Abstract
Ideally, the inflammatory response occurs rapidly to terminate infection. It also must halt in a timely manner to stop this reaction from inflicting self damage. Such a highly regulated process results from altering balances in pro- and anti-inflammatory signals orchestrated by multiple cell types and factors within the tissue microenvironment. The discovery of new substrates of metalloproteinases within this microenvironment has disclosed a new function in inflammation. The role of these proteases now extends beyond extracellular matrix remodelling enzymes to that of mediators of inflammatory signals involving various chemokines and cytokines. As natural inhibitors of these metalloproteinases, TIMPs have the potential of regulating the inflammatory response and affecting diseases such as rheumatoid arthritis. TIMP-3, in particular, stands out as an important regulator of inflammation with its ability to specifically inhibit proinflammatory cytokines and tissue destruction in the joint.
Full Text
The Full Text of this article is available as a PDF (223.7 KB).
Figure 1.
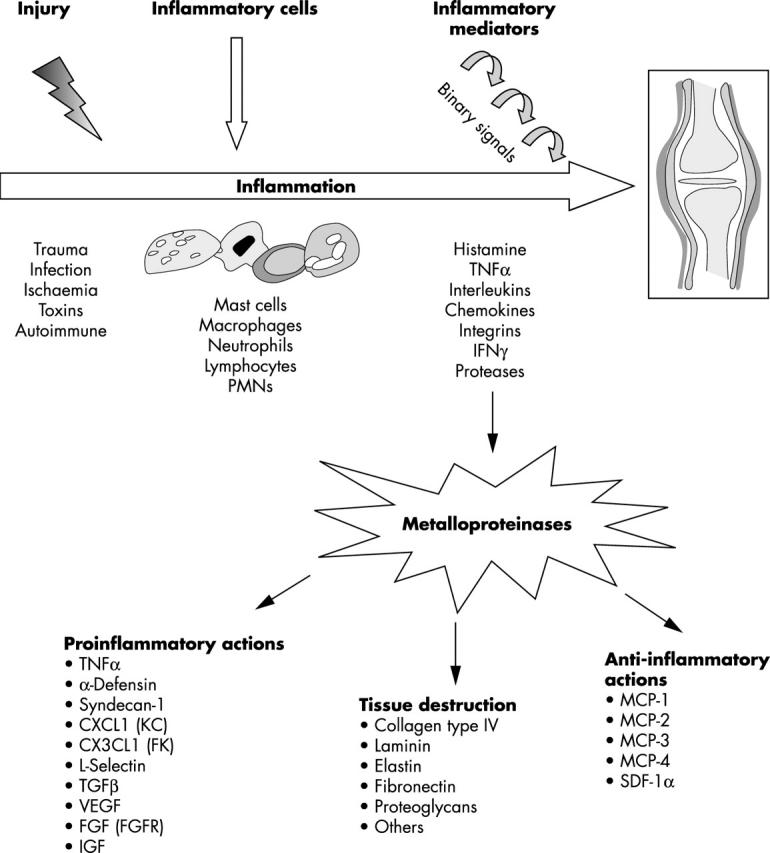
Molecular signals involved in the generation of an inflammatory reaction. Upon injury, the host inflammatory cells respond by releasing various factors that further trigger the release of other inflammatory cues. A system of binary or higher order signals culminates in the inflammatory reaction. A role for the family of metalloproteinases is now recognised, which not only mediates tissue destruction but also generates both pro- and anti-inflammatory responses.
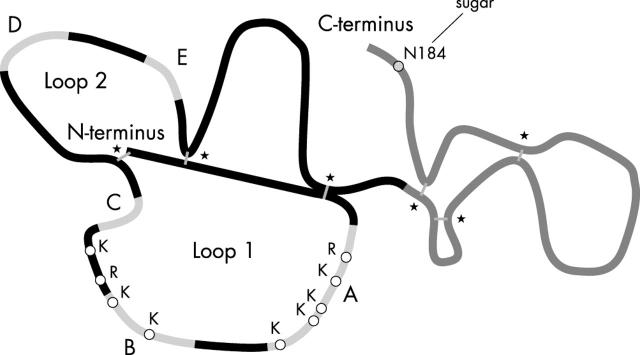
Figure 2.
