Abstract
The atrial natriuretic peptide (ANP), a member of the natriuretic peptide family, is a cardiovascular hormone which possesses well defined natriuretic, diuretic, and vasodilating properties. Most of the biological effects of ANP are mediated through its guanylyl cyclase coupled A receptor. Because ANP and its receptors have been shown to be expressed and differentially regulated in the immune system, it has been suggested that ANP has an immunomodulatory potency. Much investigation of the effects of ANP on the activation of macrophages has been carried out. ANP was shown to inhibit the lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) in macrophages in an autocrine fashion. ANP in this context was shown to reduce significantly the activation of NF-κB and to destabilise iNOS mRNA. ANP, furthermore, can significantly reduce the LPS-induced secretion of tumour necrosis factor α (TNFα) in macrophages. The relevance of these findings on a regulatory role for ANP on TNFα in humans was shown by the fact that ANP significantly reduces the release of TNFα in whole human blood. It was furthermore shown to attenuate the release of interleukin 1β (IL1β). Interestingly, ANP did not affect the secretion of the anti-inflammatory cytokines IL10 and IL1 receptor antagonist (IL1ra). In summary, ANP was shown to reduce the secretion of inflammatory mediators in macrophages. Therefore, this cardiovascular hormone may possess anti-inflammatory potential.
Full Text
The Full Text of this article is available as a PDF (149.6 KB).
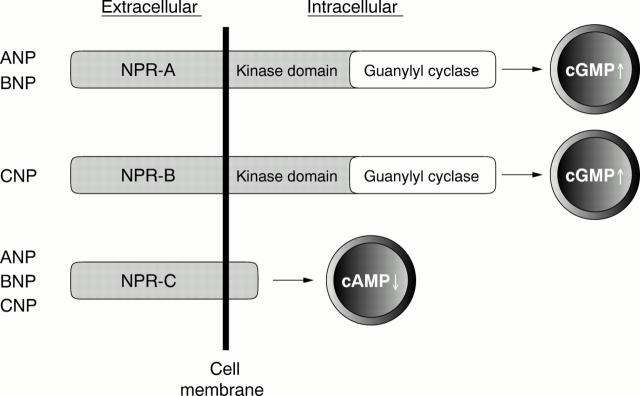
Figure 1 .
Natriuretic peptide receptors (NPR). The guanylyl cyclases, NPR-A and NPR-B, contain an extracellular ligand binding domain. NPR-A binds ANP and BNP, whereas NPR-B binds CNP. NPR-C has the potency to internalise and clear the natriuretic peptides and exerts other biological effects by inhibiting the production of cAMP.
Figure 2 .
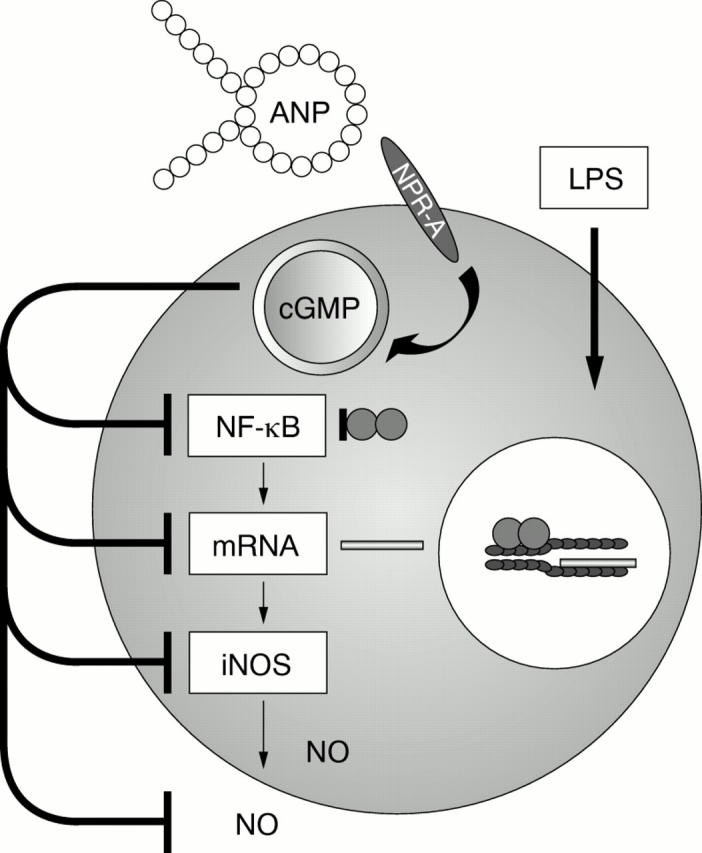
The inhibitory action of ANP on the induction of iNOS. The LPS induction of iNOS is inhibited by ANP through binding to the NPR-A. The inhibitory action involves the destabilisation of iNOS mRNA and inhibition of the activation of NF-κB.
Figure 3 .
