Abstract
The title L-glutathione derivatives, containing acid- and base-labile esters, respectively, were obtained in good overall yields. N-tBoc L-glutathione dimethyl ester was prepared via Fischer esterification of L-glutathione disulfide (GSSG) using HCl in dry methanol, protection of the amine with tBoc2O, and tributylphosphine cleavage of the disulfide in wet isopropanol. Alternatively, Fischer esterification and tBoc-protection of L-glutathione (GSH) also furnished N-tBoc glutathione dimethyl ester accompanied by a small amount of S-tBoc that was removed chromatographically. The di-tert--butyl ester was obtained by S-palmitoylation of GSH in TFA as solvent, N-tBoc-protection, esterification using tBuOH mediated by diisopropylcarbodiimide/copper(I) chloride, and saponification of the thioester. These L-glutathione derivatives are versatile synthetic building blocks for the preparation of S-glutathione adducts.
Keywords: Amino acids and derivatives, Thioesters, Protecting groups, Peptides
1. Introduction
The tripeptide L-glutathione (L-γ-glutamyl-L-cysteinyl-glycine; GSH) (4) is a common constituent in most animal cells1 and many bacteria.2 It participates in a variety of physiologic functions, inter alia, signal transduction,3 immunity,4 maintenance of cellular osmoilality,5 defense against reactive ozygen species (ROS) and free radicals,6 and protein folding. Of particular interest is the bioactivation8 or inactivation of xenobiotics, drugs, and endogenous substrates by GSH-S- conjugation mediated by a widely distributed family of GSH-S-transferases.9 The latter category of substrates includes metabolites of the cyclooxygenase, lipoxygenase, and cytochrome P450 branches of the arachidonate cascade.10 As part of our longstanding interest in the structure elucidation and total synthesis of GSH-S-conjugates of eicosanoids,11 we required N-protected L-glutathione derivatives bearing orthogonally protected esters as synthetic intermediates. Herein, we report reliable, multi-gram preparations of the base-labile building block N-tBoc L-glutathione dimethyl ester12 (3) and its acid-labile di-tert-butyl ester analog (9).
2. Results and Discussion
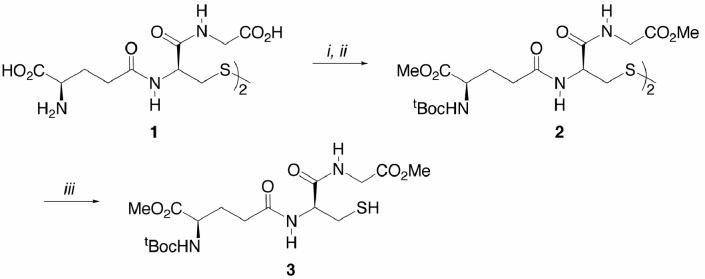
The synthesis of 3 began by dissolving commercial L-glutathione disulfide (1) in MeOH and saturating with dry HCl gas (Scheme 1).13 After several days, all volatiles removed in vacuo and, typically, the crude tetramethyl ester dihydrochloride salt13 were was directly N-carbamoylated using tBoc-anhydride in the presence of NaHCO3 to give 2.14 Disulfide cleavage15 using n-Bu3P proceeded smoothly and furnished N-tBoc L-glutathione dimethyl ester12 (3) as a white, crystalline solid in 54% overall yield.
Scheme 1.
Reagents and conditions: (i) HCL, MeOH, 23°C, 80 h; (ii) tBoc2O, NaHCO3, THF/H2O, 23°C, 29 h, 75% from 1; (iii) Bu3P, n-PrOH/H2(2:1), 23°C, 4 h, 72%.
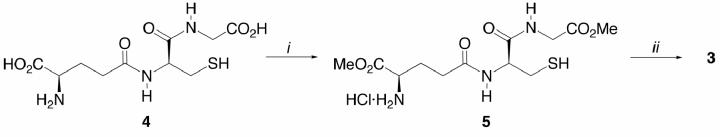
Fischer esterification of 4 as conducted above and subsequent N-carbamoylation of the resultant dimethyl ester hydrochloride salt16 5 led to 3 (Scheme 2).17 The somewhat superior yield of the route in Scheme 2 (64% overall) versus that in Scheme 1 (54% overall) is counterbalanced by the need to remove chromatographically a small amount of N,S-di-tBoc dimethyl ester by-product.
