Abstract
Methods: A list of 26 potential domains was prepared through literature review and email discussions amongst the GRAPPA steering committee members and scored by rheumatologists identified through membership of the CASPAR study and the steering committee. Each participant was emailed an up to date review of outcome measures in psoriatic arthritis and asked to distribute 100 points amongst each potential domain. In two subsequent rounds the group median, interquartile range, and earlier responses were emailed to each respondent to provide an opportunity to revise their scoring.
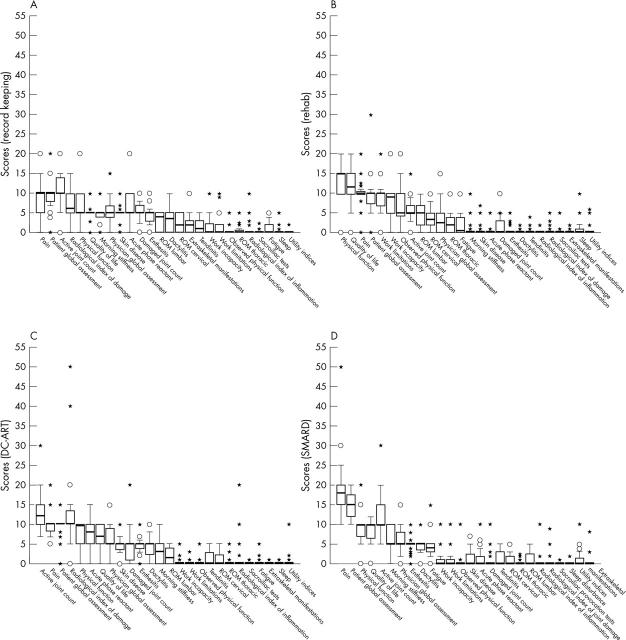
Results: Thirty two participants responded to the first round, of whom 30 responded to the third round. For DC-ART, the highest scoring domains were actively inflamed joint count, radiological damage score, patient global assessment, pain, physical function, acute phase response, and quality of life (scores 7 to 12). For SMARD, the highest scoring domains were pain, patient global assessment, physical function, quality of life, and active joint count (scores 10 to 18). For clinical record keeping, three domains scored highly at 10 (pain, patient global assessment, and active joint count). For rehabilitation, the highest scoring domains were physical function, quality of life, pain, patient global assessment, work limitations, and work incapacity (scores 10 to 15).
Conclusion: Amongst rheumatologists with an interest in psoriatic arthritis, a reduced list of potential standard outcome domains have been defined by Delphi consensus methods.
Full Text
The Full Text of this article is available as a PDF (65.5 KB).
Figure 1.
Box plot distribution of scores for each domain (median, interquartile range (IQR), 90% confidence interval, outliers (1.5–3 IQR beyond median) and extremes (beyond 3 IQR from median)). (A) Clinical record keeping; (B) rehabilitation; (C) disease controlling antirheumatic therapy (DC-ART); (D) symptom modifying antirheumatic drugs (SMARD). ROM, range of motion.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy N., Kirwan J., Boers M., Brooks P., Strand V., Tugwell P., Altman R., Brandt K., Dougados M., Lequesne M. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol. 1997 Apr;24(4):799–802. [PubMed] [Google Scholar]
- Gladman Dafna D., Helliwell Philip, Mease Philip J., Nash Peter, Ritchlin Christopher, Taylor William. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum. 2004 Jan;50(1):24–35. doi: 10.1002/art.11417. [DOI] [PubMed] [Google Scholar]
- Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995 Aug 5;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor William J. Epidemiology of psoriatic arthritis. Curr Opin Rheumatol. 2002 Mar;14(2):98–103. doi: 10.1097/00002281-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Tugwell P., Boers M. Developing consensus on preliminary core efficacy endpoints for rheumatoid arthritis clinical trials. OMERACT Committee. J Rheumatol. 1993 Mar;20(3):555–556. [PubMed] [Google Scholar]
- van der Heijde D., Bellamy N., Calin A., Dougados M., Khan M. A., van der Linden S. Preliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working Group. J Rheumatol. 1997 Nov;24(11):2225–2229. [PubMed] [Google Scholar]