Abstract
Despite recent advances in antibiotic therapy and intensive care, sepsis remains a widespread problem in critically ill patients. The high mortality from sepsis is in part mediated by bacterial endotoxin, which stimulates macrophages/monocytes to sequentially release early (e.g., tumor necrosis factor, interleukin-1, and interferon-γ) and late [e.g., high mobility group box 1 protein (HMGB1)] proinflammatory cytokines. Our discovery of HMGB1 as a late mediator of lethal systemic inflammation has initiated a new field of investigation for the development of experimental therapeutics. A popular Chinese herb, Angelica sinensis (also known as Dang Gui or Dong Quai) has been used traditionally for treating women with gynecological disorders (such as dysmenorrheal and hot flashes). Here we examined the effect of Angelica sinensis extract on endotoxin-induced HMGB1 release in vitro, and explored its therapeutic potential in animal models of lethal endotoxemia and sepsis [induced by cecal ligation and puncture (CLP)] in vivo. We demonstrated that a low-molecular-weight (<10 kDa) fraction of A. sinensis extract significantly attenuated endotoxin-induced HMGB1 release in part through interfering with its cytoplasmic translocation in macrophage cultures. Prophylactic administration of an aqueous extract of A. sinensis significantly attenuated systemic HMGB1 accumulation in vivo, and conferred a dose-dependent protection against lethal endotoxemia. Furthermore, delayed administration of A. sinensis extract beginning 24 h after CLP attenuated systemic HMGB1 accumulation, and significantly rescued mice from lethal sepsis. Taken together, these data suggest that A. sinensis contains water-soluble components that exert protective effects against lethal endotoxemia and experimental sepsis in part by attenuating systemic accumulation of a late proinflammatory cytokine, HMGB1.
Keywords: cecal ligation and puncture, HMGB1, macrophages, cytoplasmic translocation
“Severe sepsis” refers to an overwhelming systemic inflammatory response to infection, and is defined by signs of organ dysfunction that include abnormalities in body temperature, heart rate, respiratory rate, and leukocyte counts. Despite recent advances in antibiotic therapy and intensive care, sepsis is still the most common cause of death in intensive care units, claiming ∼225,000 victims annually in the United States alone. Initiated by an infection, the pathogenesis of sepsis is attributable, at least in part, to dysregulated systemic inflammatory responses characterized by excessive accumulation of various proinflammatory cytokines (1-4).
In response to bacterial toxins [e.g., lipopolysaccharide (LPS)], macrophages/monocytes release nitric oxide (5), and various proinflammatory cytokines such as tumor necrosis factor (TNF) (6), interleukin (IL)-1 (7), interferon-γ (8), and macrophage migration inhibitory factor (MIF) (9), which individually, or in combination, contribute to the pathogenesis of lethal endotoxemia or sepsis. For instance, neutralizing antibodies against TNF, the first cytokine elaborated in the inflammatory cascade, reduces lethality in an animal model of endotoxemic/bacteremic shock (6). However, the early release of TNF makes it difficult to target therapeutically in a clinical setting (6), prompting the search for late proinflammatory cytokines that may offer a wider therapeutic window for the treatment of lethal systemic inflammatory diseases.
We discovered that a ubiquitous protein, high mobility group box 1 (HMGB1), is released by activated macrophages/monocytes (10-12) and functions as a late mediator of lethal endotoxemia and sepsis (10,13-15). First, circulating HMGB1 levels are elevated in a delayed fashion (after 16–32 h) in endotoxemic and septic mice (10,13), and in patients with sepsis (10). Second, administration of recombinant HMGB1 to mice recapitulates many clinical signs of sepsis, including fever (16), allodynia (17), derangement of intestinal barrier function (18), lung injury (19), and lethal multiple organ failure (10). Third, administration of anti-HMGB1 antibodies or inhibitors (e.g., ethyl pyruvate, nicotine, or stearoyl lysophosphatidylcholine) significantly protects mice against LPS-induced acute lung injury (19,20), and lethal endotoxemia (10,14,21-23). Notably, these anti-HMGB1 reagents are also capable of rescuing mice from lethal experimental sepsis even when the first doses are given 24 h after the onset of sepsis (13,14,24), indicating a wider window for HMGB1-targeted therapeutic strategies. Therefore, agents proven clinically safe, and yet still capable of attenuating HMGB1 release, may hold potential in the prevention and treatment of inflammatory diseases.