Scheme 2.
Reagents and conditions: (i) HCL, MeOH, 23°C, 80 h; (ii) tBoc2O, NaHCO3, THF/H2O, 23°C, 15 h, 75%.
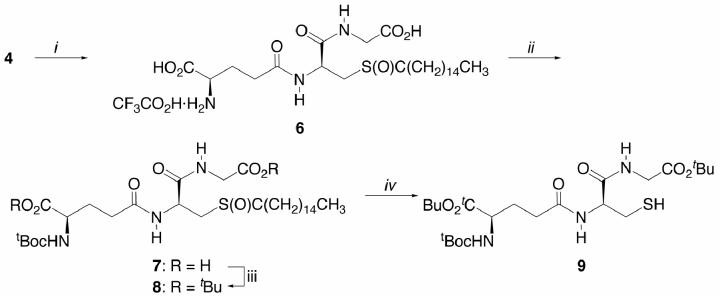
Due in large part to its poor solubility in even polar organic solvents, e.g., DMF, DMSO, dioxane, and THF, the conversion of 4 to its di-tert-butyl ester proved problematic. Little or no esterification was noted using a variety of chemical and enzymatic procedures: isobutylene/H2SO4 or CH3SO3H in dioxane,18 tBuOAc/HClO4,19 tBoc2O/DMAP/tBuOH,20 BuBr/K2CO3/(PhCH2)Et3NCl in DMF or THF,21 tBuOC(O)F/Et3N/DMAP in tBuOH under reflux,22 Me2NC(OtBu)2H,23 tBuOH/EDCI/DMAP,24 tBuOH/CDI/DBU,25 transesterification of 5 with tBuOH/H2SO4, tBuOH/Amano AK lipase, and tBuOH/pig liver esterase.26 To improve its solubility characteristics as well as obviate the inherent nucleophilicity of the thiol, 4 was dissolved in a minimum of TFA and S-acylated with acid chlorides of varying chain lengths.27,28 The palmitoyl thioester 6 displayed satisfactory behavior and could be isolated as the solid trifluoroacetate salt (Scheme 3). No N-palmitoylation under the acidic conditions was noted.29 Sequential N-carbamoylation and esterification of 6 with tBuOH afforded N-Boc 7 and N-Boc di-tert-butyl ester 8, respectively. The latter esterification, however, could only be effected in satisfactory yield using N,N′-diisopropylcarbodiimide in the presence of catalytic CuCl according to Zhu et al. 30 Methanolysis31 of 8 with NaOMe/MeOH gave rise to 9 without event.
Scheme 3.
Reagents and conditions: (i) H3C(CH2)14C(O)Cl, F3CCO2H, 23°C, 20 min, then 40°C, 40 min, 91%; (ii) O(CO2tBu)2, NaH2O, 23°C, 15 h, 64%; (iii) tBuOH, (iPrHN=)2C, CuCl, 23°C, 12 h, then add 7 in CH2Cl2, 40°C, 48 h, 70%; (iv) NaOMe, MeOH, 1 h, 23°C, 71%.
3. Conclusions
Herein, we provide convenient and efficient preparations of the dimethyl and di-tert-butyl esters of N-tBoc L-glutathione. Since these synthetic building blocks can be orthogonally deprotected under basic and acidic conditions, respectively, we anticipate they will find utility in the preparation of S-glutathione conjugates, peptide synthesis, and library development.
4. Experimental
4.1. General Procedures
1H and 13C spectra were recorded in CDCl3 unless otherwise specified using tetramethylsilane as internal reference. The Michigan State University Mass Spectroscopy Facility provided high-resolution mass spectra. All reactions were maintained under an argon atmosphere. Anhydrous solvents were freshly distilled from sodium benzophenone ketyl, except for CH2Cl2, which was distilled from CaH2. Extracts were dried over anhydrous Na2SO4 and filtered prior to removal of all volatiles under reduced pressure.
