Abstract
Chemotactic migration of leucocytes largely depends on adhesive interaction with the substratum and recognition of a chemoattractant gradient. Both aspects, cell adhesion and chemotaxis, are regulated by members of the family of chemotactic cytokines (chemokines) comprising structurally related and secreted proteins of 67–127 amino acids in length. Breakdown in the control of leucocyte mobilisation contributes to chronic inflammatory diseases and, hence, interference with chemokine function is a promising approach for the development of novel anti-inflammatory medication. Chemokines target all types of leucocyte, including haematopoietic precursors, mature leucocytes of the innate immune system as well as naive, memory, and effector lymphocytes. The combinatorial diversity in responsiveness to chemokines ensures proper tissue distribution of distinct leucocyte subsets under normal and inflammatory/pathological conditions. Here, we discuss recent views on the role of chemokines in controlling tissue localisation of human memory T cells under steady state (non-inflamed) conditions. Emphasis is placed on a concept describing distinct subsets of memory T cells according to their primary residence in peripheral blood, secondary lymphoid tissues, or peripheral (extralymphoid) tissues.
Full Text
The Full Text of this article is available as a PDF (267.9 KB).
Figure 1.
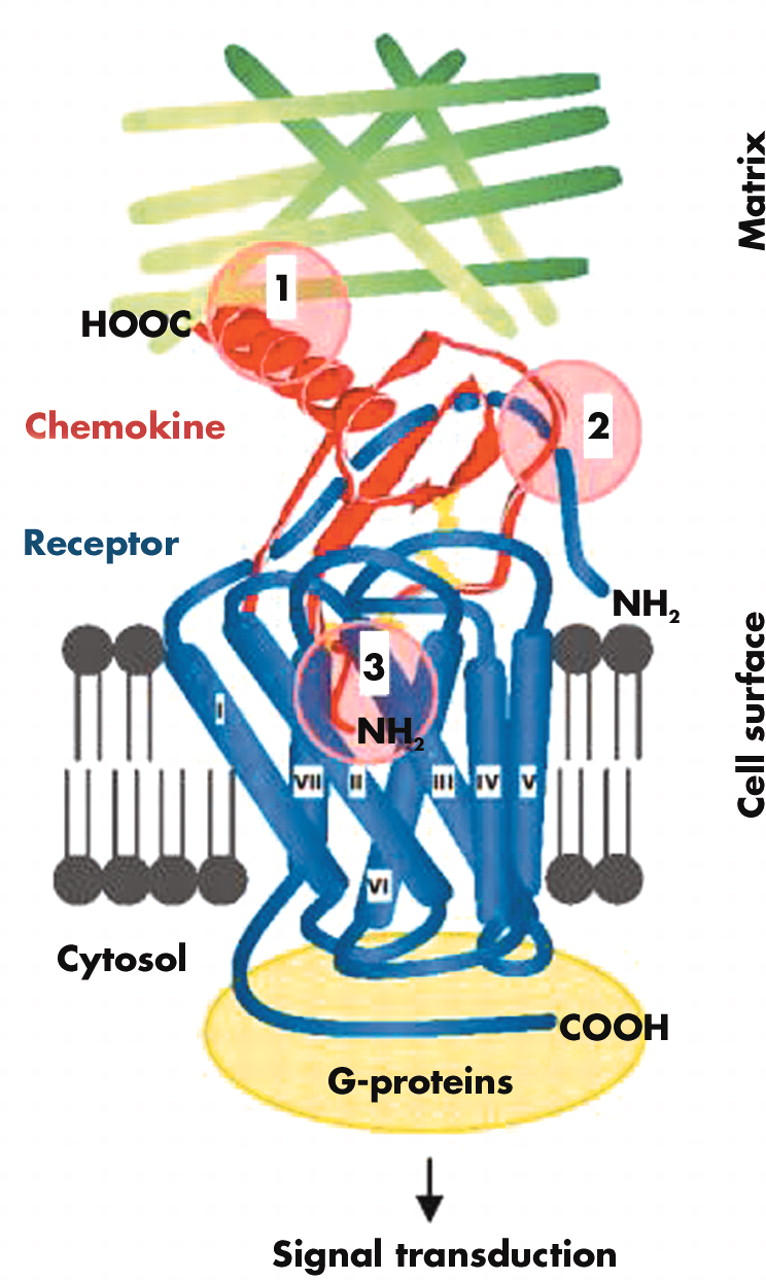
Chemokine–receptor interactions. Chemokine receptors are embedded into the membrane by seven transmembrane domains. The NH2-terminus and three extracellular loop regions are involved in chemokine binding, and the COOH-terminus and three intracellular loop regions participate in G-protein mediated signal transduction. The three sites in chemokines that are essential for function include (1) the matrix fixation site in the COOH terminal α-helix or core structure, (2) the N loop region enabling initial receptor contact, and (3) the NH2-terminus, which interacts with the chemokine binding pocket formed by the transmembrane regions of the chemokine receptor.