Various Chinese herbs [e.g., Bai Shu (white atractylodes root, or Atractylodes macrocephalae), Bai Shao (white peony root, or Paeoniae lactiflora), and Dang Gui (also known as “Dong Quai,” or Angelica sinensis)] have been used alone, or in combination with others, in the treatment of various inflammatory diseases (25-29). For instance, the Chinese name of “Dang Gui,” literally means “state of return,” and refers to its ability to regulate “qi” and “blood” to maintain a normal state of well-being. Its medical use was first recorded in the Shen Nong Ben Cao Jing during the 1st century; it was subsequently listed in the 22nd edition of the United States Dispensatory (30). A. sinensis is often referred to as the female ginseng, and has been traditionally used to treat gynecological disorders such as abnormal, painful menstruation (dysmenorrhea), pelvic pain, or uterine bleeding. In fact, a clinical study of a combinational therapy containing soy isoflavones (60 mg), A. sinensis (100 mg), and black cohosh (50 mg), revealed efficacy in reducing the frequency and severity of menstrual migraines after 1 mo of therapy (31).
In addition, A. sinensis has been used as an analgesic for the treatment of abdominal pain, anemia, and chronic hepatitis, but its mechanism of action remains largely elusive. Recently, a number of preclinical studies documented its beneficial effects in animal models of bacteria-induced pneumonia (27), carrageenan-induced edema (29), and ethanol-induced hemorrhagic tissue damage (32). However, it was previously not known whether A. sinensis extract is capable of attenuating HMGB1 and protecting mice against lethal systemic inflammatory diseases. In this study, we examined the effects of A. sinensis on bacterial endotoxin–induced HMGB1 release, and explored its therapeutic potential in animal models of lethal endotoxemia and sepsis.
MATERIALS AND METHODS
Cell culture
Murine macrophage-like RAW 264.7 cells were obtained from the American Type Culture Collection and cultured in RPMI 1640 medium (Gibco BRL) supplemented with 10% fetal bovine serum and 2 mmol/L glutamine. At 80–90% confluence, RAW 264.7 cells were washed twice with, and subsequently cultured in, serum-free OPTI-MEM I medium (Gibco BRL) before stimulation with bacterial endotoxin (LPS, E. coli 0111:B4, Sigma-Aldrich). Cell viability was assessed by trypan blue exclusion and propidium iodide uptake assays as previously described (11,33).
LPS stimulation
Macrophage cultures were stimulated with LPS alone, or in the presence of herbal extract or ferulic acid (no. 4628, Sigma-Aldrich). At 16 or 24 h after LPS stimulation, HMGB1 levels in the culture medium were determined as previously described (10-12).
Preparation of herbal extract
Dry root slices of 3 popular Chinese herbs, Bai Shao (Paeoniae lactiflora), Bai Shu (Atractylodes macrocephalae), and Dang Gui (Angelica sinensis), were obtained from NY-Tongrentang and extracted in water (85°C) for 4 h. The water-soluble fraction was cleared sequentially by centrifugation (3300 × g, 20 min, 4°C) and filtration (through a 0.2-μm filter). The clear water-soluble filtrate fraction was subsequently fractionated using the Centriprep YM-10 Centrifugal Filter unit following the manufacturer's instructions (Catalogue no. 4305, Millipore). The resulted low- (<10 kDa) and high-molecular-weight (>10 kDa) fractions were examined for HMGB1-suppressing activities. From 10 g dry Angelica sinensis root, ∼0.3 g yellow, oily substance was recovered from the low-molecular-weight (<10 kDa) fraction (LMWF) after lyophilization.
Animal models of endotoxemia and sepsis
This study was approved and performed in accordance with the guidelines for the care and use of laboratory animals at the Feinstein Institute for Medical Research, Manhasset, New York. Endotoxemia was induced in Balb/C mice (male, 7–8 wk old) by i.p. injection of bacterial endotoxin (LPS, 15 mg/kg) as previously described (10,22). To study the efficacy of herbal extract for the treatment of sepsis, a clinically relevant, standardized animal model of sepsis induced by cecal ligation and puncture (CLP) was employed as previously described (13,14). Briefly, the cecum of Balb/C mice was ligated at 5.0 mm from the cecal tip, and then punctured once with a 22-gauge needle. Herbal extract was administered i.p. into mice at indicated doses and time points, and mice were monitored for survival for up to 2 wk. In parallel experiments, mice were killed to collect blood at 28 h (after 2 doses of herbal extract at −0.5 and +24 h) after endotoxemia, and at 52 h (after 2 doses of herbal extract at +24 and +48 h) after CLP. Serum HMGB1 levels were determined by Western blotting analysis as previously described (10).
TNF ELISA
The levels of TNF in the culture medium or serum were determined using commercially available ELISA kit (no. MTA00, R & D Systems) with reference to standard curves of purified recombinant TNF at various dilutions as previously described (11,12).