4.2. Chemistry
4.2.1. Bis-N-tBoc L-glutathione tetramethyl ester disulfide (2)
Dry HCl gas was bubbled through a 0°C solution of L-glutathione disulfide (1) (6.0 g, 9.8 mmol) in anhydrous MeOH (500 mL) until 48 g of HCl was absorbed. Stirring was continued at 0 °C for 80 h, then all volatiles were removed in vacuo. The residue was further dried using a mechanical vacuum pump for 24 h to give the corresponding tetramethyl ester dihydrochloride salt as a colorless gum that was used directly in the next step.
A solution of the above tetramethyl ester salt (6.0 g), NaHCO3 (3.3 g, 39.2 mmol, 4 equiv), and tBoc2O (5.16 g, 23 mmol, 2.4 equiv) in THF/H2O (2.4:1, 150 mL) was stirred at room temperature. After 29 h, the pH was adjusted to 3 with conc. HCl, and the solution was extracted with EtOAc (5 × 10 mL). The combined extracts were concentrated in vacuo and the residue purified by SiO2 column chromatography to give 2 (4.0 g, 75%) as a white solid, mp 135°C. TLC: 10% MeOH/CH2Cl2, Rf ∼ 0.35; [α]23d +30.5 (c 2.25, CHCl3); 1H NMR (400 MHz) δ 8.46-8.58 (m, 1H) 6.72 (d, 1H, J = 8.6 Hz), 5.51-5.56 (m, 1H), 5.32 (d, 1H, J = 8.2 Hz), 4.34-4.44 (m, 1H), 4.15 (dd, 1H, J = 5.8,18 Hz), 4.09 (dd, 1H, J = 4.9,18 Hz), 3.76 (s, 3H), 3.74 (s, 3H), 3.06 (dd, 1H, J = 4, 15 Hz), 2.84-2.97 (m, 1H), 2.32-2.44 (m, 2H), 2.14-2.26 (m, 1H), 1.95-2.20 (m, 1H),1.43 (s, 9H); 13C NMR (100 MHz) δ 173.2, 172.8, 171.2, 170.1, 155.9, 80.2, 53.2, 53.1, 52.5, 46.1, 41.5, 32.4, 28.5.
4.2.2. N-tBoc L-Glutathione dimethyl ester (3) from 2
Bu3P (500 ⌊L, 617 mg, 3.05 mmol) was added to a solution of disulfide 2 (1.6 g, 1.84 mmol) in n-PrOH/H2O (2:1, 6 mL, degassed with argon) under argon atmosphere. After stirring for 4 h, the n-PrOH was removed and the aqueous solution was extracted with CH2Cl2 (4 × 40 mL). The combined extracts were washed with water, dried, and concentrated. The residue was purified by SiO2 column chromatography to give 3 (1.16 g, 72%) as a white, crystalline solid, mp 94 °C. TLC: 10% MeOH/CH2Cl2, Rf ∼ 0.38; [α]23d −6.8 (c 1.3, CHCl3); 1H NMR (400 MHz) δ 6.97 (t, 1H, J = 5.5 Hz), 6.83 (d, 1H, J = 7 Hz), 5.29 (d, 1H, J = 8.5 Hz), 4.68 (ddd, 1H, J = 8.5, 6.0, 4.6 Hz), 4.30-4.40 (m, 1H), 4.08 (dd, 1H, J = 5.8 Hz, 18 Hz), 4.00 (dd, 1H, J = 18 Hz, 5.2 Hz), 3.76 (s, 3H), 3.75 (s, 3H), 3.14 (ddd, 1H, J = 14, 7.9, 4.6 Hz), 2.74-2.84 (m, 1H), 2.38 (t, 2H, J = 6.5 Hz), 2.20-2.26 (m, 1H), 1.90-1.99 (m, 1H), 1.83 (dd, 1H, J = 8 Hz, 11Hz), 1.43 (s, 9H); 13C NMR (100 MHz) δ 173.1, 172.8, 170.4, 170.2, 86.04, 80.3, 54.1, 52.7, 52.5, 41.4, 32.1, 28.5, 28.3, 26.4.
