Abstract
A role for metalloproteinases in the pathological destruction in diseases such as rheumatoid arthritis and osteoarthritis, and the irreversible nature of the ensuing cartilage and bone damage, have been the focus of much investigation for several decades. This has led to the development of broad spectrum metalloproteinase inhibitors as potential therapeutics. More recently it has been appreciated that several families of zinc dependent proteinases play significant and varied roles in the biology of the resident cells in these tissues, orchestrating development, remodelling, and subsequent pathological processes. They also play key roles in the activity of inflammatory cells. The task of elucidating the precise role of individual metalloproteinases is therefore a burgeoning necessity for the final design of metalloproteinase inhibitors if they are to be employed as therapeutic agents.
Full Text
The Full Text of this article is available as a PDF (110.6 KB).
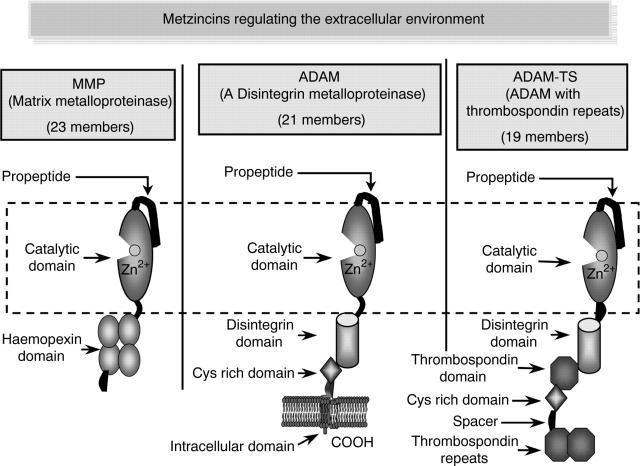
Figure 1.
Three metzincin families, matrix metalloproteinases (MMPs), disintegrin metalloproteinases (ADAMs), and disintegrin metalloproteinases with thrombospondin repeats (ADAM TSs) have been identified as having roles in the biology and pathology of cartilage and bone. They have in common the zinc containing catalytic domain which is rendered inactive in the presence of an N-terminal propeptide. Activation is usually effected by the proteolytic removal of the propeptide at cellular sites specific to individual proteinases. Most of these metalloproteinases have other domains conferring specificity with respect to substrate cleavage or cellular or ECM localisation. Some members of the MMP family and all the ADAM family are membrane associated.
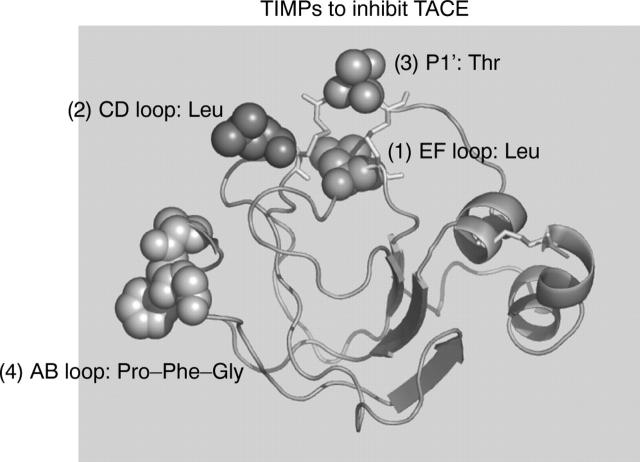
Figure 2.
