Abstract
Oncostatin M (OM) is a pleiotropic cytokine of the interleukin 6 family, whose in vivo properties and physiological function remain in dispute and poorly defined. These in vivo studies strongly suggest that OM is anabolic, promoting wound healing and bone formation, and anti-inflammatory. In models of inflammation OM is produced late in the cytokine response and protects from lipopolysaccharide (LPS)-induced toxicities, promoting the re-establishment of homoeostasis by cooperating with proinflammatory cytokines and acute phase molecules to alter and attenuate the inflammatory response. Administration of OM inhibited bacterial LPS-induced production of tumour necrosis factor α and septic lethality in a dose dependent manner. Consistent with these findings, in animal models of chronic inflammatory disease OM potently suppressed inflammation and tissue destruction in murine models of rheumatoid arthritis and multiple sclerosis. T cell function and antibody production were not impaired by OM treatment. Taken together, these data indicate that the activities of this cytokine in vivo are anti-inflammatory without concordant immunosuppression.
Full Text
The Full Text of this article is available as a PDF (146.6 KB).
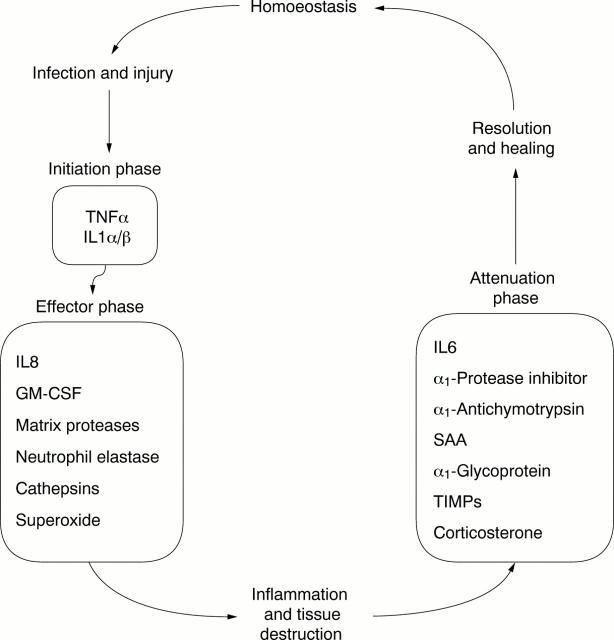
Figure 1 .
The cycle of inflammation.
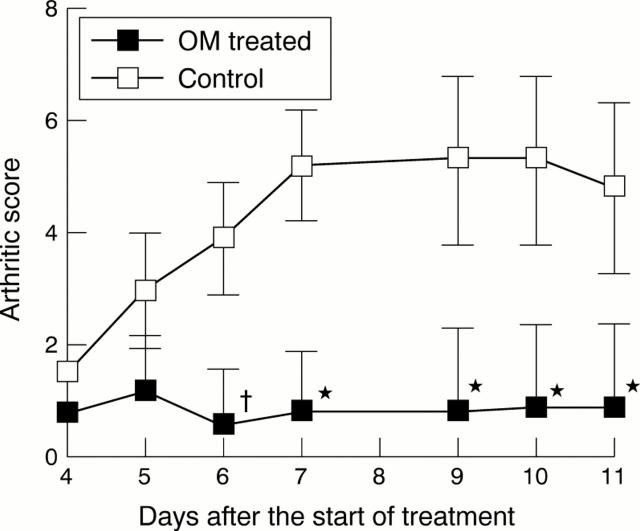
Figure 2 .