4.2.3. L-Glutathione dimethyl ester hydrochloride (5)
L-Glutathione (4) (2.0 g, 6.51 mmol) was esterified in acidified MeOH as described for 2 to give 5 (2.2 g, 91%) as a free flowing, white solid, mp 98 °C. TLC: 10% MeOH/CH2Cl2, Rf ∼ 0.25; [α]23D –26 (c 1.1, EtOH); 1H NMR (400 MHz, CD3OD) δ 4.58 (t, 1H, J = 6.4 Hz), 4.01 (d, 1H, J = 5.5 Hz), 3.78 (s, 3H), 3.76 (s, 3H), 3.62 (t, 1H, J = 6.8 Hz), 3.32-3.37 (m, 1H), 2.95 (dd, 1H, J = 6.7, 14.2 Hz), 2.87 (dd, 1H, J = 7.2, 13.9 Hz), 2.43-2.51 (m, 2H), 2.06-2.15 (m, 1H), 1.93-2.04 (m, 2H); 13C NMR (100 MHz, CD3OD) δ 176.3, 175.3, 173.0, 171.7, 57.0, 54.7, 52.9, 52.81, 42.0, 32.9, 30.7, 27.1.
4.2.4. N-tBoc L-Glutathione dimethyl ester (3) from 5
A solution of 5 (2.2 g, 6.6 mmol), NaHCO3 (1.2 g, 14.3 mmol, 2.2 equiv), and tBoc2O (1.4g, 6.6 mmol, 1.0 equiv) in THF/H2O (1:2.4, 25 mL) was stirred at room temperature. After 15 h, the solution was extracted with EtOAc (4 × 50 mL). The combined extracts were concentrated in vacuo and the residue was purified by SiO2 column chromatography to give 3 (1.8 g, 70%) as described above and the by-product S-tBoc,N-tBoc dimethyl ester (0.39 g, 11%) as a white solid, mp 91 °C; lit.17 mp 92 °C. TLC of S-tBoc,N-tBoc dimethyl ester: 5% MeOH/CH2Cl2, Rf ∼ 0.25; [α]23D –32 (c 1.0, EtOH); lit.17 [α]23D –37.2 (c 1.0, EtOH); 1H NMR (400 MHz) δ 7.29 (t, 1H, J = 5.2 Hz), 6.93 (d, 1H, J = 7.2 Hz), 5.44 (d, 1H, J = 7.9 Hz), 4.63-4.69 (m, 1H) , 4.25 (d, 1H, J = 4.7 Hz), 4.02 (dd, 1H, J = 5.7, 17.9 Hz), 3.95 (dd, 1H, J = 4.7, 18 Hz), 3.69 (s, 3H), 3.68 (s, 3H), 3.22 (dd, 1H, J = 4.8, 15 Hz), 3.13 (dd, 1H, J = 7.0, 14.6 Hz), 2.29 (t, 2H, J = 7.2 Hz), 2.08-2.14 (m, 1H), 1.92-2.00 (m, 1H), 1.43 (s, 9H), 1.38 (s, 9H); 13C NMR (100 MHz) δ 173.0, 172.7, 170.5, 170.2, 169.9, 155.8, 85.8, 80.2, 53.7, 53.1, 53.0, 52.6, 52.5, 41.4, 32.3, 32.2, 28.4, 28.2.
4.2.5. S-Palmitoyl L-Glutathione trifluoroacetate (6)
Palmitoyl chloride (2.4 mL, 7.8 mmol, 2.4 equiv) was added dropwise to a solution of L-glutathione (4) (1.0 g, 3.25 mmol) in trifluoroacetic acid (11 mL) under an argon atmosphere. After stirring at rt for 20 min and at 40 °C for 30 min, the reaction was quenched by the addition of water (0.25 mL, 14 mmol, 4.3 equiv) and stirred for an additional 1 h at 40 °C. The trifluoroacetic acid was removed in vacuo, ethyl acetate (20 mL) was added, the mixture was cooled to 10 °C, and the precipitated trifluoroacetate salt of 6 (1.6 g, 91%) was collected by filtration, mp 179-181 °C. TLC: MeOH/H2O (2:1), Rf ∼ 0.67; [α]23D –15 (c 0.66, EtOH/DMSO (2:1)); 1H NMR (400 MHz, CD3SOCD3) δ 8.24-8.37 (m, 1H), 4.36-4.45 (m, 1H), 3.60-3.86 (m, 1H), 3.24-3.38 (m, 1H), 2.92-2.99 (m, 1H), 2.84-2.97 (m, 1H), 2.25-2.56 (m, 2H), 2.16 (t, 1H, J = 7.3 Hz), 1.10-1.38 (m, 18H), 0.83 (t, 3H, J = 6.8 Hz); ESMS m/z 546 (M++1); HRMS (FAB-CI, NBA) Calcd. for C26H47N3O7S [M+H]+ 546.3214, found 546.3213.