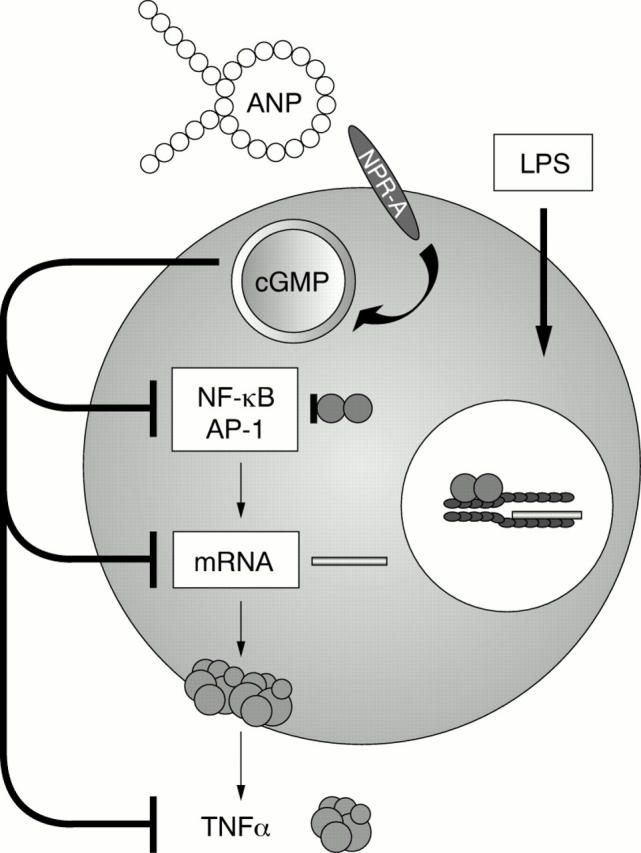
The inhibitory action of ANP on the induction of TNFα. The LPS-induced expression of TNFα is inhibited by ANP through binding to the NPR-A. The inhibitory action involves transcriptional processes with a reduced activation of NF-κB and AP1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiura K., Ueda M., Endo M., Kitajima M. Circulating concentrations and physiologic role of atrial natriuretic peptide during endotoxic shock in the rat. Crit Care Med. 1995 Nov;23(11):1898–1906. doi: 10.1097/00003246-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Amin A. R., Attur M., Abramson S. B. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol. 1999 May;11(3):202–209. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Of microbes, macrophages and nitric oxide. Behring Inst Mitt. 1997 Mar;(99):58–72. [PubMed] [Google Scholar]
- Bovy P. R. Structure activity in the atrial natriuretic peptide (ANP) family. Med Res Rev. 1990 Jan-Mar;10(1):115–142. doi: 10.1002/med.2610100105. [DOI] [PubMed] [Google Scholar]
- Chabrier P. E., Demerlé-Pallardy C., Auguet M. Nitric oxide synthases: targets for therapeutic strategies in neurological diseases. Cell Mol Life Sci. 1999 Jul;55(8-9):1029–1035. doi: 10.1007/s000180050353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn T. G., Davies P. L. The biochemistry and molecular biology of atrial natriuretic factor. Biochem J. 1985 Dec 1;232(2):313–321. doi: 10.1042/bj2320313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund T., Hannonen P., Reitamo S., Fyhrquist F. Hypertension in cyclosporin A-treated patients is independent of circulating endothelin levels. J Intern Med. 1995 Jul;238(1):71–75. doi: 10.1111/j.1365-2796.1995.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch Pharmacol. 1995 Oct;352(4):351–364. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Geng Y., Lotz M. Increased intracellular Ca2+ selectively suppresses IL-1-induced NO production by reducing iNOS mRNA stability. J Cell Biol. 1995 Jun;129(6):1651–1657. doi: 10.1083/jcb.129.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Topper J. N., Nagel T., Anderson K. R., Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000 May;902:230–240. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Gutkowska J., Nemer M. Structure, expression, and function of atrial natriuretic factor in extraatrial tissues. Endocr Rev. 1989 Nov;10(4):519–536. doi: 10.1210/edrv-10-4-519. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Gross S. S. Cycloheximide induces nitric oxide synthase mRNA in vascular smooth muscle cells by prolonging mRNA lifetime. Biochem Mol Biol Int. 1995 Oct;37(3):439–445. [PubMed] [Google Scholar]
- Kiemer A. K., Hartung T., Vollmar A. M. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J Immunol. 2000 Jul 1;165(1):175–181. doi: 10.4049/jimmunol.165.1.175. [DOI] [PubMed] [Google Scholar]
- Kiemer A. K., Vollmar A. M. Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide. J Biol Chem. 1998 May 29;273(22):13444–13451. doi: 10.1074/jbc.273.22.13444. [DOI] [PubMed] [Google Scholar]
- Kiemer A. K., Vollmar A. M. Effects of different natriuretic peptides on nitric oxide synthesis in macrophages. Endocrinology. 1997 Oct;138(10):4282–4290. doi: 10.1210/endo.138.10.5459. [DOI] [PubMed] [Google Scholar]
- Kiemer A. K., Vollmar A. M. Elevation of intracellular calcium levels contributes to the inhibition of nitric oxide production by atrial natriuretic peptide. Immunol Cell Biol. 2001 Feb;79(1):11–17. doi: 10.1046/j.1440-1711.2001.00969.x. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Goeddel D. V. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992 Oct;86(4):1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- Levin E. R., Gardner D. G., Samson W. K. Natriuretic peptides. N Engl J Med. 1998 Jul 30;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- Levin E. R. Natriuretic peptide C-receptor: more than a clearance receptor. Am J Physiol. 1993 Apr;264(4 Pt 1):E483–E489. doi: 10.1152/ajpendo.1993.264.4.E483. [DOI] [PubMed] [Google Scholar]
- Maack T. Role of atrial natriuretic factor in volume control. Kidney Int. 1996 Jun;49(6):1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- Morishita Y., Sano T., Ando K., Saitoh Y., Kase H., Yamada K., Matsuda Y. Microbial polysaccharide, HS-142-1, competitively and selectively inhibits ANP binding to its guanylyl cyclase-containing receptor. Biochem Biophys Res Commun. 1991 May 15;176(3):949–957. doi: 10.1016/0006-291x(91)90374-g. [DOI] [PubMed] [Google Scholar]
