Abstract
Current concepts and basic principles of neurogastroenterology in relation to functional gastrointestinal disorders are reviewed. Neurogastroenterology is emphasized as a new and advancing subspecialty of clinical gastroenterology and digestive science. As such, it embraces the investigative sciences dealing with functions, malfunctions, and malformations in the brain and spinal cord, and the sympathetic, parasympathetic and enteric divisions of the autonomic innervation of the digestive tract. Somatomotor systems are included insofar as pharyngeal phases of swallowing and pelvic floor involvement in defecation, continence, and pelvic pain are concerned. Inclusion of basic physiology of smooth muscle, mucosal epithelium, and the enteric immune system in the neurogastroenterologic domain relates to requirements for compatibility with neural control mechanisms. Psychologic and psychiatric relations to functional gastrointestinal disorders are included because they are significant components of neurogastroenterology, especially in relation to projections of discomfort and pain to the digestive tract. Keywords: enteric nervous system; brain-gut axis; autonomic nervous system; nausea; gut motility; mast cells; gastrointestinal pain; Rome II
Full Text
The Full Text of this article is available as a PDF (145.4 KB).
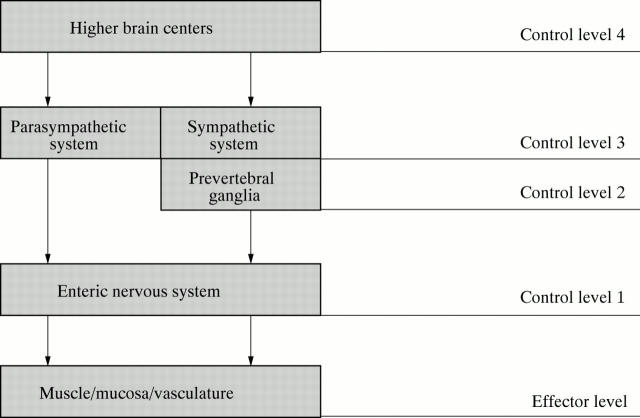
Figure 1 .
Neural control of the gut is hierarchic with four basic levels of integrative organization. Level 1 is the enteric nervous system (ENS) which behaves like a local minibrain. The second level of integrative organization is in the prevertebral sympathetic ganglia. The third and fourth levels are within the central nervous system (CNS). Sympathetic and parasympathetic signals to the digestive tract originate at level 3 and represent the final common pathways for outflow of information from the CNS to the gut. The fourth level includes higher brain centers that provide input for integrative functions at level 3.
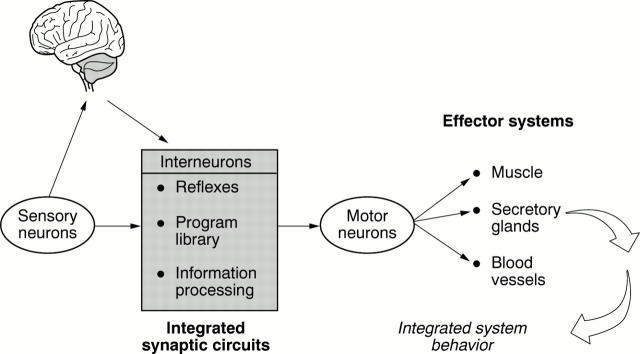
Figure 2 .
The conceptual model for the enteric nervous system (ENS) is the same as for the central nervous system (CNS). Sensory neurons, interneurons, and motor neurons are connected synaptically for flow of information from sensory neurons to interneuronal integrative networks to motor neurons to effector systems. The ENS organizes and coordinates the activity of each effector system into meaningful behavior of the integrated organ. Bi-directional communication occurs between the CNS and ENS.
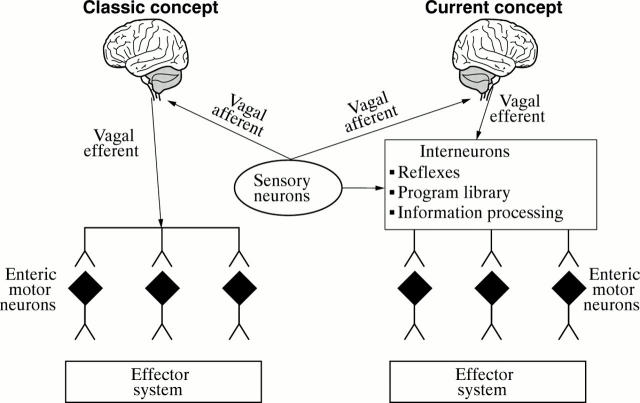
Figure 3 .