Modification of the metalloproteinase binding ridge of tissue inhibitors of metalloproteinases (TIMPs) to generate potent inhibitors of TACE. Based on any of the four TIMP basic scaffolds a tight binding inhibitor of the disintegrin metalloproteinase (ADAM) tumour necrosis factor α converting enzyme (TACE) can be made by the modification of four positions on the active site binding ridge. (1) a leucine residue on the EF ß strand loop; (2) a leucine residue on the CD ß strand loop; (3) a threonine residue at the P1' binding site; hand (4) a proline-phenylalanine-glycine triad at the tip of the AB ß strand loop. Adapted from Lee et al.20
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen Thomas L., del Carmen Ovejero Maria, Kirkegaard Tove, Lenhard Thomas, Foged Niels T., Delaissé Jean-Marie. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004 Nov;35(5):1107–1119. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Baker Andrew H., Kritz Angelika, Work Lorraine M., Nicklin Stuart A. Cell-selective viral gene delivery vectors for the vasculature. Exp Physiol. 2004 Nov 12;90(1):27–31. doi: 10.1113/expphysiol.2004.028126. [DOI] [PubMed] [Google Scholar]
- Clark Ian M., Parker Andrew E. Metalloproteinases: their role in arthritis and potential as therapeutic targets. Expert Opin Ther Targets. 2003 Feb;7(1):19–34. doi: 10.1517/14728222.7.1.19. [DOI] [PubMed] [Google Scholar]
- Coussens Lisa M., Fingleton Barbara, Matrisian Lynn M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002 Mar 29;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Dickinson Sally C., Vankemmelbeke Mireille N., Buttle David J., Rosenberg Krisztina, Heinegård Dick, Hollander Anthony P. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003 May;22(3):267–278. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Flannery C. R., Little C. B., Caterson B., Hughes C. E. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999 Jun;18(3):225–237. doi: 10.1016/s0945-053x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Stanton H., Little C. B., Atley L. M. Neoepitopes as biomarkers of cartilage catabolism. Inflamm Res. 2003 Jun;52(7):277–282. doi: 10.1007/s00011-003-1177-5. [DOI] [PubMed] [Google Scholar]
- Garnero Patrick, Mazières Bernard, Guéguen Alice, Abbal Michel, Berdah Laurent, Lequesne Michel, Nguyen Minh, Salles Jean-Pierre, Vignon Eric, Dougados Maxime. Cross-sectional association of 10 molecular markers of bone, cartilage, and synovium with disease activity and radiological joint damage in patients with hip osteoarthritis: the ECHODIAH cohort. J Rheumatol. 2005 Apr;32(4):697–703. [PubMed] [Google Scholar]
- Kevorkian Lara, Young David A., Darrah Clare, Donell Simon T., Shepstone Lee, Porter Sarah, Brockbank Sarah M. V., Edwards Dylan R., Parker Andrew E., Clark Ian M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004 Jan;50(1):131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Lee Meng-Huee, Rapti Magdalini, Murphy Gillian. Total conversion of tissue inhibitor of metalloproteinase (TIMP) for specific metalloproteinase targeting: fine-tuning TIMP-4 for optimal inhibition of tumor necrosis factor-{alpha}-converting enzyme. J Biol Chem. 2005 Feb 15;280(16):15967–15975. doi: 10.1074/jbc.M500897200. [DOI] [PubMed] [Google Scholar]
- Mengshol John A., Mix Kimberlee S., Brinckerhoff Constance E. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 2002 Jan;46(1):13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mix Kimberlee S., Sporn Michael B., Brinckerhoff Constance E., Eyre David, Schurman David J. Novel inhibitors of matrix metalloproteinase gene expression as potential therapies for arthritis. Clin Orthop Relat Res. 2004 Oct;(427 Suppl):S129–S137. doi: 10.1097/01.blo.0000144483.62033.8b. [DOI] [PubMed] [Google Scholar]
- Mohammed F. F., Smookler D. S., Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003 Nov;62 (Suppl 2):ii43–ii47. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott Joni D., Werb Zena. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004 Oct;16(5):558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase Hideaki, Kashiwagi Masahide. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003 Feb 14;5(2):94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall Christopher Mark, López-Otín Carlos. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002 Sep;2(9):657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Patwari P., Gao G., Lee J. H., Grodzinsky A. J., Sandy J. D. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage. 2005 Apr;13(4):269–277. doi: 10.1016/j.joca.2004.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. Robin, Nelson Fred, Dahlberg Leif, Tchetina Elena, Kobayashi Masahiko, Yasuda Tadashi, Laverty Sheila, Squires Ginette, Kojima Toshihisa, Wu William. Proteolysis of the collagen fibril in osteoarthritis. Biochem Soc Symp. 2003;(70):115–123. doi: 10.1042/bss0700115. [DOI] [PubMed] [Google Scholar]
- Puente Xose S., Sánchez Luis M., Overall Christopher M., López-Otín Carlos. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003 Jul;4(7):544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- Rao B. Govinda. Recent developments in the design of specific Matrix Metalloproteinase inhibitors aided by structural and computational studies. Curr Pharm Des. 2005;11(3):295–322. doi: 10.2174/1381612053382115. [DOI] [PubMed] [Google Scholar]
- Verrier S., Hogan A., McKie N., Horton M. ADAM gene expression and regulation during human osteoclast formation. Bone. 2004 Jul;35(1):34–46. doi: 10.1016/j.bone.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Vincenti M. P., Brinckerhoff C. E. The potential of signal transduction inhibitors for the treatment of arthritis: Is it all just JNK? J Clin Invest. 2001 Jul;108(2):181–183. doi: 10.1172/JCI13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voils Stacy A., Evans Martin E., Lane Matthew T., Schosser Robert H., Rapp Robert P. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann Pharmacother. 2004 Nov 23;39(1):86–94. doi: 10.1345/aph.1E282. [DOI] [PubMed] [Google Scholar]
- van der Laan W. H., Quax P. H. A., Seemayer C. A., Huisman L. G. M., Pieterman E. J., Grimbergen J. M., Verheijen J. H., Breedveld F. C., Gay R. E., Gay S. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003 Feb;10(3):234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]