Abstract
Studies conducted over the past decade have demonstrated a central role for tumour necrosis factor α (TNFα) in inflammatory diseases. As a result of this work, a number of biological agents that neutralise the activity of this cytokine have entered the clinic. The recent clinical data obtained with etanercept and infliximab highlight the relevance of this strategy. TNFα converting enzyme (TACE) is the metalloproteinase that processes the 26 kDa membrane bound precursor of TNFα (proTNFα) to the 17 kDa soluble component. Although a number of proteases have been shown to process proTNFα, none do so with the efficiency of TACE. A series of orally bioavailable, selective, and potent TACE inhibitors are currently in clinical development. These inhibitors effectively block TACE mediated processing of proTNFα and can reduce TNF production by lipopolysaccharide stimulated whole blood by >95%. Through a series of studies it is shown here that >80% of the unprocessed proTNFα is degraded intracellularly. The remainder appears to be transiently expressed on the cell surface. Although, in vitro, TACE inhibition has also been implicated in shedding of p55 and p75 surface TNFα receptors, the in vivo data cast doubt on the consequences of this finding. In a mouse model of collagen-induced arthritis, the inhibitors are efficacious both prophylactically and therapeutically. The efficacy seen is equivalent to strategies that neutralise TNFα. In many studies greater efficacy is observed with the TACE inhibitors, presumably owing to greater penetration to the site of TNFα production.
Full Text
The Full Text of this article is available as a PDF (166.9 KB).
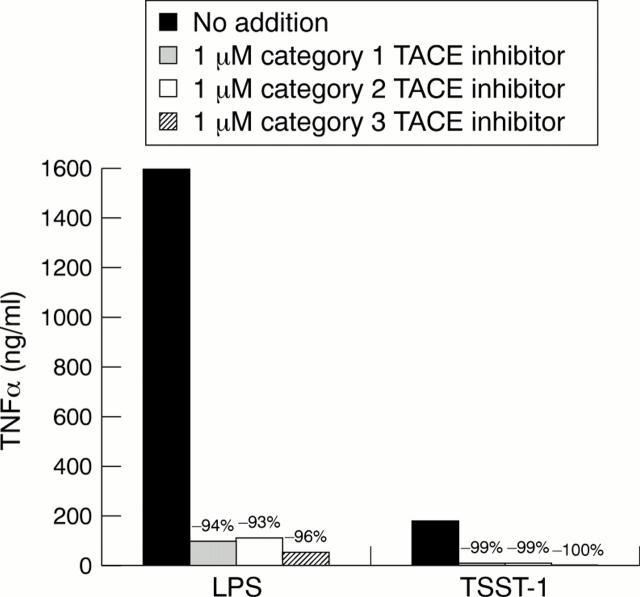
Figure 1 .
Effect of the addition of TACE inhibitors on soluble TNFα produced by LPS stimulated human monocytes or TSST-1 activated T lymphocytes. The respective cell type was stimulated in the presence of no addition, 1 µM category 1 TACE inhibitor , 1 µM category 2 TACE inhibitor, or 1 µM category 3 TACE inhibitor (see "Materials and methods" for category description).
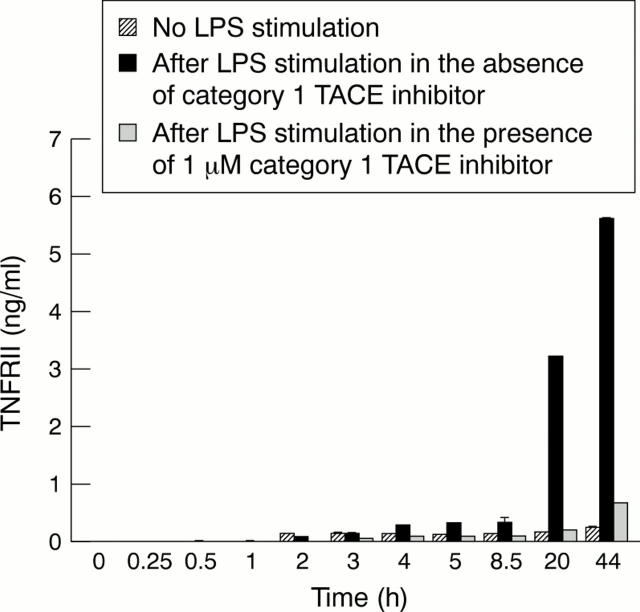
Figure 2 .