Nitric oxide assay
The levels of nitric oxide in the culture medium were determined indirectly by measuring NO2− production with a colorimetric assay based on the Griess reaction as previously described (11). NO2− concentrations were determined with reference to a standard curve generated with sodium nitrite at various dilutions.
HMGB1 Western blotting analysis
The levels of HMGB1 in the culture medium or serum were determined by Western blotting analysis as previously described (10-12). The relative band intensity was quantified by using the NIH image 1.59 software to determine HMGB1 levels with reference to standard curves generated with purified HMGB1.
HMGB1 immunostaining
At 6 h after LPS stimulation, cellular HMGB1 was immunostained with anti-HMGB1 polyclonal antibodies, and images were acquired using a confocal microscope (Fluoroview, Olympus) as previously described (11,12).
Statistical analysis
Data are expressed as means ± SD of 2 independent experiments performed in triplicate. One-way ANOVA was used for comparison among the different groups. When the ANOVA was significant, post hoc testing of differences between groups was performed using Duncan's test. The Kaplan-Merer method was used to compare the differences in mortality rates among groups. A P-value < 0.05 was considered significant.
RESULTS
Effect of A. sinensis extract on bacterial endotoxin-induced HMGB1 release
The aqueous extract of Bai Shao and Bai Shu, at a wide range of concentrations, did not significantly attenuate endotoxin-induced release of nitric oxide and HMGB1. In contrast, the aqueous extract of A. sinensis dose dependently attenuated bacterial endotoxin–induced release of nitric oxide and HMGB1 (data not shown). As a first step in gaining insight into the molecular properties of active principles responsible for inhibiting HMGB1 release, the aqueous extract of A. sinensis was size-fractionated by ultrafiltration through membranes with a 10-kDa molecular weight cut-off. The high-molecular-weight (>10 kDa) fraction did not significantly attenuate bacterial endotoxin-induced release of TNF, nitric oxide, and HMGB1. In contrast, the LMWF dose dependently and significantly attenuated bacterial endotoxin–induced release of TNF (Fig. 1A), nitric oxide (Fig. 1B), and HMGB1 (Fig. 1C).
FIGURE 1.
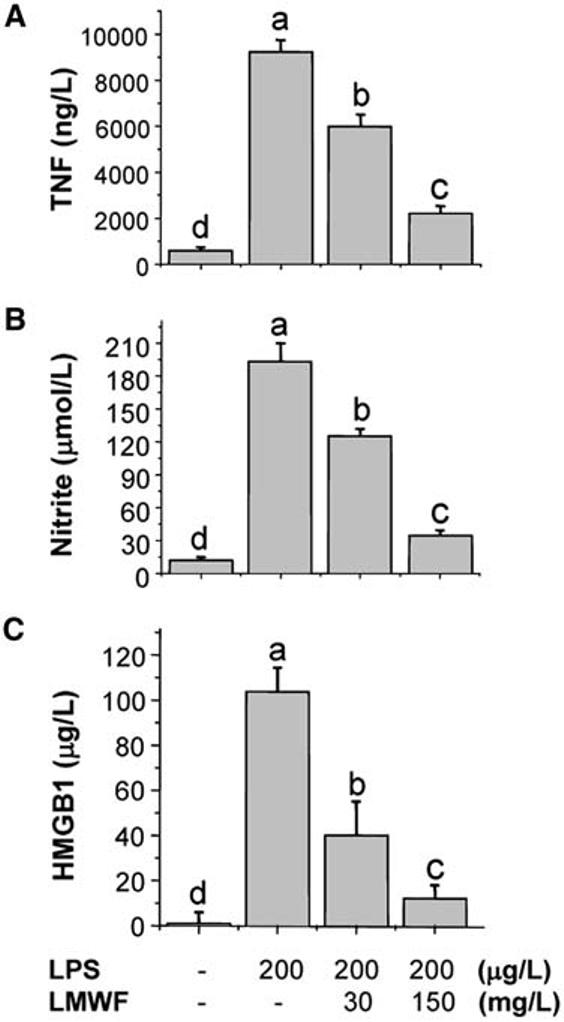
Aqueous extract of A. sinensis suppressed endotoxin-induced release of TNF (A), nitric oxide (B) and HMGB1 (C). Murine macrophage-like RAW 264.7 cells were stimulated with LPS in the absence or presence of the LMWF of A. sinensis extract at various concentrations. At 16 h after stimulation, levels of TNF, nitric oxide, and HMGB1 in the culture medium were determined and expressed as means ± SD of 2 independent experiments. Means not sharing a common letter differ, P < 0.05 (ANOVA, Duncan's test).