Figure 2.
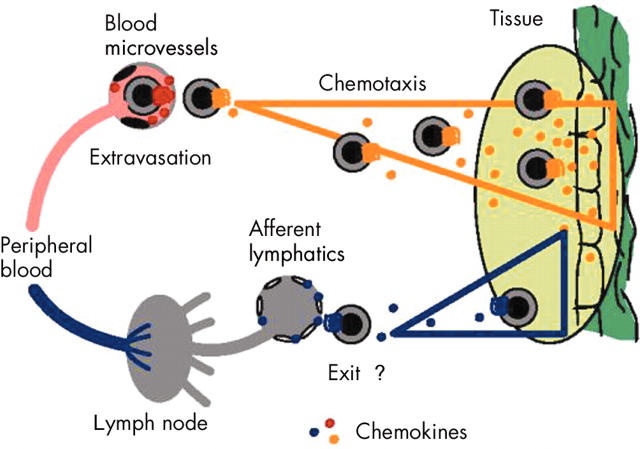
Recruitment, localisation, and tissue exit of circulating leucocytes. Tissue localisation of leucocytes involves two distinct and sequential processes, termed extravasation and chemotaxis. During extravasation, blood leucocytes interact with adhesion molecules on the luminal side of blood vessels and, upon chemokine receptor triggering, become firmly attached and transmigrate through the epithelial barrier. Subsequent chemotaxis guides the perivascular leucocytes to the cellular source(s) of chemokines, which enables cellular colocalisation and subsequent execution of leucocyte function. Eventually, leucocytes exit the tissue via afferent lymphatic vessels to reach draining lymph nodes (LNs) and peripheral blood.
Figure 3.
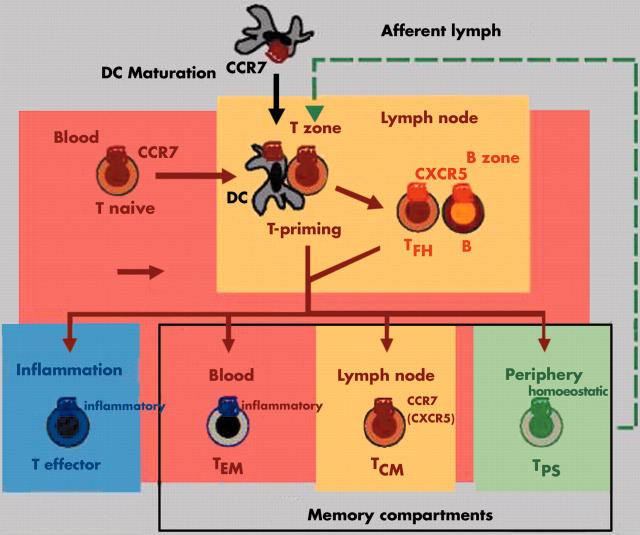
Chemokine receptors define distinct migratory T cell subsets. Naive T cells develop into effector and memory T cells accompanied by major changes in chemokine receptor expression and responsiveness to chemokines. Effector T cells are short lived and efficiently home to sites of inflammation. Memory T cells, in contrast, are long lived and are classified here according to their principal site of residence. TEM cells mainly reside in peripheral blood and are ready to respond to inflammatory chemokines at sites of immune response. Resting TEM cells lack CCR7 and are excluded from lymph nodes (LNs) and Peyer's patches (PPs). TCM cells express CCR7 and adhesion molecules for homing to T zones of secondary lymphoid tissues. Their principal function is the screening of LNs and PPs for the presence of recall antigens presented by local dendritic cells (DCs). Finally, TPS cells primarily reside in healthy (extralymphoid) peripheral tissues whereas they are rare in peripheral blood, LNs, and PPs. Their primary function is immune surveillance at sites of previous antigen encounter. B, B cells; T naive; naive T cells; T effector, effector T cells, TCM, central memory T cells; TEM, effector memory T cells; TFH, follicular B helper T cells; TPS, peripheral immune surveillance T cells; inflammatory and homoeostatic, receptors for inflammatory and homoeostatic chemokines, respectively.
Figure 4.