Inhibition of joint inflammation by oncostatin M (OM). Groups of 10 Balb/c mice were injected intravenously with 1 mg each of four different anticollagen monoclonal antibodies. At 72 hours after injection with the monoclonal antibodies an intravenous boost of 25 µg lipopolysaccharide (LPS) was given to accelerate the progression of disease. Joint and limb inflammation was apparent within 24 hours. Treatment of animals with OM (10 µg/day) or control diluent began 24 hours after LPS and was continued until day 10. Arthritic disease was assessed as described.18 The median (SD) arthritic scores of control and OM treated animals are shown. *p⩽0.001; †p⩽0.005.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992 Jul 27;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- Baker D., Butler D., Scallon B. J., O'Neill J. K., Turk J. L., Feldmann M. Control of established experimental allergic encephalomyelitis by inhibition of tumor necrosis factor (TNF) activity within the central nervous system using monoclonal antibodies and TNF receptor-immunoglobulin fusion proteins. Eur J Immunol. 1994 Sep;24(9):2040–2048. doi: 10.1002/eji.1830240916. [DOI] [PubMed] [Google Scholar]
- Benigni F., Fantuzzi G., Sacco S., Sironi M., Pozzi P., Dinarello C. A., Sipe J. D., Poli V., Cappelletti M., Paonessa G. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood. 1996 Mar 1;87(5):1851–1854. [PubMed] [Google Scholar]
- Breedveld F. C., van der Lubbe P. A. Monoclonal antibody therapy of inflammatory rheumatic diseases. Br Med Bull. 1995 Apr;51(2):493–502. doi: 10.1093/oxfordjournals.bmb.a072975. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Rowe J. M., Liu J. W., Shoyab M. Regulation of IL-6 expression by oncostatin M. J Immunol. 1991 Oct 1;147(7):2175–2180. [PubMed] [Google Scholar]
- Cichy J., Potempa J., Chawla R. K., Travis J. Regulation of alpha 1-antichymotrypsin synthesis in cells of epithelial origin. FEBS Lett. 1995 Feb 13;359(2-3):262–266. doi: 10.1016/0014-5793(95)00064-g. [DOI] [PubMed] [Google Scholar]
- Cichy J., Potempa J., Chawla R. K., Travis J. Stimulatory effect of inflammatory cytokines on alpha 1-antichymotrypsin expression in human lung-derived epithelial cells. J Clin Invest. 1995 Jun;95(6):2729–2733. doi: 10.1172/JCI117975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Burger D. Interleukin-1, tumor necrosis factor and their specific inhibitors. Eur Cytokine Netw. 1994 Nov-Dec;5(6):563–571. [PubMed] [Google Scholar]
- Edwards D. R., Beaudry P. P., Laing T. D., Kowal V., Leco K. J., Leco P. A., Lim M. S. The roles of tissue inhibitors of metalloproteinases in tissue remodelling and cell growth. Int J Obes Relat Metab Disord. 1996 Mar;20 (Suppl 3):S9–15. [PubMed] [Google Scholar]
- Elliott S., Rowan A. D., Carrère S., Koshy P., Catterall J. B., Cawston T. E. Esculetin inhibits cartilage resorption induced by interleukin 1alpha in combination with oncostatin M. Ann Rheum Dis. 2001 Feb;60(2):158–165. doi: 10.1136/ard.60.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. Protéines de la phase aiguë de l'inflammation. C R Seances Soc Biol Fil. 1995;189(4):563–578. [PubMed] [Google Scholar]
- Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994 Oct 1;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Garcia I., Miyazaki Y., Araki K., Araki M., Lucas R., Grau G. E., Milon G., Belkaid Y., Montixi C., Lesslauer W. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995 Aug;25(8):2401–2407. doi: 10.1002/eji.1830250841. [DOI] [PubMed] [Google Scholar]
- Jay P. R., Centrella M., Lorenzo J., Bruce A. G., Horowitz M. C. Oncostatin-M: a new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology. 1996 Apr;137(4):1151–1158. doi: 10.1210/endo.137.4.8625883. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L., Johnson J. L., Nickbarg E. B., Wang Z. M., Clifford T. F., Banach M., Cooperman B. S., Douglas S. D., Rubin H. Inhibition of human neutrophil superoxide generation by alpha 1-antichymotrypsin. J Immunol. 1991 Apr 1;146(7):2388–2393. [PubMed] [Google Scholar]
- Kishimoto T., Akira S., Narazaki M., Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995 Aug 15;86(4):1243–1254. [PubMed] [Google Scholar]
- Langdon C., Kerr C., Hassen M., Hara T., Arsenault A. L., Richards C. D. Murine oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo. Am J Pathol. 2000 Oct;157(4):1187–1196. doi: 10.1016/S0002-9440(10)64634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C., Brouckaert P., Fiers W. Protection by alpha 1-acid glycoprotein against tumor necrosis factor-induced lethality. J Exp Med. 1994 Oct 1;180(4):1571–1575. doi: 10.1084/jem.180.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. A., Juan T. S., Welcher A. A., Sun Y., Cupples R., Guthrie B., Fletcher F. A. Cloning and characterization of a specific receptor for mouse oncostatin M. Mol Cell Biol. 1998 Jun;18(6):3357–3367. doi: 10.1128/mcb.18.6.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisignoli G., Piacentini A., Toneguzzi S., Grassi F., Cocchini B., Ferruzzi A., Gualtieri G., Facchini A. Osteoblasts and stromal cells isolated from femora in rheumatoid arthritis (RA) and osteoarthritis (OA) patients express IL-11, leukaemia inhibitory factor and oncostatin M. Clin Exp Immunol. 2000 Feb;119(2):346–353. doi: 10.1046/j.1365-2249.2000.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz H. M., Antoni C., Valerius T., Repp R., Grünke M., Schwerdtner N., Nüsslein H., Woody J., Kalden J. R., Manger B. In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol. 1996 Feb 15;156(4):1646–1653. [PubMed] [Google Scholar]