To determine whether the herbal extract merely shifted the kinetics of LPS-induced cytokine release, we also determined cytokine levels at a later time point. Even at 24 h post-LPS stimulation, the LMWF of A. sinensis extract similarly suppressed LPS-induced release of HMGB1 (by >80 ± 15%, n = 2) and nitric oxide (by >90 ± 10%, n = 2), indicating that the herbal extract promoted a long-lasting suppression of LPS-induced cytokine release. Even at concentrations that almost completely abrogated HMGB1 release, the LMWF of A. sinensis extract did not exhibit any cytotoxicity to macrophage cultures because cell viability, as assessed by trypan blue exclusion or propidium iodide uptake (data not shown), was not reduced (98 ± 2% in the presence of LPS + herbal extract, 150 mg/L, compared with 94 ± 6% in the presence of LPS + vehicle). This is not unexpected because it was demonstrated that necrotic cells passively release HMGB1 (34), thereby potentially increasing (rather than decreasing) extracellular HMGB1 levels.
Aqueous A. sinensis extract suppressed HMGB1 release by interfering with its cytoplasmic translocation
To investigate the mechanisms of A. sinensis–mediated suppression of HMGB1 release, we determined its effect on endotoxin-induced HMGB1 translocation, an essential step for HMGB1 release (11,12,35). Consistent with previous reports (11,12,35), quiescent macrophages constitutively expressed HMGB1 and maintained an intracellular “pool” of HMGB1 predominantly in the nucleus (Fig. 2A). At 16 h post-LPS stimulation, there was a noticeable amount of HMGB1 staining in cytoplasmic vesicles (Fig. 2C), confirming that LPS stimulates macrophages to actively translocate nuclear HMGB1 to the cytoplasm before releasing it into the extracellular milieu. Although the LMWF of A. sinensis extract did not affect the nuclear localization of HMGB1 in resting cells (Fig. 2B), it dramatically attenuated LPS-induced cytoplasmic translocation in 70–80% endotoxin-stimulated cells (Fig. 2D), indicating that A. sinensis extract attenuates LPS-induced HMGB1 release by interfering with its cytoplasmic translocation.
FIGURE 2.

Aqueous extract of A. sinensis suppressed endotoxin-induced HMGB1 translocation. Quiescent macrophage-like RAW 264.7 cells (A, B) were stimulated with LPS alone (C), or in the presence of the LMWF of A. sinensis extract (150 mg/L, D), and immunostained with HMGB1-specific antibodies. Upper panels are light-translucent microscopic photographs of cells in the corresponding lower panels. Arrows indicate the nuclear regions of representative cells. Bar, 7.5 μm.
A component of A. sinensis, ferulic acid, did not inhibit endotoxin-induced HMGB1 release in vitro
A. sinensis contains a number of anti-inflammatory components including ferulic acid (36). To determine whether ferulic acid is responsible for the HMGB1-suppressing activities of A. sinensis, we explored its capacity to inhibit endotoxin-induced HMGB1 release. Consistent with earlier reports (37,38), ferulic acid almost completely abolished endotoxin-induced nitric oxide release (190 ± 20 μmol/L, in the presence of LPS alone, compared with 28 ± 7 μmol/L in the presence of LPS plus ferulic acid, 500 mg/L; n = 2, P < 0.05). However, it did not attenuate endotoxin-induced release of TNF or HMGB1 (data not shown), suggesting that ferulic acid is not the active principle of A. sinensis responsible for inhibiting HMGB1 release. In addition, these results suggest that nitric oxide may not be important in the regulation of HMGB1 cytoplasmic translocation (such as through S-nitrosylation) and release (11).
Aqueous extract of A. sinensis protects against lethal endotoxemia
In light of capacity of the LMWF of A. sinensis extract (but not ferulic acid) in attenuating LPS-induced HMGB1 release, we explored the therapeutic potential of A. sinensis extract in an animal model of lethal endotoxemia. Administration of a single dose of the LMWF of A. sinensis extract 30 min before a 70% lethal dose of LPS did not significantly improve animal survival rate (30% for control administered saline, n = 20 mice/group, compared with 37.5% for the experimental group administered the herbal extract at 200 mg/kg, n = 20 mice/group; P < 0.05). After treatment of the mice with 3 additional doses of herbal extract (+24, +48, and +72 h), there was a dose-dependent improvement in survival (from 30 to 90%, P < 0.05, Fig. 3A). All of these mice were observed for at least 2 wk, and no late deaths occurred, indicating that A. sinensis extract does not merely delay the onset of LPS lethality, but provides long-lasting protection.