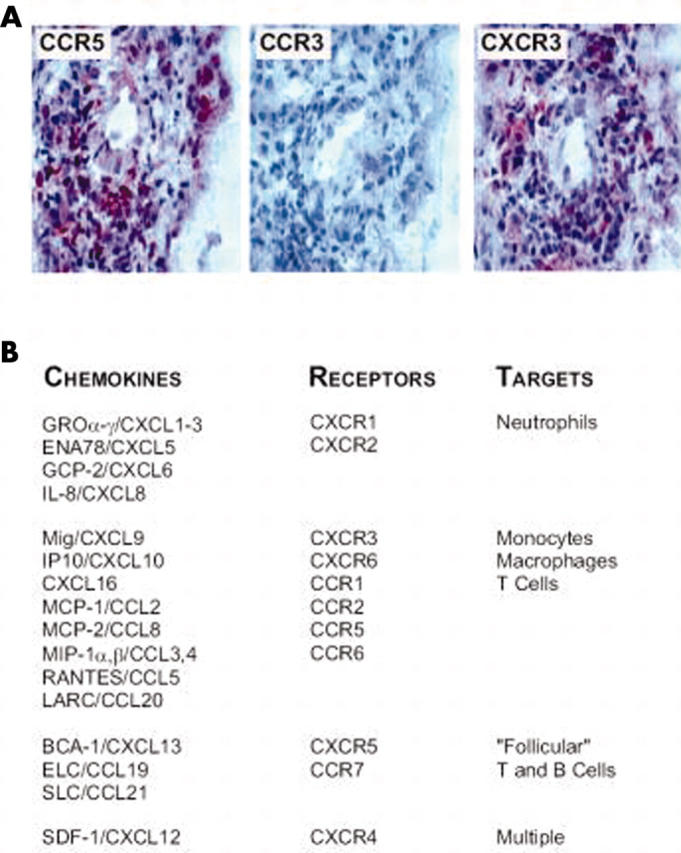
Complex composition of chemokines and chemokine receptors in rheumatoid arthritis. (A) As an example, inflammatory cells in the affected synovial tissue express Th 1 typical chemokine receptors CXCR3 and CCR5 but not the CCR3, which is more prominent on Th 2 cells. (B) A multitude of chemokines were detected in the synovial fluid and inflamed synovial tissue. The chemokines are listed according to their receptor selectivity and target cells. Of note, the condition of rheumatoid arthritis leads to the generation of all chemokines necessary for recruitment of the full complement of effector cells. BCA, B cell attracting chemokine; ELC, EBI-1-ligand chemokine; ENA, epithelial cell derived neutrophil activating protein; GCP, granulocyte chemotactic protein; GRO, growth related oncogene; IP, interferon α inducible protein; LARC, liver and activation regulated chemokine; MCP, monocyte chemoattractant protein; Mig, monokine induced by interferon γ; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; SDF-1, stromal cell derived factor 1; SLC, secondary lymphoid tissue chemokine.
Figure 5.
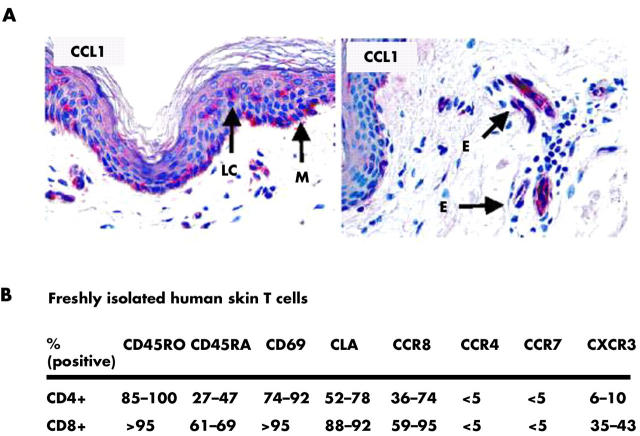
CCR8 marks skin selective immune surveillance T (TPS) cells. (A) CCL1, the only ligand chemokine for CCR8, is expressed in small amounts by epidermal Langerhans cells (LC) and melanocytes (M) as well as blood vessels in the superficial dermal plexus of normal (non-inflamed) human skin. (B) Summary of phenotypic characteristics of freshly isolated T cells from normal human skin. Please note the abundance of CCR8, which is in striking contrast to the low level of expression of other chemokine receptors that are frequently found on effector/memory T cells in peripheral blood. E, microvascular endothelia.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dewald B., Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Blanpain C., Migeotte I., Lee B., Vakili J., Doranz B. J., Govaerts C., Vassart G., Doms R. W., Parmentier M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999 Sep 15;94(6):1899–1905. [PubMed] [Google Scholar]
- Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000 Dec 4;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico G., Frascaroli G., Bianchi G., Transidico P., Doni A., Vecchi A., Sozzani S., Allavena P., Mantovani A. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat Immunol. 2000 Nov;1(5):387–391. doi: 10.1038/80819. [DOI] [PubMed] [Google Scholar]
- Foxman E. F., Campbell J. J., Butcher E. C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997 Dec 1;139(5):1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley T. J., Peiper S. C. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997 May 1;89(9):3077–3091. [PubMed] [Google Scholar]
- Kaech Susan M., Wherry E. John, Ahmed Raft. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002 Apr;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Langenkamp Anja, Nagata Kinya, Murphy Kristine, Wu Lijun, Lanzavecchia Antonio, Sallusto Federica. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003 Feb;33(2):474–482. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia Antonio, Sallusto Federica. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002 Dec;2(12):982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Lasagni Laura, Francalanci Michela, Annunziato Francesco, Lazzeri Elena, Giannini Stefano, Cosmi Lorenzo, Sagrinati Costanza, Mazzinghi Benedetta, Orlando Claudio, Maggi Enrico. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003 Jun 2;197(11):1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna Carlo, Kim Ji Yun, Constantin Gabriela, Butcher Eugene. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002 Aug;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Clark-Lewis I. Agonistic and antagonistic activities of chemokines. J Leukoc Biol. 2001 Jun;69(6):881–884. [PubMed] [Google Scholar]
- Loetscher P., Moser B., Baggiolini M. Chemokines and their receptors in lymphocyte traffic and HIV infection. Adv Immunol. 2000;74:127–180. doi: 10.1016/s0065-2776(08)60910-4. [DOI] [PubMed] [Google Scholar]