Classic outmoded and current concepts of relations between the brain and the digestive tract. The classic concept viewed parasympathetic efferents (e.g., vagal efferents) as synapsing directly with enteric motor neurons, as illustrated on the left side of the diagram. In the current concept, parasympathetic efferent fibers transmit command signals from the brain to the integrative and motor program circuitry of the enteric nervous system minibrain as shown on the right side of the diagram.
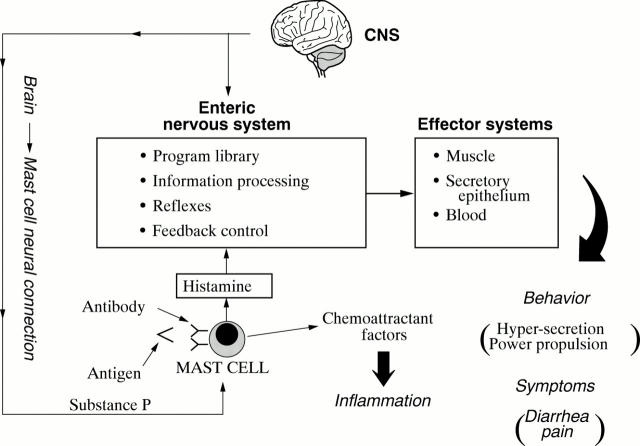
Figure 4 .
Conceptual model for enteric neuro-immunophysiology. The enteric nervous system (ENS) is a minibrain located in close apposition to the gastrointestinal effectors it controls. Enteric mast cells are in position to detect foreign antigens and signal their presence to the ENS. Stimulated mast cells release several paracrine mediators simultaneously. Some of the mediators signal the ENS whereas others act as attractant factors for polymorphonuclear leucocytes responsible for acute inflammatory responses. The ENS responds to the mast cell signal by initiating a program of coordinated secretion and propulsive motility that expels the source of antigenic stimulation from the bowel. Symptoms of abdominal pain and diarrhea result from operation of the neural program. Neural inputs to mast cells from the brain stimulate simultaneous release of chemoattractant factors for inflammatory cells and chemical signals to the ENS with symptomatic consequences that mimic antigenic stimulation. CNS, central nervous system.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. L., Bhandari P. Resinferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferret. Neuropharmacology. 1993 Aug;32(8):799–806. doi: 10.1016/0028-3908(93)90189-a. [DOI] [PubMed] [Google Scholar]
- Aziz Q., Thompson D. G. Brain-gut axis in health and disease. Gastroenterology. 1998 Mar;114(3):559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- Bench C. J., Frackowiak R. S., Dolan R. J. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995 Mar;25(2):247–261. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Camilleri M., Phillips S. F. Disorders of small intestinal motility. Gastroenterol Clin North Am. 1989 Jun;18(2):405–424. [PubMed] [Google Scholar]
- Cervero F. Afferent activity evoked by natural stimulation of the biliary system in the ferret. Pain. 1982 Jun;13(2):137–151. doi: 10.1016/0304-3959(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Cervero F., Jänig W. Visceral nociceptors: a new world order? Trends Neurosci. 1992 Oct;15(10):374–378. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- Coelho A. M., Fioramonti J., Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci. 1998 Apr;43(4):727–737. doi: 10.1023/a:1018853728251. [DOI] [PubMed] [Google Scholar]
- Cooke H. J. Role of the "little brain" in the gut in water and electrolyte homeostasis. FASEB J. 1989 Feb;3(2):127–138. doi: 10.1096/fasebj.3.2.2464517. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Wang Y. Z., Rogers R. Coordination of Cl- secretion and contraction by a histamine H2-receptor agonist in guinea pig distal colon. Am J Physiol. 1993 Nov;265(5 Pt 1):G973–G978. doi: 10.1152/ajpgi.1993.265.5.G973. [DOI] [PubMed] [Google Scholar]
- Frieling T., Cooke H. J., Wood J. D. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol. 1994 Dec;267(6 Pt 1):G1087–G1093. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- Frieling T., Palmer J. M., Cooke H. J., Wood J. D. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology. 1994 Dec;107(6):1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Kunze W. A., Bertrand P. P., Clerc N., Bornstein J. C. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998 Jan;54(1):1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gottwald T., Lhoták S., Stead R. H. Effect of truncal vagotomy and capsaicin on mast cells and IgA-positive plasma cells in rat jejunal mucosa. Neurogastroenterol Motil. 1997 Mar;9(1):25–32. doi: 10.1046/j.1365-2982.1997.d01-4.x. [DOI] [PubMed] [Google Scholar]
- Gué M., Del Rio-Lacheze C., Eutamene H., Théodorou V., Fioramonti J., Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997 Dec;9(4):271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Haltia M., Somer H., Palo J., Johnson W. G. Neuronal intranuclear inclusion disease in identical twins. Ann Neurol. 1984 Apr;15(4):316–321. doi: 10.1002/ana.410150403. [DOI] [PubMed] [Google Scholar]