FIGURE 3.
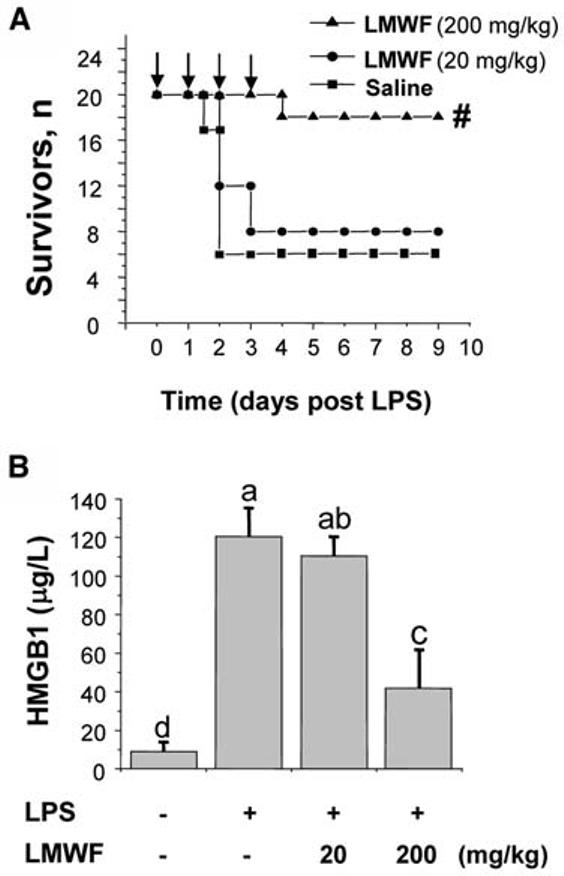
Aqueous extract of A. sinensis significantly protects mice against lethal endotoxemia (A) in part by attenuating systemic HMGB1 accumulation (B). Balb/C mice were administered (i.p.) bacterial endotoxin (LPS, 15 mg/kg), and the LMWF of A. sinensis extract was administered (i.p.) at −0.5, +24, +48, and +72 h after the onset of endotoxemia. The Kaplan-Merer method was used to compare the differences in mortality rates between groups (A). #Different from saline, P < 0.05. Serum HMGB1 levels (B) were expressed as means ± SD, n = 5. Means not sharing a common letter differ, P < 0.05 (ANOVA, Duncan's test).
To gain insight into the protective mechanism of the LMWF of A. sinensis extract against lethal endotoxemia, we evaluated its effects on the systemic accumulation of HMGB1. Repeated administration of herbal extract beginning at 30 min before the onset of endotoxemia dose dependently and significantly attenuated circulating HMGB1 levels (Fig. 3B), suggesting that A. sinensis extract protects mice against lethal endotoxemia in part through attenuating systemic accumulation of HMGB1.
Aqueous extract of A. sinensis rescues mice from lethal experimental sepsis
Although endotoxemia is useful in investigating the complex cytokine cascades, more clinically relevant animal models are necessary to explore efficacy of herbal extract for the treatment of sepsis. One well-characterized, standardized animal model of sepsis is induced by CLP. In light of the late and prolonged kinetics of HMGB1 accumulation in experimental sepsis (13), we reasoned that it might be possible to rescue mice from lethal sepsis even if the herbal extract is administered after the onset of sepsis. The first dose of the LMWF of A. sinensis extract was given 24 h after the onset of sepsis, a time point at which mice had developed clear signs of sepsis (including lethargy, diarrhea, piloerection). Intraperitoneal administration with a single dose of herbal extract 24 h after the onset of sepsis did not improve the survival rate (25% for control administered saline, n = 20 mice/group compared with 30% for experimental group administered the herbal extract at 200 mg/kg, n = 20 mice/group, P > 0.05). However, repeated administration of herbal extract beginning 24 h after the onset of sepsis (followed by 3 additional doses at +48, +72, and +96 h postsepsis) conferred a dose-dependent protection against lethal sepsis (Fig. 4A), significantly increasing animal survival rate from 25 to 70% (P < 0.05).
FIGURE 4.

Aqueous extract of A. sinensis rescues mice from lethal sepsis (A) in part by attenuating systemic HMGB1 accumulation (B). Balb/C mice were subjected to lethal sepsis by CLP, and the LMWF of A. sinensis extract was administered (i.p.) at +24, +48, +72, and +96 h post-CLP. The Kaplan-Merer method was used to compare the differences in mortality rates between groups (A). #Different from saline, P < 0.05. Serum HMGB1 levels (B) were expressed as means ± SD, n = 5. Means not sharing a common letter differ, P < 0.05 (ANOVA, Duncan's test).
