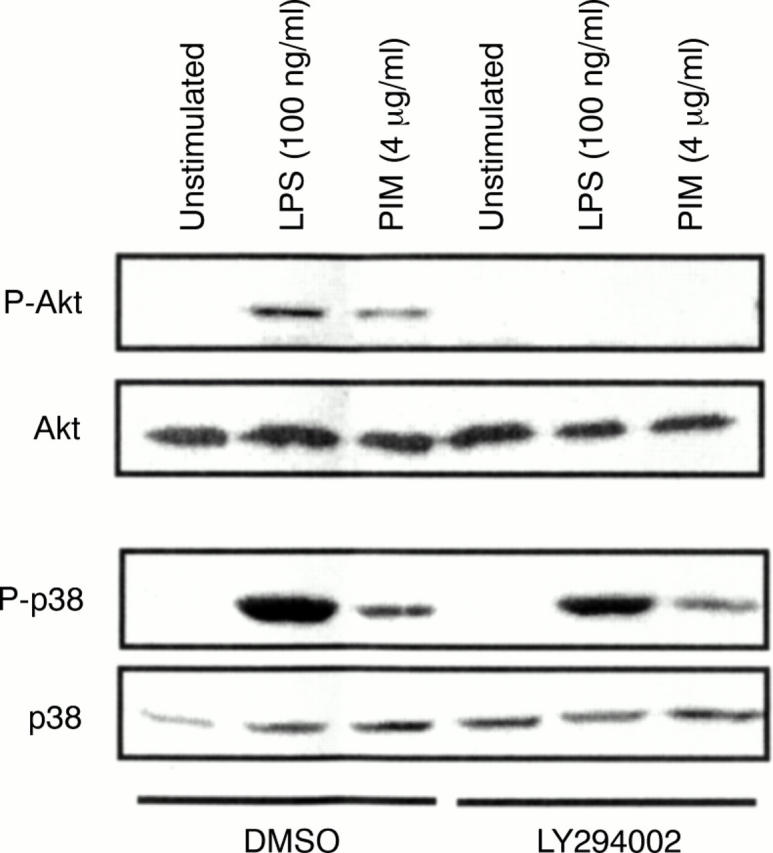
Both TLR2 and TLR4 agonists induce Akt activation in a PI-3-K dependent manner. RAW264.7 cells were pretreated for one hour with either the PI-3-K inhibitor LY294002 (25 µM) or vehicle (DMSO, 6.5 µl/ml), and then stimulated with either LPS (100 ng/ml) or PIM (4 µg/ml), for 30 minutes. After stimulation, whole cell lysates were prepared, fractionated by SDS-PAGE (50 µg lysate/lane), and transferred to nitrocellulose membranes. The membranes were then probed with antibodies against the phosphorylated forms of Akt and the p38 MAP kinase. Duplicate membranes were also probed with antibodies specific for the non-phosphorylated forms of Akt and p38. Bound primary antibodies were detected using secondary antibodies conjugated to HRP. LPS = lipopolysaccharide; PIM = phosphatidylinositol dimannoside.
Figure 4 .
Interferon gamma (IFNγ) confers on TLR2 agonists the ability to induce NO production. RAW264.7 macrophages were pretreated for one hour with 10 ng/ml of murine IFNγ and then stimulated for 24 hours with LPS (100 ng/ml), STF (20 µl/ml), or LAM (5 µg/ml). Supernatants were collected and analysed for NO using the Greiss assay. Assays were performed in triplicate and repeated on three separate occasions. A single representative experiment is shown and data are expressed as mean values (SEM). LPS = lipopolysaccharide; STF = soluble tuberculosis factor; LAM = lipoarabinomannan.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbibe L., Mira J. P., Teusch N., Kline L., Guha M., Mackman N., Godowski P. J., Ulevitch R. J., Knaus U. G. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000 Dec;1(6):533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Brightbill H. D., Libraty D. H., Krutzik S. R., Yang R. B., Belisle J. T., Bleharski J. R., Maitland M., Norgard M. V., Plevy S. E., Smale S. T. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999 Jul 30;285(5428):732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Kim S., Burdick M., Strieter R. M., Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997 Dec;65(12):5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Smith K. D., Ozinsky A., Hawn T. R., Yi E. C., Goodlett D. R., Eng J. K., Akira S., Underhill D. M., Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001 Apr 26;410(6832):1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000 Dec 7;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M., Ma Y., Weis J. H., Vogel S. N., Weis J. J. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000 Jul 15;165(2):618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M., Weis J. J., Toshchakov V., Salkowski C. A., Cody M. J., Ward D. C., Qureshi N., Michalek S. M., Vogel S. N. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001 Mar;69(3):1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H., Vabulas R. M., Takeuchi O., Hoshino K., Akira S., Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000 Aug 21;192(4):595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. L., Hoffmann J. A. Toll receptors in innate immunity. Trends Cell Biol. 2001 Jul;11(7):304–311. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- Jones B. W., Means T. K., Heldwein K. A., Keen M. A., Hill P. J., Belisle J. T., Fenton M. J. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001 Jun;69(6):1036–1044. [PubMed] [Google Scholar]
- Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999 Jul;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Akashi S., Shimazu R., Yoshida T., Miyake K., Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000 Jan 28;275(4):2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Popova L., Kwinn L., Haynes L. M., Jones L. P., Tripp R. A., Walsh E. E., Freeman M. W., Golenbock D. T., Anderson L. J. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000 Nov;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Lien E., Means T. K., Heine H., Yoshimura A., Kusumoto S., Fukase K., Fenton M. J., Oikawa M., Qureshi N., Monks B. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000 Feb;105(4):497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E., Sellati T. J., Yoshimura A., Flo T. H., Rawadi G., Finberg R. W., Carroll J. D., Espevik T., Ingalls R. R., Radolf J. D. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999 Nov 19;274(47):33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- Means T. K., Golenbock D. T., Fenton M. J. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000 Sep;11(3):219–232. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Means T. K., Lien E., Yoshimura A., Wang S., Golenbock D. T., Fenton M. J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999 Dec 15;163(12):6748–6755. [PubMed] [Google Scholar]
- Means T. K., Pavlovich R. P., Roca D., Vermeulen M. W., Fenton M. J. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J Leukoc Biol. 2000 Jun;67(6):885–893. doi: 10.1002/jlb.67.6.885. [DOI] [PubMed] [Google Scholar]
- Means T. K., Wang S., Lien E., Yoshimura A., Golenbock D. T., Fenton M. J. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999 Oct 1;163(7):3920–3927. [PubMed] [Google Scholar]
- O'Neill L. A., Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998 Jun;63(6):650–657. [PubMed] [Google Scholar]
- Ohashi K., Burkart V., Flohé S., Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000 Jan 15;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T. A. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001 Apr;69(4):598–604. [PubMed] [Google Scholar]
- Park Y. C., Lee C. H., Kang H. S., Chung H. T., Kim H. D. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem Biophys Res Commun. 1997 Nov 26;240(3):692–696. doi: 10.1006/bbrc.1997.7722. [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998 Dec 11;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rock F. L., Hardiman G., Timans J. C., Kastelein R. A., Bazan J. F. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L. K., Vermeulen M. W. Alveolar macrophages from C3H/HeJ mice show sensitivity to endotoxin. Am J Respir Cell Mol Biol. 1995 May;12(5):540–546. doi: 10.1165/ajrcmb.12.5.7742017. [DOI] [PubMed] [Google Scholar]
- Schröder N. W., Opitz B., Lamping N., Michelsen K. S., Zähringer U., Göbel U. B., Schumann R. R. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J Immunol. 2000 Sep 1;165(5):2683–2693. doi: 10.4049/jimmunol.165.5.2683. [DOI] [PubMed] [Google Scholar]
- Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C. J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999 Jun 18;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999 Oct;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Kaufmann A., Grote K., Kawai T., Hoshino K., Morr M., Mühlradt P. F., Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000 Jan 15;164(2):554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- Thoma-Uszynski S., Stenger S., Takeuchi O., Ochoa M. T., Engele M., Sieling P. A., Barnes P. F., Rollinghoff M., Bolcskei P. L., Wagner M. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001 Feb 23;291(5508):1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- Weinstein S. L., Finn A. J., Davé S. H., Meng F., Lowell C. A., Sanghera J. S., DeFranco A. L. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J Leukoc Biol. 2000 Mar;67(3):405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- Werts C., Tapping R. I., Mathison J. C., Chuang T. H., Kravchenko V., Saint Girons I., Haake D. A., Godowski P. J., Hayashi F., Ozinsky A. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001 Apr;2(4):346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yang H., Young D. W., Gusovsky F., Chow J. C. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000 Jul 7;275(27):20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]