- Porter J. G., Arfsten A., Fuller F., Miller J. A., Gregory L. C., Lewicki J. A. Isolation and functional expression of the human atrial natriuretic peptide clearance receptor cDNA. Biochem Biophys Res Commun. 1990 Sep 14;171(2):796–803. doi: 10.1016/0006-291x(90)91216-f. [DOI] [PubMed] [Google Scholar]
- Rhoades K. L., Golub S. H., Economou J. S. The regulation of the human tumor necrosis factor alpha promoter region in macrophage, T cell, and B cell lines. J Biol Chem. 1992 Nov 5;267(31):22102–22107. [PubMed] [Google Scholar]
- Rosenzweig A., Seidman C. E. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995 Sep;59(3):423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson W. K. Cardiac hormones and neuroendocrine function. Adv Exp Med Biol. 1990;274:177–190. doi: 10.1007/978-1-4684-5799-5_11. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Kangawa K., Minamino N., Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332(6159):78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- Sudoh T., Minamino N., Kangawa K., Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):863–870. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- Throsby M., Lee D., Huang W. Q., Yang Z. Y., Copolov D. L., Lim A. T. Evidence for atrial natriuretic peptide-(5-28) production by macrophages of the rat spleen: an immunochemical and nonradioactive in situ hybridization approach. Endocrinology. 1991 Aug;129(2):991–1000. doi: 10.1210/endo-129-2-991. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A. Nitric oxide in septic shock. Biochim Biophys Acta. 1999 May 5;1411(2-3):437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Shimizu Y., Kawata T., Hisada T., Shimizu Y., Iwamae S., Ishizuka T., Iizuka K., Dobashi K., Mori M. Atrial natriuretic peptide inhibits tumor necrosis factor-alpha production by interferon-gamma-activated macrophages via suppression of p38 mitogen-activated protein kinase and nuclear factor-kappa B activation. Regul Pept. 2001 May 5;99(1):21–29. doi: 10.1016/s0167-0115(01)00218-x. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Friedrich A., Schulz R. Immunoreactive atrial natriuretic peptide in the guinea pig spleen. Life Sci. 1989;45(14):1293–1297. doi: 10.1016/0024-3205(89)90132-x. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Förster R., Schulz R. Effects of atrial natriuretic peptide on phagocytosis and respiratory burst in murine macrophages. Eur J Pharmacol. 1997 Jan 29;319(2-3):279–285. doi: 10.1016/s0014-2999(96)00859-x. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M. Influence of atrial natriuretic peptide on thymocyte development in fetal thymic organ culture. J Neuroimmunol. 1997 Sep;78(1-2):90–96. doi: 10.1016/s0165-5728(97)00086-6. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Lang R. E., Hänze J., Schulz R. The rat thymus--a site of atrial natriuretic peptide synthesis. Peptides. 1990 Jan-Feb;11(1):33–37. doi: 10.1016/0196-9781(90)90106-f. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Schmidt K. N., Schulz R. Natriuretic peptide receptors on rat thymocytes: inhibition of proliferation by atrial natriuretic peptide. Endocrinology. 1996 May;137(5):1706–1713. doi: 10.1210/endo.137.5.8612505. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Schulz R. Atrial natriuretic peptide in lymphoid organs of various species. Comp Biochem Physiol A Comp Physiol. 1990;96(4):459–463. doi: 10.1016/0300-9629(90)90661-b. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Schulz R. Expression and differential regulation of natriuretic peptides in mouse macrophages. J Clin Invest. 1995 Jun;95(6):2442–2450. doi: 10.1172/JCI117944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar A. M., Schulz R. Gene expression and secretion of atrial natriuretic peptide by murine macrophages. J Clin Invest. 1994 Aug;94(2):539–545. doi: 10.1172/JCI117367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- de Bold A. J., Borenstein H. B., Veress A. T., Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Reprinted from Life Sci. 28:89-94, 1981. J Am Soc Nephrol. 2001 Feb;12(2):403-9; discussion 403-8, 408-9. doi: 10.1681/ASN.V122403. [DOI] [PubMed] [Google Scholar]