Time course of the release of soluble TNFRII by human monocytes without stimulation or after LPS stimulation in the absence or presence of 1 µM TACE inhibitor, category 1.
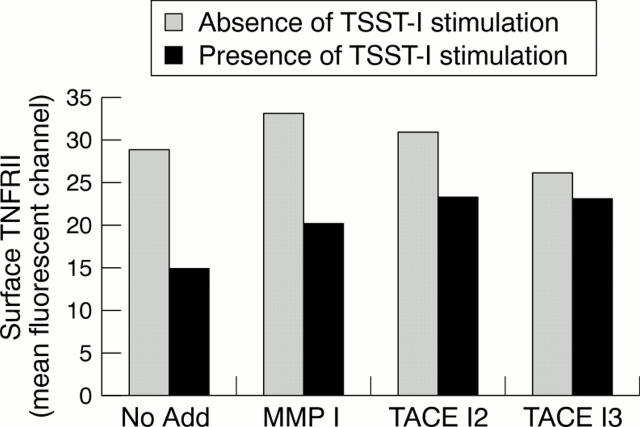
Figure 3 .
Effect of TACE inhibitors with different degrees of selectivity on residual surface expression of TNFRII after antigen activation of human T lymphocytes. Cells were incubated in the absence or in the presence of TSST-1 antigen stimulation to induce TNFRII shedding.
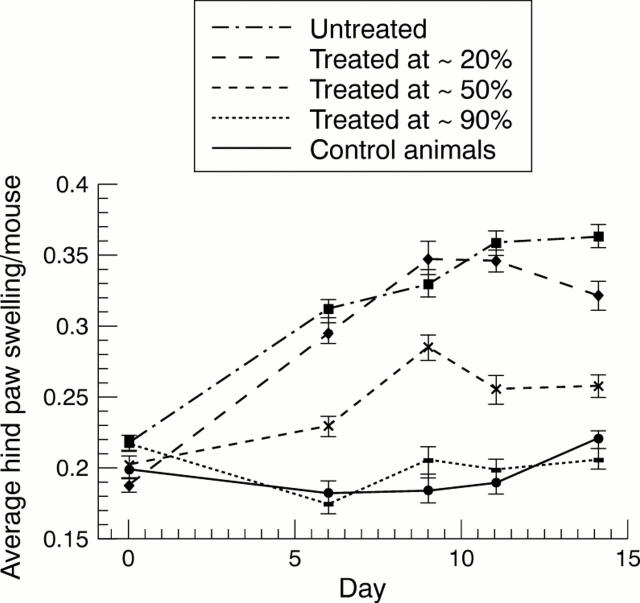
Figure 4 .
Efficacy of TACE inhibition (category 4), given by continuous infusion, in a mouse model of CIA. Animals were untreated or treated at levels of ~20%, ~50%, and ~90% inhibition of LPS-induced TNFα release. Control animals show no paw swelling.
Figure 5 .