To gain further insight into the mechanisms underlying herbal extract–mediated protection against lethal sepsis, we evaluated its effects on the systemic accumulation of TNF, nitric oxide, and HMGB1. Consistent with an early report (21), systemic TNF was barely detectable at a late stage of sepsis. Delayed administration of herbal extract did not attenuate circulating TNF levels 52 h after the onset of sepsis (TNF = 50 ± 15 ng/L, vehicle control group, n = 10 mice/group compared with TNF = 70 ± 25 ng/L, herbal group, n = 10 mice/group; P > 0.05). Similarly, delayed administration of herbal extract did not attenuate circulating nitric oxide levels 52 h after the onset of sepsis (16.1 ± 4.5 μmol/L, vehicle control group, compared with 14.3 ± 2.3 μmol/L, herbal group; n = 3, P > 0.05). In contrast, repeated administration of the herbal extract dose dependently attenuated circulating HMGB1 levels in septic mice (Fig. 4B, P < 0.05), indicating that A. sinensis extract confers protection against lethal sepsis in part by attenuating systemic accumulation of a late proinflammatory cytokine, HMGB1.
DISCUSSION
Despite recent advances in antibiotic therapy and intensive care, gram-negative bacterial infection and sepsis are widespread problems in critically ill patients. The high mortality of sepsis is mediated in part by bacterial endotoxin (39), which activates macrophages and monocytes to sequentially release early (e.g., TNF and IL-1) (40,41), nitric oxide (5), and late (e.g., HMGB1) (10) proinflammatory cytokines. HMGB1 is constitutively expressed in quiescent macrophages, and a large “pool” of HMGB1 is stored in the nucleus, due to the presence of 2 lysine-rich nuclear localization sequences (42). After LPS stimulation, activated macrophages translocate nuclear HMGB1 into cytoplasmic vesicles before its subsequent release into the extracellular milieu (11,12,35,42).
Here, we demonstrated that a LMWF of A. sinensis extract dose dependently attenuates endotoxin-induced HMGB1 release in macrophage cultures. Notably, A. sinensis extract also significantly attenuates endotoxin-induced release of other inflammatory mediators (e.g., TNF, and nitric oxide). However, its suppression of endotoxin-induced HMGB1 release is not likely dependent on its concomitant suppression of TNF or nitric oxide for the following reasons: 1) genetic disruption of TNF expression does not always lead to a reduction in HMGB1 release, particularly when macrophages are stimulated with endotoxin at high levels (>100 μg/L) (12); and 2) inhibition of nitric oxide production by ferulic acid did not significantly decrease endotoxin-induced HMGB1 release. Alternatively, A. sinensis extract attenuates HMGB1 release in part by interfering with its cytoplasmic translocation, thereby preserving its nuclear localization in endotoxin-stimulated cells.
The active principles of A. sinensis responsible for inhibiting HMGB1 release remain elusive, although it contains a number of anti-inflammatory substances including polysaccharides (32, 43), estrogen-like phytoestrogens (44-46), and ferulic acid (36). Although a randomized clinical trial using A. sinensis as an alternative hormone replacement therapy did not show efficacy in reducing postmenopausal hot flashes (47), ferulic acid has shown great promise in preventing bone loss in ovariectomized rats (48) and treating hot flashes in menopausal women (49). In light of the capacity of ferulic acid to attenuate endotoxin-induced nitric oxide release, as well as the important roles of nitric oxide in the pathogenesis of sepsis (50,51), it may be important to investigate the therapeutic potential of ferulic acid for sepsis in future studies.
In an animal model of lethal endotoxemia, repeated administration of herbal extract beginning 30 min before the onset of endotoxemia, significantly attenuated circulating HMGB1 levels, and increased animal survival rate from 30 to 90%. It suggests that A. sinensis extract protects against lethal endotoxemia in part through attenuating systemic accumulation of HMGB1. Although endotoxemia does occur in septic patients (52), an animal model of lethal endotoxemia may not be clinically relevant to human sepsis. Therefore, more clinically relevant animal models are required to explore the efficacy of herbal extracts for the treatment of sepsis.