4.2.6. N-tBoc S-Palmitoyl L-glutathione (7)
Sodium bicarbonate (984 mg, 11.72 mmol, 4 equiv) and di-tert-butyldicarbonate (767 mg, 3.5 mmol, 1.2 equiv) were added sequentially to a solution of 6 (1.6 g, 2.93 mmol) in THF/H2O (1:2.4, 10 mL) under an argon atmosphere. After stirring overnight, the pH was adjusted to 4 with 6 N HCl and the reaction mixture was extracted with EtOAc (4 × 5 mL). The combined organic extracts were dried, concentrated in vacuo, and the solid white residue was azeotroped with anhydrous benzene (20 mL) and dried under high vacuum for 1 h to yield 7 (1.2 g, 64%) as a free flowing solid, mp 104 °C, sufficiently pure to be used directly in the next step. TLC: 30% MeOH/EtOAc, Rf ∼ 0.25; [α]23D –8 (c 1.7, CHCl3); 1H NMR (400 MHz) δ 7.50-8.20 (m, 2H), 6.47 (brs, 1H), 5.65 (brs, 1H), 4.71 (d, 1H, J = 5.8 Hz), 4.13-4.35 (m, 2H), 3.72-3.88 (m, 2H), 3.18–3.36 (m, 2H), 2.55 (t, 2H, J = 7.6 Hz), 2.35 (t, 2H, J = 7.3 Hz), 1.45 (s, 9H), 1.25 (s, 26H), 0.88 (t, 3H, J = 6.7 Hz); 13C NMR (75 MHz) δ 200.1, 179.4, 175.4, 174.2, 172.8, 171.6, 156.2, 85.3, 82.2, 80.6, 53.0, 44.2, 41.6, 34.3, 32.1, 29.9, 29.7, 29.6, 24.5, 29.3, 29.2, 28.5, 27.6, 27.8, 25.0, 22.9, 14.3; ESMS m/z 668 (M++23); HRMS (FAB-CI, NBA) Calcd. for C31H55N3O9S [M+H]+ 646.3737, found 646.3737.
4.2.7. N-tBoc S-Palmitoyl L-Glutathione di-tert-butyl ester (8)
tert-Butanol (2.18 mL, 23 mmol, 10.6 equiv) and N,N′-diisopropylcarbodiimide (3.2 mL, 20.8 mmol, 9.6 equiv) were stirred overnight in the presence of a catalytic amount of CuCl (25 mg) under an argon atmosphere. The resulting O-tert-butyl N,N′-diisopropylisourea solution was diluted with dry dichloromethane (10 mL) followed by the addition of 7 (1.4 g, 2.16 mmol) and then the reaction mixture was heated under refluxed. After 2 days, the reaction mixture was filtered through Celite® and the filter cake was washed with dichloromethane (150 mL). The filtrate was concentrated in vacuo and the residue purified by column chromatography on silica gel impregnated with triethylamine (2 mL Et3N/100 g SiO2) using 30% EtOAc/hexanes as eluant to give 8 (1.15 g, 70%) as a thick syrup. TLC: 5% MeOH/CH2Cl2, Rf ∼ 0.66; [α]23D –12.5 (c 2.06, CHCl3); 1H NMR (300 MHz) δ 6.96 (t, 1H, J = 5.2 Hz), 6.77 (d, 1H, J = 7.0 Hz), 5.24 (d, 1H, J = 7.6 Hz), 4.60 (dt, 1H, J = 4.8, 7.9 Hz), 4.16-4.22 (m, 1H), 3.90 (dd, 1H, J = 5.5, 12.6 Hz), 3.34 (dd, 1H, J = 4.6, 14.3 Hz), 3.25 (dd, 1H, J = 7.94, 14.3 Hz), 2.56 (t, 2H, J = 7.3 Hz), 2.12-2.38 (m, 2H), 1.63 (t, 2H, J = 7.1 Hz), 1.46 (s, 9H), 1.45 (s, 9H), 1.43 (s, 9H), 1.24 (br s, 26H), 0.87 (t, 3H, J = 7.0 Hz); 13C NMR (75 MHZ) δ 200.5, 172.8, 171.5, 170.1, 168.6, 155.9, 82.3, 80.0, 53.6, 44.1, 42.2, 32.4, 32.0, 30.4, 29.78, 29.77, 29.75, 29.71, 29.54, 29.47, 29.3, 29.1, 28.8, 28.5, 28.2, 28.1, 25.7, 22.8, 14.3; ESMS m/z 780 (M++23); HRMS (FAB-CI, NBA) Calcd. for C39H71N3O9S [M+H]+ 758.4997, found 758.4989.