Structure of the TIMP-3 protein. TIMP-3 is glycosylated at the asparagine amino acid, N184 and contains six disulphide bonds marked by asterisks. The gray sections represent the five strands (A-E) of the N-terminus ß-barrel. Strands A and B can each strongly bind heparan sulphate, which is suggested to result from the net positive charge in these regions. They contain nine basic amino acids indicated by open circles and only one acidic residue. TIMP-1 and TIMP-2 contain several acidic residues that neutralise the charge from their basic residues residing in this region.
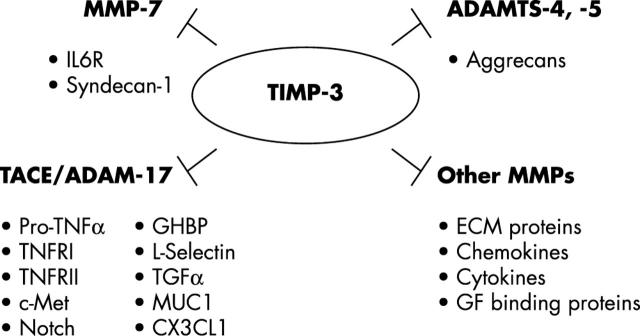
Figure 3.
The broader inhibition profile of TIMP-3. TIMP-3 stands unique among the TIMP family of inhibitors, as it can inhibit ADAM and ADAMTS enzymes in addition to MMPs. These metalloproteinases are known to proteolytically process several factors involved in the normal inflammatory response and in inflammatory diseases.
Figure 4.
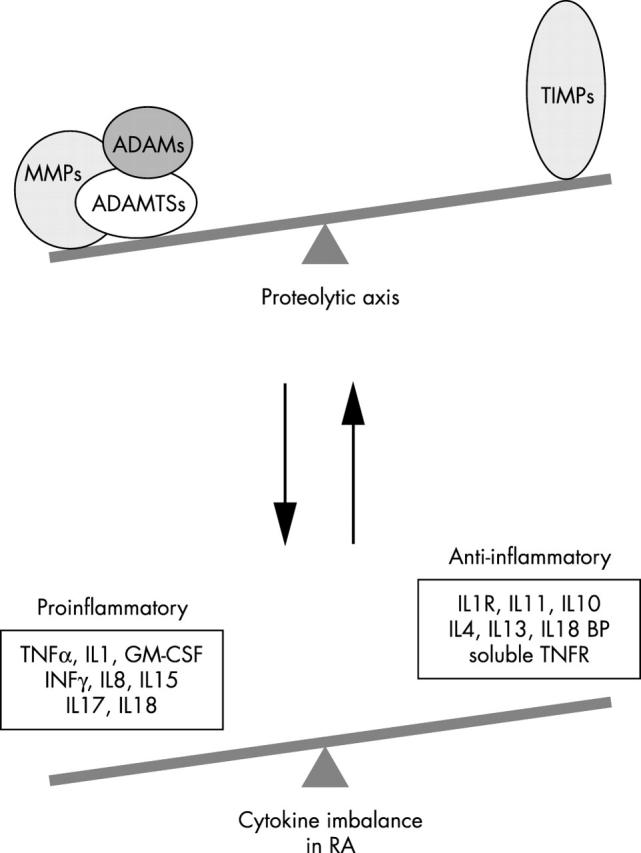
The projected interactions between the metalloproteinase axis and the cytokine axis in rheumatoid disease. Metalloproteinase activity is linked to the activation of various cytokines involved in inflammation. The net metalloproteinase activity can shift the balance between some pro- and anti-inflammatory cytokines. Cytokines, in turn, also induce the production and activation of various metalloproteinases. An imbalance in one axis can potentially cause imbalance in the other perpetuating a loop of destructive activity in RA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992 Feb 1;175(2):323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour A., Slocombe P. M., Webster A., Butler M., Knight C. G., Smith B. J., Stephens P. E., Shelley C., Hutton M., Knäuper V. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998 Sep 11;435(1):39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Apte S. S., Olsen B. R., Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J Biol Chem. 1995 Jun 16;270(24):14313–14318. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- Arend W. P. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001 Feb;45(1):101–106. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Hughes C. E., Decicco C. P., Caterson B., Tortorella M. D. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998 May;6(3):214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- Bian Z. M., Elner S. G., Strieter R. M., Kunkel S. L., Lukacs N. W., Elner V. M. IL-4 potentiates IL-1beta- and TNF-alpha-stimulated IL-8 and MCP-1 protein production in human retinal pigment epithelial cells. Curr Eye Res. 1999 May;18(5):349–357. doi: 10.1076/ceyr.18.5.349.5353. [DOI] [PubMed] [Google Scholar]
- Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997 Feb 20;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Borden P., Heller R. A. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997;7(1-2):159–178. doi: 10.1615/critreveukargeneexpr.v7.i1-2.90. [DOI] [PubMed] [Google Scholar]
- Brew K., Dinakarpandian D., Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000 Mar 7;1477(1-2):267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Butler D. M., Maini R. N., Feldmann M., Brennan F. M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995 Jul-Dec;6(4):225–230. [PubMed] [Google Scholar]
- Coussens Lisa M., Werb Zena. Inflammation and cancer. Nature. 2002 Dec 19;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton K. J., Gough P. J., Blobel C. P., Murphy G., Greaves D. R., Dempsey P. J., Raines E. W. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001 Aug 8;276(41):37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Hashimoto G., Aoki T., Nakamura H., Tanzawa K., Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett. 2001 Apr 13;494(3):192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Aggarwal B. B. Modulation of two forms of tumor necrosis factor receptors and their cellular response by soluble receptors and their monoclonal antibodies. J Biol Chem. 1992 Oct 15;267(29):20892–20899. [PubMed] [Google Scholar]