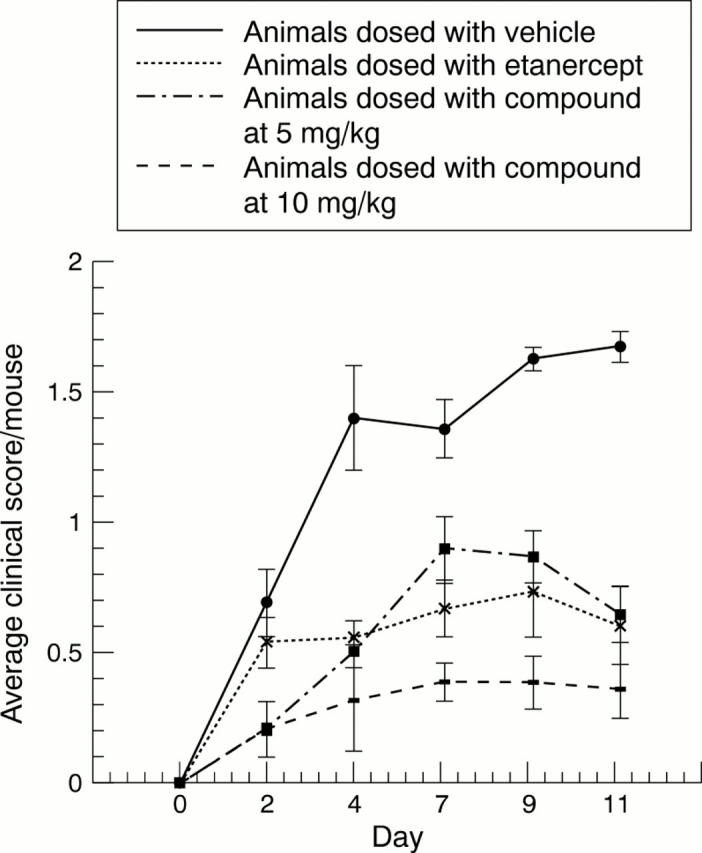
Efficacy of TACE inhibition (category 4) in a mouse model of CIA using bolus dosing in comparison with etanercept. Animals were dosed with vehicle, etanercept, or compound at 5 mg/kg or 10 mg/kg, twice a day.
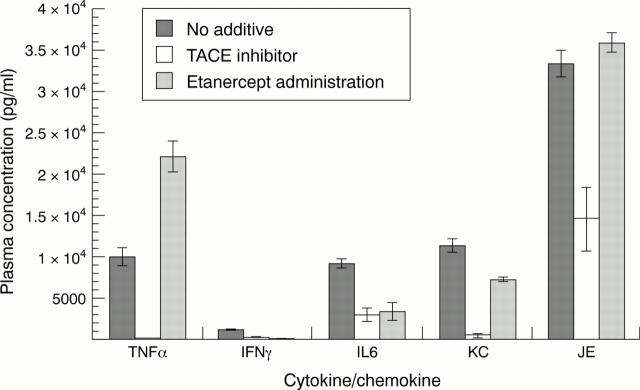
Figure 6 .
Induction of cytokines and the effect of TACE inhibitor (category 4) or etanercept administration on LPS-induced cytokine production after LPS challenge in mice. IFNλ = interferon λ.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996 Apr-Jun;7(2):93–124. [PubMed] [Google Scholar]
- Althoff K., Reddy P., Voltz N., Rose-John S., Müllberg J. Shedding of interleukin-6 receptor and tumor necrosis factor alpha. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur J Biochem. 2000 May;267(9):2624–2631. doi: 10.1046/j.1432-1327.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997 Feb 20;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel C. P. Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol. 2000 Oct;12(5):606–612. doi: 10.1016/s0955-0674(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Butler D. M., Maini R. N., Feldmann M., Brennan F. M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995 Jul-Dec;6(4):225–230. [PubMed] [Google Scholar]
- Chen W., Jin W., Cook M., Weiner H. L., Wahl S. M. Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J Immunol. 1998 Dec 1;161(11):6297–6304. [PubMed] [Google Scholar]
- Crowe P. D., Walter B. N., Mohler K. M., Otten-Evans C., Black R. A., Ware C. F. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med. 1995 Mar 1;181(3):1205–1210. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoster E., Vanhaesebroeck B., Vandenabeele P., Grooten J., Fiers W. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J Biol Chem. 1995 Aug 4;270(31):18473–18478. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- English W. R., Puente X. S., Freije J. M., Knauper V., Amour A., Merryweather A., Lopez-Otin C., Murphy G. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem. 2000 May 12;275(19):14046–14055. doi: 10.1074/jbc.275.19.14046. [DOI] [PubMed] [Google Scholar]
- Haro H., Crawford H. C., Fingleton B., Shinomiya K., Spengler D. M., Matrisian L. M. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000 Jan;105(2):143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M. Families of zinc metalloproteases. FEBS Lett. 1994 Oct 31;354(1):1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- Jarvis B., Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs. 1999 Jun;57(6):945–966. doi: 10.2165/00003495-199957060-00014. [DOI] [PubMed] [Google Scholar]
- Joe B., Griffiths M. M., Remmers E. F., Wilder R. L. Animal models of rheumatoid arthritis and related inflammation. Curr Rheumatol Rep. 1999 Dec;1(2):139–148. doi: 10.1007/s11926-999-0011-7. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Knäuper V., Becherer J. D., Murphy G. Full-length and N-TIMP-3 display equal inhibitory activities toward TNF-alpha convertase. Biochem Biophys Res Commun. 2001 Jan 26;280(3):945–950. doi: 10.1006/bbrc.2000.4192. [DOI] [PubMed] [Google Scholar]