Using a standardized animal model of sepsis induced by CLP, we demonstrated that delayed administration of A. sinensis extract beginning 24 h after CLP (a time point at which some mice have already died before receiving herbal extract) rescues mice from lethal sepsis. To our knowledge, no other proinflammatory mediators can be targeted this late in the course of experimental sepsis to rescue mice from lethality. By comparison, anti-MIF antibodies are protective if administered 8 h after CLP (9), whereas anti-TNF antibodies actually increase mortality in experimental sepsis (53). In light of our observation that delayed administration of A. sinensis extract significantly attenuated systemic accumulation of HMGB1 (but not TNF or nitric oxide), we propose that A. sinensis rescues mice from lethal sepsis in part by attenuating systemic HMGB1 accumulation.
At present, it is not known whether the aqueous extract of A. sinensis is orally effective in animal models of sepsis and whether it exerts protective effects by interfering with the availability and/or binding of LPS to macrophage cell surface receptors. The LMWF of A. sinensis extract is likely to be orally effective because prolonged incubation of this herbal extract at acidic pH (e.g., pH 4.4), or treatment with proteases (e.g., trypsin), did not affect its HMGB1-inhibiting activities in vitro (data not shown). The daily dosages used for septic mice are equivalent to daily dosages of 5–50 g A. sinensis for a person (with an average body weight of 75 kg), assuming a ∼10% recovery of active principle(s) after a 3-step purification (i.e., extraction, filtration, and ultrafiltration). These estimated daily dosages (5–50 g/person) of A. sinensis for severely septic patients are comparable to the daily dosages (3–15 g/person) recommended for elderly people as a nutrient supplement (54). With the continuous increase in the consumption of botanical supplements in the United States, it might be beneficial in future studies to explore the therapeutic potential of A. sinensis in the clinical management of human sepsis and other inflammatory diseases.
Footnotes
Presented at the American College of Emergency Physician (ACEP) Scientific Assembly, October 17–20, 2004, San Francisco, CA [Wang H, Ma G, Ochani M, Li J. Ancient Chinese herbal medicine as a modern hope for the treatment of sepsis: extract of Angelica sinensis as an antagonist for a newly discovered late mediator of sepsis. Ann Emerg Med. 2004 Oct;44(4):S52].
Supported in part by the National Institutes of Health grants (R01GM063075, R01GM070817, to H.W.) and the Feinstein Institute for Medical Research Faculty Research Award (to H.W.).
Abbreviations used: CLP, cecal ligation and puncture; HMGB1, high mobility group box 1 protein; IL, interleukin; LPS, lipopolysaccharide; LMWF, low-molecular-weight fraction; MIF, migration inhibitory factor; TNF, tumor necrosis factor.
LITERATURE CITED
- 1.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–38. [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling–regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28(Suppl 4):N3–12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–9. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–10. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 8.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–4. [PubMed] [Google Scholar]
- 9.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–7. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Li J, Ochani M, Rendon-Mitchell B, Qiang X, Susarla S, Ulloa L, Yang H, Fan S, et al. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol. 2004;76:994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al Abed Y, Wang H, Metz C, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–31. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor KA, Hansen MK, Rachal PC, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, et al. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–65. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 18.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and causes derangements in intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 19.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–6. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 21.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–7. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–7. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Czura CJ, Tracey KJ. Lipid unites disparate syndromes of sepsis. Nat Med. 2004;10:124–5. doi: 10.1038/nm0204-124. [DOI] [PubMed] [Google Scholar]
- 24.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–6. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang HL, Chen CC, Yeh CY, Huang RL. Reactive oxygen species mediation of baizhu-induced apoptosis in human leukemia cells. J Ethnopharmacol. 2005;97:21–9. doi: 10.1016/j.jep.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Chen DZ, Li J, Czura CJ, Tracey KJ, Sama AE, Wang H. Pathogenic role of HMGB1 in SARS? Med Hypotheses. 2004;63:691–5. doi: 10.1016/j.mehy.2004.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song ZJ, Johansen HK, Moser C, Faber V, Kharazmi A, Rygaard J, Hoiby N. Effects of radix Angelicae sinensis and shuanghuanglian on a rat model of chronic Pseudomonas aeruginosa pneumonia. Chin Med Sci J. 2000;15:83–8. [PubMed] [Google Scholar]