- Jin J. G., Murthy K. S., Grider J. R., Makhlouf G. M. Stoichiometry of neurally induced VIP release, NO formation, and relaxation in rabbit and rat gastric muscle. Am J Physiol. 1996 Aug;271(2 Pt 1):G357–G369. doi: 10.1152/ajpgi.1996.271.2.G357. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S., Schuffler M. D. Pathology of neuromuscular disorders of the small intestine and colon. Gastroenterology. 1987 Sep;93(3):610–639. doi: 10.1016/0016-5085(87)90926-7. [DOI] [PubMed] [Google Scholar]
- Lembo T., Munakata J., Mertz H., Niazi N., Kodner A., Nikas V., Mayer E. A. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994 Dec;107(6):1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Loening-Baucke V. A. Sensitivity of the sigmoid colon and rectum in children treated for chronic constipation. J Pediatr Gastroenterol Nutr. 1984 Jun;3(3):454–459. doi: 10.1097/00005176-198406000-00026. [DOI] [PubMed] [Google Scholar]
- MacQueen G., Marshall J., Perdue M., Siegel S., Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989 Jan 6;243(4887):83–85. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Catalioto R. M., Criscuoli M., Cucchi P., Giuliani S., Lecci A., Lippi A., Meini S., Patacchini R., Renzetti A. R. Tachykinin receptors and intestinal motility. Can J Physiol Pharmacol. 1997 Jun;75(6):696–703. [PubMed] [Google Scholar]
- Magni G. The use of antidepressants in the treatment of chronic pain. A review of the current evidence. Drugs. 1991 Nov;42(5):730–748. doi: 10.2165/00003495-199142050-00002. [DOI] [PubMed] [Google Scholar]
- Munoz-Garcia D., Ludwin S. K. Adult-onset neuronal intranuclear hyaline inclusion disease. Neurology. 1986 Jun;36(6):785–790. doi: 10.1212/wnl.36.6.785. [DOI] [PubMed] [Google Scholar]
- North C. S., Alpers D. H., Thompson S. J., Spitznagel E. L. Gastrointestinal symptoms and psychiatric disorders in the general population. Findings from NIMH Epidemiologic Catchment Area Project. Dig Dis Sci. 1996 Apr;41(4):633–640. doi: 10.1007/BF02213117. [DOI] [PubMed] [Google Scholar]
- Richter J. E., Barish C. F., Castell D. O. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology. 1986 Oct;91(4):845–852. doi: 10.1016/0016-5085(86)90685-2. [DOI] [PubMed] [Google Scholar]
- Santos J., Saperas E., Mourelle M., Antolín M., Malagelada J. R. Regulation of intestinal mast cells and luminal protein release by cerebral thyrotropin-releasing hormone in rats. Gastroenterology. 1996 Dec;111(6):1465–1473. doi: 10.1016/s0016-5085(96)70007-0. [DOI] [PubMed] [Google Scholar]
- Santos J., Saperas E., Nogueiras C., Mourelle M., Antolín M., Cadahia A., Malagelada J. R. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998 Apr;114(4):640–648. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- Silverman D. H., Munakata J. A., Ennes H., Mandelkern M. A., Hoh C. K., Mayer E. A. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997 Jan;112(1):64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- Smith V. V., Gregson N., Foggensteiner L., Neale G., Milla P. J. Acquired intestinal aganglionosis and circulating autoantibodies without neoplasia or other neural involvement. Gastroenterology. 1997 Apr;112(4):1366–1371. doi: 10.1016/s0016-5085(97)70151-3. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncon L. E., Thompson D. G., Ahluwalia N. K., Barlow J., Heggie L. Relations between upper abdominal symptoms and gastric distension abnormalities in dysmotility like functional dyspepsia and after vagotomy. Gut. 1995 Jul;37(1):17–22. doi: 10.1136/gut.37.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. W., Gonsalves S. F., Fossa A. A., McLean S., Seeger T., Obach S., Andrews P. L. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995 May;115(1):84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead W. E., Holtkotter B., Enck P., Hoelzl R., Holmes K. D., Anthony J., Shabsin H. S., Schuster M. M. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990 May;98(5 Pt 1):1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Histamine signals in enteric neuroimmune interactions. Ann N Y Acad Sci. 1992;664:275–283. doi: 10.1111/j.1749-6632.1992.tb39767.x. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Neuro-immunophysiology of colon function. Pharmacology. 1993 Oct;47 (Suppl 1):7–13. doi: 10.1159/000139836. [DOI] [PubMed] [Google Scholar]