- Loetscher P., Pellegrino A., Gong J. H., Mattioli I., Loetscher M., Bardi G., Baggiolini M., Clark-Lewis I. The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3. J Biol Chem. 2000 Nov 10;276(5):2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- Loetscher Pius, Moser Bernhard. Homing chemokines in rheumatoid arthritis. Arthritis Res. 2002 Jan 31;4(4):233–236. doi: 10.1186/ar412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian Mehrdad, Lo Charles G., Cinamon Guy, Lesneski Matthew J., Xu Ying, Brinkmann Volker, Allende Maria L., Proia Richard L., Cyster Jason G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004 Jan 22;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Moser B., Loetscher M., Piali L., Loetscher P. Lymphocyte responses to chemokines. Int Rev Immunol. 1998;16(3-4):323–344. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- Moser B., Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001 Feb;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- Moser Bernhard, Schaerli Patrick, Loetscher Pius. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002 May;23(5):250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- Moser Bernhard, Wolf Marlene, Walz Alfred, Loetscher Pius. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004 Feb;25(2):75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Muller William A. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003 Jun;24(6):327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Baggiolini M., Charo I. F., Hébert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000 Mar;52(1):145–176. [PubMed] [Google Scholar]
- Murphy Philip M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002 Jun;54(2):227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- Nibbs R. J., Kriehuber E., Ponath P. D., Parent D., Qin S., Campbell J. D., Henderson A., Kerjaschki D., Maurer D., Graham G. J. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001 Mar;158(3):867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie P., Bardi G., Clark-Lewis I., Baggiolini M., Uguccioni M. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood. 2001 Apr 1;97(7):1920–1924. doi: 10.1182/blood.v97.7.1920. [DOI] [PubMed] [Google Scholar]
- Ogilvie Patricia, Paoletti Samantha, Clark-Lewis Ian, Uguccioni Mariagrazia. Eotaxin-3 is a natural antagonist for CCR2 and exerts a repulsive effect on human monocytes. Blood. 2003 Apr 10;102(3):789–794. doi: 10.1182/blood-2002-09-2773. [DOI] [PubMed] [Google Scholar]
- Onuffer James J., Horuk Richard. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002 Oct;23(10):459–467. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- Partida-Sánchez Santiago, Goodrich Stephen, Kusser Kim, Oppenheimer Norman, Randall Troy D., Lund Frances E. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004 Mar;20(3):279–291. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- Proudfoot Amanda E. I. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002 Feb;2(2):106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph G. J., Beaulieu S., Pope M., Sugawara I., Hoffman L., Steinman R. M., Muller W. A. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6924–6929. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H., Moore A. M., Brown M. J., Hwang S. T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999 Mar 1;162(5):2472–2475. [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 Oct 14;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schaerli P., Loetscher P., Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol. 2001 Dec 1;167(11):6082–6086. doi: 10.4049/jimmunol.167.11.6082. [DOI] [PubMed] [Google Scholar]
- Schaerli P., Willimann K., Lang A. B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000 Dec 4;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli Patrick, Ebert Lisa, Willimann Katharina, Blaser Andrea, Roos Regula Stuber, Loetscher Pius, Moser Bernhard. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004 May 3;199(9):1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Tough D. F. T cell death and memory. Science. 2001 Jul 13;293(5528):245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001 Feb;2(2):129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000 Feb;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen Ester M., Gamadia Laila E., Baars Paul A., Remmerswaal Ester B., ten Berge Ineke J., van Lier René A. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002 Nov 15;169(10):5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]