4.2.8. N-tBoc L-Glutathione di-tert-butyl ester (9)
A freshly prepared 0.1 N solution of NaOMe in MeOH (14 mL, 1.45 mmol) was added to a stirring solution of 8 (1.0 g, 1.32 mmol) in methanol (14 mL) under an argon atmosphere. After 1 h, the reaction mixture was cooled to 5 °C and acidified to pH 5 using 5% acetic acid in ether. All volatiles were removed in vacuo and the residue was purified by column chromatography using 50% EtOAc/hexanes as eluant to furnish 9 (480 mg, 71%), mp 41.5°C. TLC: 50% EtOAc/hexanes, Rf ∼ 0.26; [α]23D –7.6 (c 2.0, CHCl3); 1H NMR (400 MHz) δ 6.93 (d, 1H, J = 6.4 Hz), 6.80-6.85 (m, 1H), 5.26 (d, 2H, J = 7.9 Hz), 4.64-4.72 (m, 1H), 4.16-4.28 (m, 1H), 3.96 (dd, 1H, J = 5.5, 13 Hz), 3.87 (dd, 1H, J = 5.4, 11.7 Hz), 3.06-3.18 (m, 2H), 2.78-2.86 (m, 1H), 2.36 (t, 2H, J = 7.4 Hz), 2.18-2.26 (m, 1H), 1.82 (dd, 1H, J = 7.9, 9.8 Hz), 1.47 (s, 18H), 1.45 (s, 9H); 13C NMR (75 MHz) δ 172.6, 171.6, 170.1, 168.7, 156.0, 82.4, 80.1, 54.6, 53.5, 42.2, 32.5, 29.1, 28.4, 28.14, 28.09, 26.7; ESMS m/z 542 (M++23); HRMS (FAB-CI, NBA) Calcd. for C23H41N3O8S [M+H]+ 520.2693, found 520.2692.
Acknowledgments
Financial support provide by the Robert A. Welch Foundation and NIH (GM31278, DK38226, GM37922).
References and notes
- 1.Reid M, Jahoor F. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3:385–390. doi: 10.1097/00075197-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Masip L, Veeravalli K, Georgiou G. Antiox. Redox Signaling. 2006;8:753–762. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- 3.Reid M, Jahoor F. Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:65–71. doi: 10.1097/00075197-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Droge W, Breitkreutz R. Proc. Nutr. Soc. 2000;59:595–600. doi: 10.1017/s0029665100000847. [DOI] [PubMed] [Google Scholar]
- 5.Lorenson MY, Jacobs LS. Endocrinology. 1987;120:365–372. doi: 10.1210/endo-120-1-365. [DOI] [PubMed] [Google Scholar]
- 6.Cnubben NHP, Rietjens IMCM, Wortelboer H, van Zanden J, van Bladeren PJ. Environ. Toxicol. Pharm. 2001;10:141–152. doi: 10.1016/s1382-6689(01)00077-1. [DOI] [PubMed] [Google Scholar]
- 7.Shackelford RE, Heinloth AN, Heard SC, Paules RS. Antiox. Redox Signaling. 2005;7:940–950. doi: 10.1089/ars.2005.7.940. [DOI] [PubMed] [Google Scholar]
- 8.(a) Vamvakas S, Anders MW. Adv. Exp. Med. Biol. 1991;283:13–24. doi: 10.1007/978-1-4684-5877-0_2. [DOI] [PubMed] [Google Scholar]; (b) Lauterburg BH. Prog. Pharm. Clin. Pharm. 1991;8:201–213. [Google Scholar]
- 9.Ingelman-Sundberg M. Chem.-Biol. Interact. 2001;133:84–86. [Google Scholar]
- 10.Murphy RC, Zarini S. Prostag. Oth. Lipid M. 2002;68–69:471–482. doi: 10.1016/s0090-6980(02)00049-7. Review: [DOI] [PubMed] [Google Scholar]