- 28.Xue L, Zhang HY, Qin L, Wang XC, Wang L. Effect of chuanwu and baishao used separately or in combination on adjuvant arthritis in rats [in Chinese] Zhongguo Zhong Yao Za Zhi. 2000;25:175–8. [PubMed] [Google Scholar]
- 29.Hu H, Hang B, Wang P. Anti-inflammatory effect of radix Angelicae sinensis [in Chinese] Zhongguo Zhong Yao Za Zhi. 1991;16:684–6. 704. [PubMed] [Google Scholar]
- 30.Wood H, LaWell CH. The dispensatory of the United States of America. 22nd ed. J. B. Lippincott Company; Philadelphia: 1937. p. 1894. [Google Scholar]
- 31.Burke GL, Legault C, Anthony M, Bland DR, Morgan TM, Naughton MJ, Leggett K, Washburn SA, Vitolins MZ. Soy protein and isoflavone effects on vasomotor symptoms in peri- and postmenopausal women: the Soy Estrogen Alternative Study. Menopause. 2003;10:147–53. doi: 10.1097/00042192-200310020-00006. [DOI] [PubMed] [Google Scholar]
- 32.Cho CH, Mei QB, Shang P, Lee SS, So HL, Guo X, Li Y. Study of the gastrointestinal protective effects of polysaccharides from Angelica sinensis in rats. Planta Med. 2000;66:348–51. doi: 10.1055/s-2000-8552. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Harrison-Shostak DC, Lemasters JJ, Herman B. Contribution of pH-dependent group II phospholipase A2 to chemical hypoxic injury in rat hepatocytes. FASEB J. 1996;10:1319–25. doi: 10.1096/fasebj.10.11.8836046. [DOI] [PubMed] [Google Scholar]
- 34.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 35.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:955–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu GH, Chan K, Leung K, Chan CL, Zhao ZZ, Jiang ZH. Assay of free ferulic acid and total ferulic acid for quality assessment of Angelica sinensis. J Chromatogr A. 2005;1068:209–19. doi: 10.1016/j.chroma.2005.01.082. [DOI] [PubMed] [Google Scholar]
- 37.Murakami A, Nakamura Y, Koshimizu K, Takahashi D, Matsumoto K, Hagihara K, Taniguchi H, Nomura E, Hosoda A, et al. FA15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumor promotion: comparison with ferulic acid. Cancer Lett. 2002;180:121–9. doi: 10.1016/s0304-3835(01)00858-8. [DOI] [PubMed] [Google Scholar]
- 38.Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002;22:2711–7. [PubMed] [Google Scholar]
- 39.Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit Care Med. 2000;28:2949–55. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Czura CJ, Tracey KJ. TNF. In: Thomson A, Lotze MT, editors. The cytokine handbook. 4th ed. Academic Press; Oxford: 2003. pp. 837–60. [Google Scholar]
- 41.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 42.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choy YM, Leung KN, Cho CS, Wong CK, Pang PK. Immunopharmacological studies of low molecular weight polysaccharide from Angelica sinensis. Am J Chin Med. 1994;22:137–45. doi: 10.1142/S0192415X94000176. [DOI] [PubMed] [Google Scholar]
- 44.Piersen CE. Phytoestrogens in botanical dietary supplements: implications for cancer. Integr Cancer Ther. 2003;2:120–38. doi: 10.1177/1534735403002002004. [DOI] [PubMed] [Google Scholar]
- 45.Russell L, Hicks GS, Low AK, Shepherd JM, Brown CA. Phytoestrogens: a viable option? Am J Med Sci. 2002;324:185–8. doi: 10.1097/00000441-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–9. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 47.Hirata JD, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril. 1997;68:981–6. doi: 10.1016/s0015-0282(97)00397-x. [DOI] [PubMed] [Google Scholar]
- 48.Sassa S, Kikuchi T, Shinoda H, Suzuki S, Kudo H, Sakamoto S. Preventive effect of ferulic acid on bone loss in ovariectomized rats. In Vivo. 2003;17:277–80. [PubMed] [Google Scholar]
- 49.Philp HA. Hot flashes—a review of the literature on alternative and complementary treatment approaches. Altern Med Rev. 2003;8:284–302. [PubMed] [Google Scholar]
- 50.Vincent JL, Zhang H, Szabo C, Preiser JC. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med. 2000;161:1781–5. doi: 10.1164/ajrccm.161.6.9812004. [DOI] [PubMed] [Google Scholar]
- 51.Fink MP, Payen D. The role of nitric oxide in sepsis and ARDS: synopsis of a roundtable conference held in Brussels on 18–20 March 1995. Intensive Care Med. 1996;22:158–65. doi: 10.1007/BF01720723. [DOI] [PubMed] [Google Scholar]
- 52.Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE. Endotoxemia in human septic shock. Chest. 1991;99:169–75. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- 53.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–30. [PubMed] [Google Scholar]
- 54. Camline [The evidence-based complementary and alternative medicine website for healthcare professionals] Natural Health Products: Dong Quai Dose, Dosage Forms/Formulations [cited 2005 Oct 31]. Available from: http://www.camline.ca/professionalreview/pr_dose.php?NHPID=9.