- Lunn C. A., Fan X., Dalie B., Miller K., Zavodny P. J., Narula S. K., Lundell D. Purification of ADAM 10 from bovine spleen as a TNFalpha convertase. FEBS Lett. 1997 Jan 6;400(3):333–335. doi: 10.1016/s0014-5793(96)01410-x. [DOI] [PubMed] [Google Scholar]
- McGeehan G. M., Becherer J. D., Bast R. C., Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., Kidao S. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994 Aug 18;370(6490):558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997 Feb 20;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Mueller C., Corazza N., Trachsel-Løseth S., Eugster H. P., Bühler-Jungo M., Brunner T., Imboden M. A. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. J Biol Chem. 1999 Dec 31;274(53):38112–38118. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- Newton R. C., Decicco C. P. Therapeutic potential and strategies for inhibiting tumor necrosis factor-alpha. J Med Chem. 1999 Jul 1;42(13):2295–2314. doi: 10.1021/jm980541n. [DOI] [PubMed] [Google Scholar]
- Onrust S. V., Lamb H. M. Infliximab: a review of its use in Crohn's disease and rheumatoid arthritis. BioDrugs. 1998 Nov;10(5):397–422. doi: 10.2165/00063030-199810050-00006. [DOI] [PubMed] [Google Scholar]
- Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N. An essential role for ectodomain shedding in mammalian development. Science. 1998 Nov 13;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Pradines-Figueres A., Raetz C. R. Processing and secretion of tumor necrosis factor alpha in endotoxin-treated Mono Mac 6 cells are dependent on phorbol myristate acetate. J Biol Chem. 1992 Nov 15;267(32):23261–23268. [PubMed] [Google Scholar]
- Probert L., Akassoglou K., Alexopoulou L., Douni E., Haralambous S., Hill S., Kassiotis G., Kontoyiannis D., Pasparakis M., Plows D. Dissection of the pathologies induced by transmembrane and wild-type tumor necrosis factor in transgenic mice. J Leukoc Biol. 1996 Apr;59(4):518–525. doi: 10.1002/jlb.59.4.518. [DOI] [PubMed] [Google Scholar]
- Rosemurgy A., Harris J., Langleben A., Casper E., Goode S., Rasmussen H. Marimastat in patients with advanced pancreatic cancer: a dose-finding study. Am J Clin Oncol. 1999 Jun;22(3):247–252. doi: 10.1097/00000421-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Solomon K. A., Covington M. B., DeCicco C. P., Newton R. C. The fate of pro-TNF-alpha following inhibition of metalloprotease-dependent processing to soluble TNF-alpha in human monocytes. J Immunol. 1997 Nov 1;159(9):4524–4531. [PubMed] [Google Scholar]
- Solomon K. A., Pesti N., Wu G., Newton R. C. Cutting edge: a dominant negative form of TNF-alpha converting enzyme inhibits proTNF and TNFRII secretion. J Immunol. 1999 Oct 15;163(8):4105–4108. [PubMed] [Google Scholar]
- Williams L. M., Gibbons D. L., Gearing A., Maini R. N., Feldmann M., Brennan F. M. Paradoxical effects of a synthetic metalloproteinase inhibitor that blocks both p55 and p75 TNF receptor shedding and TNF alpha processing in RA synovial membrane cell cultures. J Clin Invest. 1996 Jun 15;97(12):2833–2841. doi: 10.1172/JCI118739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Ortho M. P., Will H., Atkinson S., Butler G., Messent A., Gavrilovic J., Smith B., Timpl R., Zardi L., Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997 Dec 15;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]