- 11.Spearman ME, Prough RA, Estabrook RW, Falck JR, Manna S, Leibman KC, Murphy RC, Capdevila J. Arch. Biochem. Biophys. 1985;242:225–230. doi: 10.1016/0003-9861(85)90496-5. [DOI] [PubMed] [Google Scholar]
- 12.(a) Threadgill MD, Gledhill AP. J. Org. Chem. 1989;54:2940–2949. For a multi-step total synthesis of 3 from L-cysteine and an unsuccessful attempt directly from L-glutathione see. [Google Scholar]; (b) Crich D, Krishnamurthy V, Hutton TK. J. Am. Chem. Soc. 2006;128:2544–2545. doi: 10.1021/ja057521c. Using L-glutathione see. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su D, Ren X, You D, Li D, Mu Y, Yan G, Zhang Y, Luo Y, Xue Y, Shen J, Liu Z, Luo G. Arch. Biochem. Biophysics. 2001;395:177–184. doi: 10.1006/abbi.2001.2551. [DOI] [PubMed] [Google Scholar]
- 14.Arisawa M, Ono T, Yamaguchi M. Tetrahedron Lett. 2005;46:5669–5671. [Google Scholar]
- 15.Kedrowski BL, Heathcock CH. Heterocycles. 2002;58:601–634. [Google Scholar]
- 16.Anderson ME, Powrie F, Puri RN, Meister A. Arch. Biochem. Biophys. 1985;239:538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- 17.Muraki M, Mizoguchi T. Chem. Pharm. Bull. 1971;19:1708–1713. [Google Scholar]
- 18.(a) Anderson GW, Callahan FM. J. Am. Chem. Soc. 1960;82:3359–3363. [Google Scholar]; (b) Valerio RM, Alewood PF, Johns RB. Synthesis. 1988:786–789. [Google Scholar]
- 19.Liu L, Tanke RS, Miller MJ. J. Org. Chem. 1986;51:5332–5337. [Google Scholar]
- 20.Takeda K, Akiyama A, Nakamura H, Takizawa S, Mizuno Y, Takayanagi H, Harigaya Y. Synthesis. 1994 [Google Scholar]
- 21.Chevallet P, Garrouste P, Malawska B, Martinez J. Tetrahedron Lett. 1993;34:7409–7412. [Google Scholar]
- 22.Loffet A, Galeotti N, Jouin P, Castro B. Tetrahedron Lett. 1989;30:6859–6860. [Google Scholar]
- 23.Widmer U. Synthesis. 1983:135–136. [Google Scholar]
- 24.Dhaon MK, Olsen RK, Ramasamy K. J. Org. Chem. 1982;47:1962–1965. [Google Scholar]
- 25.Ohta S, Shimabayashi A, Aona M, Okamoto M. Synthesis. 1982:833–834. [Google Scholar]
- 26.Shih I-L, Chiu L-C, Lai CT, Liaw W-C, Tai D-F. Biotech. Lett. 1997;19:857–859. [Google Scholar]
- 27.Galzigna L. PCT Int. Appl. 1992 WO 9200320 A1 19920109; CAN 116:152418; AN 1992:152418. [Google Scholar]
- 28. A similar strategy to improve the solubility of GSH by preparing a lipophilic S-derivative prior to esterification was published while this manuscript was in preparation see ref. 12b.
- 29.Vignais PV, Zabin I. Biochim. Biophys. Acta. 1958;29:263–269. doi: 10.1016/0006-3002(58)90183-5. A related procedure produced a mixture of 54% S-palmitoyl and 46% N-palmitoyl glutathione: [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Hu X, Dizin E, Pei D. J. Am. Chem. Soc. 2003;125:13379–13381. doi: 10.1021/ja0369663. [DOI] [PubMed] [Google Scholar]
- 31.Zervas L, Photaki I, Ghelis N. J. Am. Chem. Soc. 1963;85:1337–1341. [Google Scholar]