- Modur V., Feldhaus M. J., Weyrich A. S., Jicha D. L., Prescott S. M., Zimmerman G. A., McIntyre T. M. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest. 1997 Jul 1;100(1):158–168. doi: 10.1172/JCI119508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori L., Iselin S., De Libero G., Lesslauer W. Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1-deficient mice. J Immunol. 1996 Oct 1;157(7):3178–3182. [PubMed] [Google Scholar]
- Mosley B., De Imus C., Friend D., Boiani N., Thoma B., Park L. S., Cosman D. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem. 1996 Dec 20;271(51):32635–32643. doi: 10.1074/jbc.271.51.32635. [DOI] [PubMed] [Google Scholar]
- Nemoto O., Yamada H., Mukaida M., Shimmei M. Stimulation of TIMP-1 production by oncostatin M in human articular cartilage. Arthritis Rheum. 1996 Apr;39(4):560–566. doi: 10.1002/art.1780390404. [DOI] [PubMed] [Google Scholar]
- Opdenakker G., Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol Today. 1994 Mar;15(3):103–107. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Multiple sclerosis: TNF revisited, with promise. Nat Med. 1995 Mar;1(3):211–214. doi: 10.1038/nm0395-211. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Langdon C., Botelho F., Brown T. J., Agro A. Oncostatin M inhibits IL-1-induced expression of IL-8 and granulocyte-macrophage colony-stimulating factor by synovial and lung fibroblasts. J Immunol. 1996 Jan 1;156(1):343–349. [PubMed] [Google Scholar]
- Richards C. D., Shoyab M., Brown T. J., Gauldie J. Selective regulation of metalloproteinase inhibitor (TIMP-1) by oncostatin M in fibroblasts in culture. J Immunol. 1993 Jun 15;150(12):5596–5603. [PubMed] [Google Scholar]
- Sandborn W. J. Transcending conventional therapies: the role of biologic and other novel therapies. Inflamm Bowel Dis. 2001 May;7 (Suppl 1):S9–16. doi: 10.1002/ibd.3780070504. [DOI] [PubMed] [Google Scholar]
- Scholz W. Interleukin 6 in diseases: cause or cure? Immunopharmacology. 1996 Mar;31(2-3):131–150. doi: 10.1016/0162-3109(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Berlyne G., Zimlichman S., Sorin H. R., Nyska M., Danon A. Acute phase protein, serum amyloid A, inhibits IL-1- and TNF-induced fever and hypothalamic PGE2 in mice. Scand J Immunol. 1991 Aug;34(2):179–183. doi: 10.1111/j.1365-3083.1991.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Steel D. M., Whitehead A. S. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994 Feb;15(2):81–88. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Terato K., Harper D. S., Griffiths M. M., Hasty D. L., Ye X. J., Cremer M. A., Seyer J. M. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22(3):137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- Tilg H., Trehu E., Atkins M. B., Dinarello C. A., Mier J. W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994 Jan 1;83(1):113–118. [PubMed] [Google Scholar]
- Wallace P. M., MacMaster J. F., Rouleau K. A., Brown T. J., Loy J. K., Donaldson K. L., Wahl A. F. Regulation of inflammatory responses by oncostatin M. J Immunol. 1999 May 1;162(9):5547–5555. [PubMed] [Google Scholar]
- Wallace P. M., Macmaster J. F., Rillema J. R., Rouleau K. A., Hanson M. B., Burstein S. A., Shoyab M. In vivo properties of oncostatin M. Ann N Y Acad Sci. 1995 Jul 21;762:42–54. doi: 10.1111/j.1749-6632.1995.tb32313.x. [DOI] [PubMed] [Google Scholar]
- Wooley P. H., Whalen J. D., Chapman D. L., Berger A. E., Richard K. A., Aspar D. G., Staite N. D. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993 Sep;36(9):1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]
- Yonemura Y., Kawakita M., Masuda T., Fujimoto K., Takatsuki K. Effect of recombinant human interleukin-11 on rat megakaryopoiesis and thrombopoiesis in vivo: comparative study with interleukin-6. Br J Haematol. 1993 May;84(1):16–23. doi: 10.1111/j.1365-2141.1993.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Ichihara M., Kinjyo I., Moriyama M., Copeland N. G., Gilbert D. J., Jenkins N. A., Hara T., Miyajima A. Mouse oncostatin M: an immediate early gene induced by multiple cytokines through the JAK-STAT5 pathway. EMBO J. 1996 Mar 1;15(5):1055–1063. [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Shoyab M., Marquardt H., Hanson M. B., Lioubin M. N., Todaro G. J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995 Oct;27(5):537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]