- Kashiwagi M., Tortorella M., Nagase H., Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001 Jan 23;276(16):12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996 Jan 19;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Langton K. P., Barker M. D., McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem. 1998 Jul 3;273(27):16778–16781. doi: 10.1074/jbc.273.27.16778. [DOI] [PubMed] [Google Scholar]
- Li Qinglang, Park Pyong Woo, Wilson Carole L., Parks William C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002 Nov 27;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- McQuibban G. A., Butler G. S., Gong J. H., Bendall L., Power C., Clark-Lewis I., Overall C. M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001 Sep 24;276(47):43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- McQuibban G. A., Gong J. H., Tam E. M., McCulloch C. A., Clark-Lewis I., Overall C. M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000 Aug 18;289(5482):1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- McQuibban G. Angus, Gong Jiang-Hong, Wong Julie P., Wallace John L., Clark-Lewis Ian, Overall Christopher M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002 Aug 15;100(4):1160–1167. [PubMed] [Google Scholar]
- Mort J. S., Billington C. J. Articular cartilage and changes in arthritis: matrix degradation. Arthritis Res. 2001 Sep 6;3(6):337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997 Feb 20;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Murphy Gillian, Knäuper Vera, Atkinson Susan, Butler George, English William, Hutton Mike, Stracke Jan, Clark Ian. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002 May 9;4 (Suppl 3):S39–S49. doi: 10.1186/ar572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan Carl. Points of control in inflammation. Nature. 2002 Dec 19;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Reddy P., Slack J. L., Davis R., Cerretti D. P., Kozlosky C. J., Blanton R. A., Shows D., Peschon J. J., Black R. A. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000 May 12;275(19):14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- Ruth J. H., Volin M. V., Haines G. K., 3rd, Woodruff D. C., Katschke K. J., Jr, Woods J. M., Park C. C., Morel J. C., Koch A. E. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001 Jul;44(7):1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Vu T. H., Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000 Sep 1;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Weber B. H., Vogt G., Pruett R. C., Stöhr H., Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994 Dec;8(4):352–356. doi: 10.1038/ng1294-352. [DOI] [PubMed] [Google Scholar]
- Wilson C. L., Ouellette A. J., Satchell D. P., Ayabe T., López-Boado Y. S., Stratman J. L., Hultgren S. J., Matrisian L. M., Parks W. C. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999 Oct 1;286(5437):113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Yu W. H., Yu S., Meng Q., Brew K., Woessner J. F., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000 Oct 6;275(40):31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- van der Laan W. H., Quax P. H. A., Seemayer C. A., Huisman L. G. M., Pieterman E. J., Grimbergen J. M., Verheijen J. H., Breedveld F. C., Gay R. E., Gay S. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003 Feb;10(3):234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]